Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections (SSTI) have become increasingly common. This study's objectives were to describe the clinical spectrum of MRSA in a community health center and to determine whether the use of specific antimicrobials correlated with increased probability of clinical resolution of SSTI. A retrospective chart review of 399 sequential cases of culture-confirmed S. aureus SSTI, including 227 cases of MRSA SSTI, among outpatients at Fenway Community Health (Boston, MA) from 1998 to 2005 was done. The proportion of S. aureus SSTI due to MRSA increased significantly from 1998 to 2005 (P < 0.0001). Resistance to clindamycin was common (48.2% of isolates). At the beginning of the study period, most patients with MRSA SSTI empirically treated with antibiotics received a beta-lactam, whereas by 2005, 76% received trimethoprim-sulfamethoxazole (TMP-SMX) (P < 0.0001). Initially, few MRSA isolates were sensitive to the empirical antibiotic, but 77% were susceptible by 2005 (P < 0.0001). A significantly higher percentage of patients with MRSA isolates had clinical resolution on the empirical antibiotic by 2005 (P = 0.037). Use of an empirical antibiotic to which the clinical isolate was sensitive was associated with increased odds of clinical resolution on empirical therapy (odds ratio = 5.91), controlling for incision and drainage and HIV status. MRSA now accounts for the majority of SSTI due to S. aureus at Fenway, and improved rates of clinical resolution on empirical antibiotic therapy have paralleled increasing use of empirical TMP-SMX for these infections. TMP-SMX appears to be an appropriate empirical antibiotic for suspected MRSA SSTI, especially where clindamycin resistance is common.
Although methicillin-resistant Staphylococcus aureus (MRSA) is recognized as a significant cause of nosocomial infections, it is also becoming an increasingly common cause of infections presenting in the community (2, 5, 7, 10, 17, 20, 24). Patients with community-associated MRSA (CA-MRSA) infections have typically lacked traditional risk factors for MRSA infection, such as hospitalization, residency in a chronic-care facility, dialysis, and indwelling devices or catheters (2, 5, 7, 10, 17, 18, 20, 24). Risk factors and transmission of CA-MRSA remain incompletely understood, but outbreaks of CA-MRSA infections have been reported in diverse groups, including prisoners, military recruits, sports teams, and men who have sex with men (MSM). Most often, CA-MRSA causes skin and soft tissue infections (SSTI), such as abscesses and furunculosis, but other presentations, such as necrotizing pneumonia, have been reported (2, 5, 7, 10, 17, 20, 24).
CA-MRSA can be distinguished from classic hospital-associated MRSA based on genetic features, such as the presence of the staphylococcal cassette chromosome, mec type IV, and Panton-Valentine leukocidin production (2, 5, 7, 10, 17, 18, 20, 24). The antibiotic resistance profiles of community and hospital-associated MRSA also differ. Whereas hospital-associated MRSA usually has broad resistance to a number of diverse antibiotics, CA-MRSA has tended to have a narrower resistance profile and may often be sensitive to clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), and/or tetracyclines (2, 5, 10, 17, 20, 24). Guidelines for the treatment of CA-MRSA have been published, but data are limited regarding optimal outpatient antibiotic treatment regimens for MRSA SSTI (2, 3, 5, 10, 17, 19, 20, 24).
The purpose of this study was to examine the relationship between trends in choice of empirical antibiotic therapy for suspected S. aureus SSTI at an ambulatory care center and the likelihood of clinical resolution of these infections on the empirical antibiotic(s). We hypothesized that increasing usage of alternative antistaphylococcal drugs, such as TMP-SMX, would be associated with improved rates of clinical resolution among patients with suspected S. aureus SSTI.
MATERIALS AND METHODS
Study setting.
Fenway Community Health (FCH) is located in Boston, MA, and provides health care to a large urban patient population, more than half of whom are MSM, including more than 1,000 human immunodeficiency virus (HIV)-infected patients (15). More than 9,000 patients were seen at the ambulatory gynecology, medical, and podiatry clinics in 2005.
As this was a retrospective chart review, a waiver for informed consent was obtained from the FCH Institutional Review Board.
Study population.
The study population included all patients with at least one medical-clinic visit to FCH from 1 January 1998 through 31 December 2005 that was recorded in the clinic's electronic medical-record system (Centricity EMR; GE Healthcare). Patients with wound cultures positive for S. aureus were identified by querying the electronic medical-record database. Patients were included in the analysis of S. aureus skin and soft tissue infections only if they had a positive wound culture with antibiotic sensitivities available, as well as a clinic visit where they presented with signs and/or symptoms of skin or soft tissue infection. We also identified patients with other types of clinical cultures (e.g., urine, blood, or joint aspirates) positive for S. aureus to assess the spectrum of S. aureus infections seen among outpatients at FCH. Data from patients initially diagnosed at an outside hospital were included, provided these patients had a subsequent clinic visit at FCH and clinical cultures and antibiotic sensitivities were available. Cases diagnosed at outside hospitals without any supporting culture and sensitivity data in the record were excluded, as were cases in which only nasal cultures were obtained to detect asymptomatic staphylococcal carriage.
Clinical management.
Wound cultures were obtained at the discretion of FCH clinicians when there was clinical suspicion that the patient was at risk for infection with an antibiotic-resistant organism. The choice of antibiotic and duration of antibiotic therapy were at the discretion of the patient's physician.
Definitions.
The empirical antibiotic regimen was defined as the initial antibiotic regimen instituted prior to availability of culture and sensitivity data. Cases were considered to have clinically resolved only if the patient had a follow-up medical visit documenting the resolution of all symptoms and signs of the infection or if the infection was not listed as a current active problem and the documented physical findings did not include any persistent signs of the infection. Persons without any follow-up clinic visit were not counted as having clinical resolution of their infection. For individuals with multiple infection-related visits, recurrent MRSA skin or soft tissue infections were diagnosed if the patient presented to FCH with a new clinical infection more than 2 weeks after clinical resolution of a previous infection and/or clinical infections involving a noncontiguous anatomic site.
Highly active antiretroviral therapy (HAART) was defined as a medication regimen comprising at least three antiretroviral medications, including at least two nucleoside or nucleotide reverse transcriptase inhibitors.
Assessment of changes in health care providers' ordering of clinical cultures was done by first searching the electronic medical record for International Classification of Diseases 9 codes (680, 682, and 041.1) thought to be relevant to deep SSTI that were likely to yield specimens for culture and then checking the medical record for the presence of a wound culture report with antibiotic sensitivity results.
Although the exact number of persons at risk could not be precisely known, a crude adjustment for the size of the clinic patient population was done by dividing the number of yearly S. aureus infection cases by the number of visits by distinct patients during that year. This denominator included all medical, podiatry, and gynecology visits, excluding visits for intrauterine insemination and for mental health care.
Prespecified comparisons between patients with infections by MRSA and methicillin-susceptible S. aureus (MSSA) isolates included age, race, ZIP code of residence, HIV status, and HIV status within 1 year after diagnosis of an S. aureus SSTI.
Statistical analysis.
Statistical analysis was performed using Stata version 9.2 (Statacorp, College Station, TX). All P values are two sided, and exact confidence limits were calculated for all odds ratios (OR). Tests of categorical variables were done using Fisher's exact test. Two sample t tests or the Wilcoxon rank-sum test were used to compare means or medians as appropriate. Linear trends were computed with the Mantel Score Test (12). A logistic regression model was constructed to assess the effects of antibiotic sensitivity, incision and drainage, and HIV status on the odds of clinical resolution. Likelihood ratio tests were used to compare regression models.
RESULTS
Characteristics of MRSA and MSSA patients.
Almost all patients were men (97.6% versus 95.0% for MRSA and MSSA, respectively) (Table 1), and the mean age was the late 30s (38.8 ± 8.3 years for MRSA and 37.2 ± 8.9 years for MSSA). Locations of residences were similar for the two groups. A significantly higher percentage of MRSA patients were known to be HIV infected at the time of their first positive S. aureus culture (43.3% versus 15.0% for MRSA and MSSA, respectively; P < 0.0001). Among patients with a known diagnosis of HIV infection, a smaller proportion of patients with MRSA isolates were on HAART at the time of presentation, but the difference was not statistically significant (56.3% of HIV+ patients infected with MRSA isolates and 75% of HIV+ patients infected with MSSA isolates; P = 0.15). Significant differences in median viral loads and CD4 counts were also not observed. Of the 93 patients with an initial MRSA isolate infection and without a known diagnosis of HIV infection, 26 (28%) had a documented HIV test within the next year. Of the 134 patients with an initial MSSA isolate infection and without a known diagnosis of HIV infection, 21 (16%) had a documented HIV test within the subsequent year (OR for having a documented HIV test comparing MRSA and MSSA, 2.08; 95% confidence interval, 1.04 to 4.22). There was a trend toward a higher percentage of patients being diagnosed with HIV infection within the following year among persons infected with MRSA isolates (5.5% versus 1.3% for MRSA and MSSA, respectively; P = 0.061). The percentage of MRSA patients who were known to be HIV+ did not change significantly from 2001 to 2005 (years with more than one patient infected with a MRSA isolate; P = 0.30 for trend).
TABLE 1.
Comparison of patients with MRSA and MSSA isolate infections at initial presentation
| Parameter | Valuea
|
Unadjusted P value | ||
|---|---|---|---|---|
| MRSA | MSSA | OR (95% CI) | ||
| No. of persons | 164 | 160 | ||
| Age (mean ± SD) | 38.8 ± 8.3 | 37.2 ± 8.9 | NA | 0.090 |
| Male n (%) | 160 (97.6) | 152 (95.0) | 2.11 (0.55-9.73) | 0.253 |
| White n (%) | 110 (67.1) | 118 (73.8) | 0.73 (0.44-1.20) | 0.224 |
| Black n (%) | 7 (4.3) | 5 (3.1) | 1.38 (0.37-5.64) | 0.770 |
| Hispanic n (%) | 6 (3.7) | 6 (3.8) | 0.97 (0.25-3.73) | 1.000 |
| Other n (%) | 7 (4.3) | 4 (2.5) | 1.74 (0.43-8.25) | 0.542 |
| Unknown n (%) | 34 (20.7) | 27 (16.9) | 1.29 (0.71-2.35) | 0.397 |
| Boston n (%) | 93 (56.7) | 85 (53.1) | 1.16 (0.73-1.83) | 0.577 |
| Other MA n (%) | 62 (37.8) | 64 (40.0) | 0.91 (0.57-1.46) | 0.733 |
| Outside MA n (%) | 9 (5.5) | 11 (6.9) | 0.79 (0.28-2.16) | 0.650 |
| Known HIV+n (%) | 71 (43.3) | 24 (15.0) | 4.33 (2.47-7.70) | <0.0001 |
| HIV+ subsequent year n (%) | 9 (5.5) | 2 (1.3) | 4.59 (0.93-44.11) | 0.061 |
| HIV+ patients | ||||
| On HAART n (%) | 40 (56.3) | 18 (75.0) | 0.43 (0.13-1.32) | 0.15 |
| Median CD4 | 475 | 407 | NA | 0.27 |
| Median log viral load | 2.58 | 0.90 | NA | 0.57 |
CI, confidence interval; NA, not applicable.
In 2005, compared to the general population of patients presenting for medical care at FCH, patients with a MRSA infection were significantly more likely to be male (99% [100/101] versus 70.3% [6,675/9,498]; P < 0.001), to be older (mean age, 38.9 ± 7.9 years versus 35.8 ± 11.9 years; P = 0.0003), and to have a known diagnosis of HIV infection (45.5% [46/101] versus 11.8% [1,123/9,498]; P < 0.001) (data not shown).
S. aureus skin and soft tissue infections.
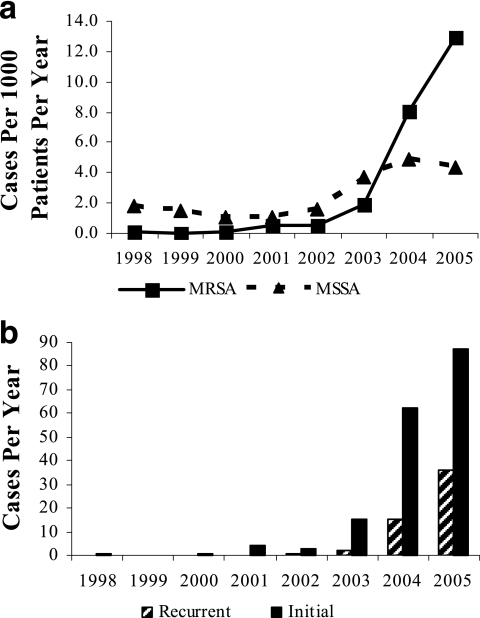
A total of 232 distinct culture-confirmed MRSA clinical infections were identified in Fenway outpatients between 1 January 1998 and 31 December 2005. Of these, 227 were SSTI, including folliculitis, furuncles, carbuncles, abscesses, and unclassified infections (Fig. 1a). Of the other five MRSA infections, four were urinary tract infections and one was due to bacteremia in a patient with a prostatic abscess. The 227 MRSA SSTI occurred in 173 different patients, 40 of whom (23.1%) had a subsequent culture-confirmed MRSI SSTI during the study period. Overall, 54/227 (23.8%) MRSA SSTI cases occurred in patients with a previous culture-confirmed MRSA SSTI (Fig. 1b). There was a trend toward a higher proportion of recurrent cases from 2001, the first year with more than one MRSA SSTI case, through 2005 (P = 0.06 for trend). The mean number of distinct MRSA infections per year among patients infected with MRSA isolates was 1.1 (range, 1.0 to 1.3 mean infections per year from 1998 to 2005). Over the study period, a total of 179 culture-confirmed MSSA infections were identified in Fenway outpatients. Of these, 172 were SSTI, and they occurred in 165 different patients. The other seven MSSA infections comprised five urinary tract infections, one bacteremia, and one case of septic bursitis. No endovascular infections occurred in this study. Fourteen patients had at least one MRSA and at least one MSSA skin and soft tissue infection during the study. Altogether, 324 different patients experienced any skin or soft tissue infection due to S. aureus during the study period; 164 persons had an initial MRSA isolate infection, whereas 160 persons had an initial MSSA isolate infection.
FIG. 1.
(a) S. aureus skin and soft tissue infections at Fenway Community Health, 1998 to 2005. (b) Initial and recurrent MRSA skin and soft tissue infections.
The numbers of culture-confirmed MRSA SSTI increased significantly, with most cases identified in 2004 and 2005. The numbers of culture-confirmed MSSA SSTI also increased, but to a lesser extent. The increase in MRSA cases continued to be significant after adjustment for the number of patients presenting each year for medical care. The proportion of all culture-confirmed S. aureus cases due to MRSA increased significantly, from 7.7% in 1998 to 75% in 2005 (P < 0.0001 for trend).
Antibiotic resistance profiles of MRSA isolates.
Resistance to TMP-SMX was uncommon (Table 2). Of the 399 S. aureus SSTI, 386 had TMP-SMX sensitivities available, with only 10 isolates (2.6%) resistant. Of these 10 patients, 5 were HIV infected, and 3 of the HIV-infected patients were receiving prophylactic TMP-SMX. Nine isolates were MSSA, and one was MRSA. Only 1 (0.5%) of the 216 MRSA skin and soft tissue isolates with TMP-SMX sensitivities available was resistant to TMP-SMX. Inducible clindamycin resistance could not be assessed, as disk diffusion testing was not performed routinely (11). Notably, constitutive clindamycin resistance was present in nearly half (48.2%) of the MRSA isolates. Only a few (13/399) had tetracycline sensitivities available, since susceptibility to tetracycline was not routinely tested for at the clinical laboratory to which wound cultures were most often sent. Of these 13 isolates, 53.8% were resistant to tetracycline. Testing for resistance to doxycycline and minocycline was not routinely performed. Among MRSA isolates, resistance to ciprofloxacin (78.5%) or erythromycin (93.8%) was common, but the isolates were almost uniformly sensitive to chloramphenicol, gentamicin, rifampin, and vancomycin.
TABLE 2.
Antibiotic susceptibilities of MRSA and MSSA isolatesa
| Antibiotic | No. (%) of MRSA resistant (227 total isolates) | No. (%) of MSSA resistant (172 total isolates) | Overall no. (%) resistant |
|---|---|---|---|
| Cephazolin | 214/214 (100) | 0/171 (0) | 214/385 (55.6) |
| Chloramphenicol | 0/198 (0) | 0/108 (0) | 0/306 (0) |
| Ciprofloxacin | 168/214 (78.5) | 15/170 (8.8) | 183/384 (47.7) |
| Clindamycin | 108/224 (48.2) | 12/171 (7.0) | 120/395 (30.4) |
| Erythromycin | 213/227 (93.8) | 99/171 (57.9) | 312/398 (78.4) |
| Gentamicin | 1/226 (0.4) | 0/149 (0) | 1/375 (0.3) |
| Rifampin | 0/211 (0) | 0/108 (0) | 0/319 (0) |
| TMP-SMX | 1/216 (0.5) | 9/170 (5.3) | 10/386 (2.6) |
| Vancomycin | 0/226 (0) | 0/170 (0) | 0/396 (0) |
| Tetracycline | 7/13 (53.8) | 0 (0) | 7/13 (53.8) |
| Levofloxacin | 7/21 (33.3) | 0/31 (0) | 7/52 (13.5) |
Not all isolates were tested for susceptibility to all the antibiotics listed.
Empirical antibiotic choice and isolate susceptibility.
We next investigated whether clinicians' choice of oral empirical antibiotic therapy changed over time as MRSA SSTI became more common. Of the 227 MRSA SSTI, 203 were treated with an empirical oral antibiotic (Fig. 2a). In 2002, the first year with more than one antibiotic-treated case, beta-lactams were used exclusively, whereas by 2005, TMP-SMX was used as empirical therapy in more than 76% of MRSA cases (P < 0.0001 for trend). Similar shifts toward the choice of TMP-SMX for empirical therapy were also seen for MSSA cases (P = 0.003 for trend). Patients with recurrent MRSA SSTI were generally treated empirically with TMP-SMX (25/32; 71.4% of cases in 2005), with linezolid used for refractory cases (data not shown).
FIG. 2.
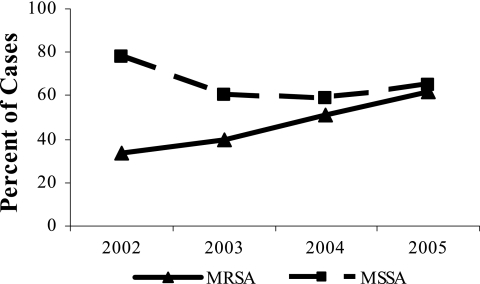
(a) Empirical oral antibiotics for MRSA skin and soft tissue infections. (b) In vitro sensitivity of MRSA isolates to at least one agent of an empirical antibiotic regimen. Other, not tested or data not available.
In parallel with the more frequent use of TMP-SMX, a significant increase in the percentage of MRSA isolates sensitive to the empirical antibiotic therapy was observed (Fig. 2b), increasing from 0% in 1998 to 77% in 2005 (P < 0.0001 for trend). The sensitivity of MSSA isolates did not change significantly (P = 0.16 for trend).
Information regarding the duration of empirical antibiotic therapy was available for 183/203 (90.1%) antibiotic-treated SSTI cases. There was a trend toward longer duration of empirical treatment for cases receiving empirical TMP-SMX than for cases treated with other empirical antibiotics (mean, 11.0 ± 3.4 days for TMP-SMX versus 10.3 ± 3.5 days for other antibiotics; P = 0.06).
Additional clinical management of S. aureus skin and soft tissue infections.
As MRSA became more widely recognized, clinicians tended to obtain clinical cultures of suspected S. aureus SSTI more frequently. The percentage of SSTI with a culture obtained increased significantly, from none in 1998 to nearly 73% in 2005 (P < 0.0001 for trend). In addition, the percentage of all S. aureus SSTI for which incision and drainage were performed also increased significantly, from 0 in 1998 to 45.1% in 2005 (P < 0.0001 for trend). The percentage of MRSA infections with incision and drainage performed similarly increased significantly, from 0 in 1998 to 56.1% in 2005 (P = 0.002 for trend).
Clinical outcomes on empirical antibiotic regimens.
In order to determine whether these changes in empirical antibiotic regimens for S. aureus SSTI led to improved clinical outcomes, we determined the percentage of cases that resolved clinically on the empirical antibiotic(s) (Fig. 3). The percentage of cases with clinical resolution on the empirical oral regimen increased significantly for MRSA (P < 0.04 for trend) but did not change significantly for MSSA (P = 0.75 for trend). Although a higher percentage of recurrent cases than initial cases resolved on empirical therapy early in the study period, by 2005 this difference was not significant (65.6% of recurrent cases versus 60.5% of initial cases; P = 0.67).
FIG. 3.
Clinical resolution of S. aureus skin and soft tissue infections on empirical antibiotic regimens.
In univariate analyses of cases of MRSA SSTI treated with empirical oral antibiotics, in vitro sensitivity to the empirical antibiotic was associated with increased odds of clinical resolution on empirical therapy (OR = 6.07; 95% confidence interval, 3.12 to 11.86). Incision and drainage were not significantly associated with clinical resolution on empirical therapy (OR = 1.28; 95% confidence interval, 0.71 to 2.31), whereas HIV seropositivity was associated with increased odds of clinical resolution on empirical therapy (OR = 1.85; 95% confidence interval, 1.01 to 3.36).
In the multivariate analysis, isolate sensitivity to empirical antibiotics remained significantly associated with clinical resolution on empirical therapy when incision and drainage and HIV status were controlled for (OR = 5.91; 95% confidence interval, 3.14 to 11.13). The association between HIV seropositivity and clinical resolution on empirical therapy was no longer statistically significant after isolate sensitivity to empirical antibiotics was controlled for (OR = 1.74; 95% confidence interval, 0.94 to 3.22).
Of the 203 MRSA SSTI cases treated with oral antibiotics, 104 (51.2%) occurred in patients who were known to be HIV+. Neither use of HAART (OR = 0.68; 95% confidence interval, 0.28 to 1.65), CD4 count (OR = 1.06 for each increase of 100; 95% confidence interval, 0.92 to 1.23), nor log viral load (OR = 1.10; 95% confidence interval, 0.91 to 1.33) was significantly associated with clinical resolution on empirical therapy. In the multivariate analysis, isolate sensitivity to empirical antibiotics remained significantly associated with clinical resolution on empirical therapy after incision and drainage, HIV status, use of HAART, log viral load, and CD4 count were controlled for (OR = 6.76; 95% confidence interval, 2.57 to 17.74).
Finally, we examined adverse reactions to TMP-SMX, the most commonly prescribed empirical antibiotic for MRSA SSTI by the end of the study period. Adverse reactions, including fever and rash deemed serious enough by the provider to discontinue TMP-SMX, occurred in 8/150 (5.3%) SSTI cases treated with empirical TMP-SMX.
DISCUSSION
Over the past decade, MRSA infections have become increasingly problematic in some community-based settings, most notably among MSM (1, 2, 10, 14, 20, 24). This study reviewed the treatment and clinical outcomes of culture-confirmed SSTI due to S. aureus among outpatients at a community health care center serving a large population of MSM. The percentage of S. aureus infections due to MRSA increased significantly, with MRSA accounting for 75% of SSTI by 2005. Interestingly, MRSA infections have increasingly become the presenting condition that leads to a diagnosis of HIV+ among FCH patients. Although patients with HIV infection/AIDS are known to be at risk for serious bacterial infections (22), this association has been reemphasized with the emergence of community-associated MRSA (21).
In the late 1990s, most MRSA cases were initially treated with oral beta-lactam antibiotics, to which MRSA isolates are insensitive. By 2005, with greater recognition of MRSA as a major cause of SSTI at FCH, clinicians tended to choose TMP-SMX for empirical treatment for suspected S. aureus SSTI. In parallel, a greater percentage of clinical S. aureus isolates were sensitive to the empirical antibiotic and a greater proportion of cases resolved clinically on the empirical antibiotic. The nonsignificant decrease in the proportion of MSSA cases that clinically resolved on empirical therapy over the study period was due to clinicians changing to beta-lactam antibiotics once wound culture data were available, not to a lack of susceptibility of MSSA to TMP-SMX (Table 2).
The increase in the frequency of obtaining clinical cultures for patients with skin and soft tissue infections likely reflects growing awareness by FCH clinicians of the high prevalence of MRSA among the patient population and the importance of antimicrobial susceptibility testing for these infections. It is also possible that the increase in clinical cultures was due to an increase in deep-seated infections from which culturable material could be collected. The classification of infections as deep seated or not was not always possible with certainty based on descriptions provided in the medical record. While the observation that the percentage of S. aureus skin and soft tissue infections undergoing incision and drainage significantly increased over the study period suggests a possible increased prevalence of deep-seated infections, such a change may simply reflect the evolution of clinical management of SSTI among FCH clinicians. A prospective study would be needed to answer this question definitively.
Awareness that MRSA is an important cause of community-onset SSTI has not spread to all centers, and patients may still receive monotherapy with agents lacking activity against MRSA, such as cephalexin or dicloxacillin (9, 18, 23). The clinical importance of inactive empirical therapy has not always been clear, as one group found no significant association between inactive empirical therapy and clinical outcomes (4). However, these researchers interviewed only a subset of their total MRSA cases, and outcomes were assessed via patient interviews, which may be less reliable than medical-record review. In the current study, information regarding the clinical response to therapy was available for the vast majority of patients in the sample. Furthermore, the prevalence of HIV was only 9% among persons over 18 in the study by Fridkin et al. compared to more than 40% of MRSA patients in our investigation (4). It is possible that treatment of S. aureus SSTI with the antibiotics showing the most in vitro efficacy has a more important role in immunocompromised individuals; however, the data from the present study do not support this hypothesis. While incision and drainage are likely to be important for resolution of deep S. aureus SSTI (3, 4), we found that use of active empirical therapy was significantly associated with improved odds of clinical resolution after incision and drainage were controlled for.
Our data suggest that TMP-SMX is an appropriate empirical oral antibiotic for the outpatient treatment of MRSA SSTI. FCH clinicians have tended to choose TMP-SMX for empirical therapy of MRSA SSTI because it is inexpensive, few isolates at FCH have shown in vitro resistance to it, and clinical response has been satisfactory.
The incidence of intolerance or adverse effects severe enough to discontinue TMP-SMX therapy was low, even though high doses were routinely prescribed (one or two double-strength tablets twice daily). Moreover, TMP-SMX resistance was uncommon, consistent with previous characterizations of community-acquired MRSA resistance patterns (2, 5, 10, 17-20, 24). The role for TMP-SMX in the treatment of MRSA infections has recently been reviewed in detail (6). Although data regarding antimicrobials for community-onset MRSA are limited (3, 6, 17, 19), Markowitz et al. found that TMP-SMX had clinical efficacy similar to that of vancomycin for treatment of nonendocarditis S. aureus infections, 47% of which were MRSA in their study (13). TMP-SMX may be an attractive option for outpatient treatment of MRSA SSTI due to its oral formulation and low cost (6, 8). Clinicians should bear in mind, however, that additional antibiotic coverage is necessary if infection with group A Streptococcus is suspected (17).
The high prevalence of clindamycin resistance in this study limits clindamycin's usefulness as empirical therapy for suspected MRSA SSTI at Fenway. Data regarding inducible clindamycin resistance in isolates that appeared sensitive to clindamycin would be useful, considering the high prevalence among MRSA isolates of erythromycin resistance (Table 2), which is associated with inducible clindamycin resistance (11). One limitation of this study is the absence of additional supporting laboratory data, such as pulsed field gel electrophoresis analysis to classify strains (16).
Furthermore, it is possible that the observed increase in clinical resolution of MRSA SSTI on empirical antibiotic therapy could be due to factors not directly related to antibiotic choice, such as changes in the virulence of MRSA strains, improved clinical management, earlier presentation or clinical recognition, or other factors not considered here. Although the mean duration of therapy was modestly increased among patients treated with empirical TMP-SMX, this difference was not statistically significant. Future investigations are needed to evaluate the relative importance of active oral antibiotic therapy and incision and drainage for community-onset MRSA SSTI. The relative efficacies of the oral antibiotics commonly used to treat MRSA SSTI should be compared in a prospective trial.
In summary, this study suggests that TMP-SMX represents an appropriate choice for outpatient empirical therapy of suspected S. aureus SSTI, especially when the prevalence of MRSA in the patient population is significant and resistance of local MRSA strains to clindamycin is common.
Acknowledgments
This study was conducted without external financial support.
We gratefully acknowledge Chris Grasso for assistance with database queries and Alex Gonzalez for initial work on this project.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 2.Crum, N. F. 2005. The emergence of severe, community-acquired methicillin-resistant Staphylococcus aureus infections. Scand. J. Infect. Dis. 37:651-656. [DOI] [PubMed] [Google Scholar]
- 3.Ellis, M. W., and J. S. Lewis II. 2005. Treatment approaches for community-acquired methicillin-resistant Staphylococcus aureus infections. Curr. Opin. Infect. Dis. 18:496-501. [DOI] [PubMed] [Google Scholar]
- 4.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 5.Gorwitz, R. J., D. B. Jernigan, J. H. Powers, J. A. Jernigan, and Participants in the CDC-Convened Experts' Meeting on Management of MRSA in the Community. 2006. Strategies for clinical management of MRSA in the community: summary of an experts' meeting convened by the Centers for Disease Control and Prevention. [Online.] http://www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf. Accessed 14 December 2006.
- 6.Grim, S. A., R. P. Rapp, C. A. Martin, and M. E. Evans. 2005. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus. Pharmacotherapy 25:253-264. [DOI] [PubMed] [Google Scholar]
- 7.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874-885. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, J. R. 2003. Linezolid versus vancomycin for methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 36:236-237. [DOI] [PubMed] [Google Scholar]
- 9.King, M. D., B. J. Humphrey, Y. F. Wang, E. V. Kourbatova, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309-317. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski, T. J., E. F. Berbari, and D. R. Osmon. 2005. Epidemiology, treatment, and prevention of community-acquired methicillin-resistant Staphylococcus aureus infections. Mayo Clin. Proc. 80:1201-1207. [DOI] [PubMed] [Google Scholar]
- 11.Lewis, J. S., II, and J. H. Jorgensen. 2005. Inducible clindamycin resistance in staphylococci: should clinicians and microbiologists be concerned? Clin. Infect. Dis. 40:280-285. [DOI] [PubMed] [Google Scholar]
- 12.Mantel, N. 1963. Chi-square tests with one degree of freedom; extensions of the Mantel-Haenszel procedure. J. Am. Stat. Assoc. 58:690-700. [Google Scholar]
- 13.Markowitz, N., E. L. Quinn, and L. D. Saravolatz. 1992. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann. Intern. Med. 117:390-398. [DOI] [PubMed] [Google Scholar]
- 14.Mathews, W. C., J. C. Caperna, R. E. Barber, F. J. Torriani, L. G. Miller, S. May, and J. A. McCutchan. 2005. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J. Acquir. Immune Defic. Syndr. 40:155-160. [DOI] [PubMed] [Google Scholar]
- 15.Mayer, K., J. Appelbaum, T. Rogers, W. Lo, J. Bradford, and S. Boswell. 2001. The evolution of the Fenway Community Health model. Am. J. Public Health 91:892-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moellering, R. C., Jr. 2006. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 144:368-370. [DOI] [PubMed] [Google Scholar]
- 18.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 19.Sabol, K. E., K. L. Echevarria, and J. S. Lewis II. 2006. Community-associated methicillin-resistant Staphylococcus aureus: new bug, old drugs. Ann. Pharmacother. 40:1125-1133. [DOI] [PubMed] [Google Scholar]
- 20.Said-Salim, B., B. Mathema, and B. N. Kreiswirth. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect. Control Hosp. Epidemiol. 24:451-455. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, K., and F. Torriani. 2006. Community-associated methicillin-resistant Staphylococcus aureus in the patient with HIV infection. Curr. HIV/AIDS Rep. 3:107-112. [DOI] [PubMed] [Google Scholar]
- 22.Witt, D. J., D. E. Craven, and W. R. McCabe. 1987. Bacterial infections in adult patients with the acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Am. J. Med. 82:900-906. [DOI] [PubMed] [Google Scholar]
- 23.Young, D. M., H. W. Harris, E. D. Charlebois, H. Chambers, A. Campbell, F. Perdreau-Remington, C. Lee, M. Mankani, R. Mackersie, and W. P. Schecter. 2004. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch. Surg. 139:947-951. [DOI] [PubMed] [Google Scholar]
- 24.Zetola, N., J. S. Francis, E. L. Nuermberger, and W. R. Bishai. 2005. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5:275-286. [DOI] [PubMed] [Google Scholar]