Abstract
Toxoplasma gondii enters host cells via an active, self-driven process to fulfill its need for intracellular replication and survival. Successful host cell invasion is governed by sequential release of secretory proteins from three specialized organelles, including the micronemes, which contribute adhesive proteins necessary for parasite attachment and penetration. Cumulative evidence from studies of Trypanosoma species and malaria parasites has shown that cysteine protease inhibitors represent potent anti-parasitic agents capable of curing infections in vivo. In this study, we screened a series of selective cysteine protease inhibitors for their effects on T. gondii cell invasion. Two of these compounds, morpholinourea-leucyl-homophenolalaninyl-phenyl-vinyl-sulfone and N-benzoxycarbonyl-(leucyl)3-phenyl-vinyl-sulfone, impaired T. gondii invasion and gliding motility at low-micromolar concentrations. Unexpectedly, these inhibitors did not affect surface proteolysis of microneme products but instead impaired an earlier step by precluding the secretion of microneme-derived adhesins to the parasite surface. Our findings suggest that cysteine protease activity is required for microneme secretion and cell invasion by T. gondii.
Toxoplasma gondii is a cosmopolitan protozoan that infects approximately one-third of the human population worldwide. Primary infection among pregnant women and recrudescence in immunodeficient patients are two major clinical presentations that arise from T. gondii infection. The standard administration for toxoplasmosis is a combination of pyrimethamine and sulfadiazine or clindamycin; however, allergic reaction to this treatment is common among patients, thereby limiting treatment in some cases (26). This problem has lingered with the ever-increasing number of individuals experiencing immunodeficiencies due to human immunodeficiency virus infection, organ transplantation, or cancer therapy in recent decades. The identification of novel antitoxoplasmic compounds effective against crucial steps in the parasite's life cycle may eventually improve the therapeutic options for managing toxoplasmosis.
As a hallmark of an obligate intracellular parasite, T. gondii relies on an efficient and robust host cell invasion strategy to support its survival and transmission. This process is driven by the parasite's actin-myosin motor system and is accomplished by a sequential release of secretory proteins from three specialized organelles, the micronemes, rhoptries, and dense granules, through its apical end (9). Unlike rhoptry and dense granule proteins, which typically associate with the parasitophorous vacuole after invasion, microneme proteins are shed from the parasite surface into the cell media after translocating toward the posterior end during cell entry (5, 15, 25). Studies on extracellular tachyzoites have demonstrated the involvement of calcium-signaling events in microneme protein secretion (6, 8, 21, 27). Although basal secretion of microneme products into the excreted/secreted antigen (ESA) fraction harvested from culture supernatants of extracellular parasites occurs in the absence of stimulation, microneme secretion is markedly induced by treatment with secretagogues such as a calcium ionophore (e.g., A23187) or short-chain alcohols (e.g., ethanol) (6, 8).
Several studies describing the identification and characterization of microneme proteins (MICs) have shown that the majority of MICs are subjected to posttranslational and postexocytic processing (11). One well-characterized example is the MIC2-M2AP hexameric protein complex, which is essential for efficient host cell invasion by T. gondii (17, 19). MIC2 is a transmembrane protein that migrates on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as a doublet or triplet due to differential trimming of its N terminus before it is ultimately shed from the parasite surface via intramembranous cleavage (28). M2AP is a MIC2 escorter protein that is secreted in two forms, proM2AP (pM2AP) and mature M2AP (mM2AP). Whereas pM2AP is released via a nonmicronemal pathway and is not subjected to additional processing on the parasite surface (16), mM2AP is secreted from the micronemes onto the parasite surface, where it is processed into a series of truncated species (M2AP-1, M2AP-2, M2AP-3, and M2AP-4). Other MICs, including MIC3, MIC4, MIC5, MIC6, MIC10, MIC11, and AMA1, also undergo similar processing.
Examination of the inhibitory profile of catalytic-type specific protease inhibitors revealed that serine and cysteine proteases are two major classes of enzymes involved in MIC processing (7, 28). Also, the ability of two serine protease inhibitors, 3,4-dichloroisocoumarin (3,4-DCI) and 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), to block T. gondii host cell invasion has been reported (10). While TgROM4 and TgROM5 (rhomboid-like integral membrane serine proteases) were recently identified as key players for MICs shedding during invasion and contributors to the parasite's sensitivity upon treatment with 3,4-DCI (4, 13), the involvement of cysteine proteases in the micronemal secretion pathway and their role in host cell invasion remain obscure.
With the development of more selective cysteine protease inhibitors, recent studies using mouse models of malaria and Chagas' disease have demonstrated that cysteine protease inhibitors can be potent anti-parasitic agents (14, 24). In light of these studies, it is reasonable to propose that similar effects might be seen for Toxoplasma if cysteine proteases participated in host cell invasion. To test this hypothesis, we screened a small library of cysteine protease inhibitors for their effects on T. gondii cell entry and motility. We found that two peptidyl vinyl sulfone (VS) compounds, morpholinourea-leucyl-homophenolalaninyl-phenyl-vinyl-sulfone (LHVS) and N-benzoxycarbonyl-(leucyl)3-phenyl-vinyl-sulfone (ZL3VS), efficiently block parasite invasion and gliding motility by selectively impairing the release of MIC contents.
MATERIALS AND METHODS
Materials.
E64 {N-[N-(l-3-trans-carboxyoxirane-2-carbonyl)-l-leucyl]-agmatine}, E64d [(2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester], and 3,4-DCI (3,4-dichloroisocoumarin) were purchased from Roche (Indianapolis, IN), and K777 was a generous gift from James McKerrow (University of California, San Francisco, CA). The papain family cysteine protease diazomethyl ketone inhibitor Z-YA-CHN2 was purchased from Enzyme Systems Products (Livermore, CA). All other cysteine protease inhibitors were synthesized in the laboratory of M. Bogyo and have been published elsewhere. Specifically, all the VS compounds other than LHVS were designed to target the proteasome but have also been shown to react with papain family cathepsins (1, 2, 23). The acyloxymethyl ketones (AOMKs) were designed to target both CA- and CD-clan cysteine proteases (20), and LHVS, LHVS-PhOH, MB-074, and JPM-OEt all target papain family cysteine proteases (3). All reagents for tissue culture were obtained from Biowhittaker Inc. (Walkersville, MD) or Gibco/BRL (Gaithersburg, MD). Fluorochrome-conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR). Unless otherwise stated, all other chemicals were obtained from Sigma (St. Louis, MO).
Parasites and host cells.
Human foreskin fibroblasts (HFF) were grown in D10 complete medium (Dulbecco's modified Eagle's medium [DMEM] containing 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES, and 50 μg/ml penicillin-streptomycin). T. gondii tachyzoites, RH or 2F1 strains, were propagated in HFF under the above conditions.
Invasion assays.
For the β-galactosidase (β-gal) invasion assay, 2F1 parasites were resuspended in invasion medium (IM; DMEM containing 1% fetal bovine serum and 10 mM HEPES) at 4 × 106/ml. One-hundred microliters of parasites was incubated in the presence of 10 μM or 50 μM of each protease inhibitor or dimethyl sulfoxide (DMSO) at room temperature for 15 min before loading onto HFF in 96-well plates. The plates were then incubated for 30 min at 37°C, with 5% CO2. Excess parasites were removed from monolayers by washing with cold phosphate-buffered saline (PBS) (containing 1 mM CaCl2 and 1 mM MgCl2) six times (2 min on the shaker per wash). After the infected HFF were lysed with 100 μl ice-cold lysis buffer (100 mM HEPES, pH 8.0, 1 mM MgSO4, 0.1% Triton X-100, 5 mM dithiothreitol), 50 μl of the lysates was aliquoted and mixed with 200 μl of assay buffer (100 mM phosphate buffer, pH 7.3, 102 mM β-mercaptoethanol, and 9 mM MgCl2) and 40 μl of 6.25 mM CPRG (chlorophenol red-β-d-galactopyranoside; Roche, Indianapolis, IN). After incubating the reaction mixtures at 37°C for 45 min, the enzymatic activity of β-galactosidase was measured at an absorbance of 550 nm using a kinetic plate reader (Molecular Devices) and Softmax pro3 alias software. Data was compiled from two independent experiments, each with duplicate samples.
For the red/green invasion assay, strain 2F1 parasites were resuspended in IM at 5 × 107/ml. Two-hundred microliters of parasites was incubated with various concentrations of protease inhibitors as indicated or DMSO at room temperature for 15 min before adding to HFF monolayers in 8-well chamber slides. After incubation for 15 min at 37°C in 5% CO2, the chamber was removed and the slide was rinsed with PBS prior to fixation with 4% formaldehyde-0.02% glutaraldehyde in PBS (20 min at room temperature). After three washes with PBS, the slides were blocked with 10% fetal bovine serum (FBS) in PBS and washed once with antigen dilution buffer (ADB; 1% fetal bovine serum and 1% normal goat serum in PBS). Attached parasites were stained with rabbit α-SAG1 (P30) polyclonal antibody (1:1,000), whereas the invaded parasites were stained with monoclonal antibody (MAb) 9E11 α-SAG1 after permeabilizing with 0.1% Triton X-100. Secondary antibodies used were goat anti-rabbit Alexa Fluoro 594 (1:1,000) and goat anti-mouse Alexa Fluoro 488 (1:500). 4′,6′-Diamidino-2-phenylindole (DAPI; 5 μg/ml) was added in the secondary solution for nuclei staining. The slides were washed three times with ADB after each staining step. Finally, the slides were mounted in Mowiol and visualized by phase contrast and epifluorescence using a Nikon Eclipse E800 equipped with an RT spot slider CCD camera. Data were compiled from three independent experiments, each from eight random fields/well under ×600 total magnification.
Preparation of excretory/secretory antigens.
To perform the screening and quantification of MIC secretion experiments, 2F1 parasites were resuspended in DGH medium (DMEM with 2 mM glutamine and 10 mM HEPES) (∼3 × 107 to 6 × 107 parasites with a total volume of 100 μl per reaction) and pretreated with 50 μM protease inhibitors or DMSO at room temperature for 15 min. For induced secretion, the parasites were incubated in a 37°C water bath for 2 min in the presence of 1% (vol/vol) ethanol. For basal secretion, the parasites were incubated in a 37°C water bath for 20 min without ethanol treatment. The parasites were then removed from the water bath and left on ice to quench microneme protein secretion. After centrifuging twice (1,000 × g, 3 min, 4°C), the supernatant (ESA) was mixed with 5× sample buffer containing 2% mercaptoethanol and boiled. The samples were stored at −20°C before use in SDS-PAGE.
Large-scale ESA (basal secretion, without ethanol treatment) used in two-dimensional differential in-gel electrophoresis (2D-DIGE) experiments was prepared using the procedure described above, with ∼4 × 109 parasites in 15 ml DGH medium.
SDS-PAGE and immunoblotting.
The ESA fractions were resolved by SDS-12.5% PAGE or SDS-15% PAGE (for MIC5, MIC10, and MIC11) prior to semidry electrotransfer to nitrocellulose membranes for immunoblotting. The primary antibodies used included MAb 40-1a S/N (β-gal; 1:100), MAb 6D10 ascites fluid (MIC2; 1:15,000), RαM2AP (M2AP; 1:10,000), TgCL7 (AMA1; 1:30,000), RαMIC5 (MIC5; 1:7,500), RαP18 (MIC10; 1:7,500), RαMIC11 (MIC11; 1:7,500), and Tg17-43 (GRA1; 1:60,000). Secondary antibodies were either goat anti-mouse- or goat anti-rabbit-conjugated horseradish peroxidase used at 1:5,000 dilution. After adding Supersignal substrate (Pierce, Rockford, IL), direct chemiluminescence was captured with the Fujifilm LAS-1000 CCD camera before conventional exposure onto films. Signal intensities were quantified using Fujifilm Image Gauge Software.
2D-DIGE.
Fifty micrograms of lyophilized DMSO-treated or 50 μM LHVS-treated large-scale ESA was dissolved in 10 μl of labeling buffer {30 mM Tris, 8 M urea, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, pH 8.5} and reacted with 400 pmol of either Cy3 or Cy5, respectively. Reactions were carried out on ice for 30 min in the dark, and the labeling reactions were quenched by adding 1 μl of 10 mM lysine. The samples were mixed together in equal parts and resolved by two-dimensional electrophoresis as described previously (28).
Gliding motility assay.
Eight-well chamber slides (Nalge-Nunc Intl., Rochester, NY) were coated with either 50% FBS in PBS (37°C for 1 h) or 0.1% poly-l-lysine (room temperature) and washed with PBS before use. Freshly harvested RH parasites were resuspended in 5 ml HHE medium (Hank's balanced salt solution supplemented with 10 mM HEPES and 1 mM EGTA). Parasites (0.5 ml) were then treated with 50 μM protease inhibitors or DMSO at room temperature for 15 min before applying onto chamber slides. The parasites were allowed to glide on the coated slides at 37°C for 15 min. The slides were then rinsed with PBS and fixed in 4% formaldehyde-0.02% glutaraldehyde in PBS at room temperature for 20 min. After blocking with 10% FBS in PBS for 30 min, trails left by gliding parasites were detected by MAb 9E11 α-SAG1 antibody (1:1,000) followed by goat anti-mouse Alexa Fluoro 594 (1:1,000). The slides were washed three times with ADB after each staining step. After the slides were mounted in Mowiol, the images were visualized and captured under epifluorescence (with ×600 total magnification) using a Nikon Eclipse E800 equipped with an RT spot slider CCD camera.
RESULTS
Two peptidyl vinyl sulfones, LHVS and ZL3VS, effectively inhibit Toxoplasma invasion and microneme protein release.
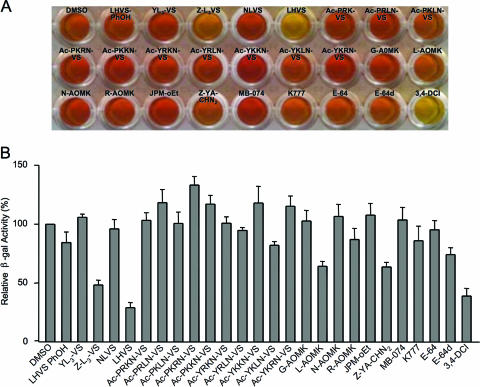
We compiled a set of 22 cysteine protease inhibitors that were synthesized initially to target either the proteasome (all vinyl sulfones [VS], except LHVS and LHVS-PhOH), papain family cysteine proteases (LHVS, LHVS-PhOH, MB-074, K777, and JPM-OEt), or general CA- and CD-clan cysteine proteases (all AOMK compounds) and three commercially available general cysteine protease inhibitors (E64, its membrane-permeable analog E64d, and Z-YA-CHN2) (Table 1). The VS compounds that were designed against the proteasome are also likely to target papain family cysteine proteases, as they contain the same reactive vinyl sulfone group as LHVS, and several (i.e., ZL3VS and YL3-VS) also contain the critical P2 leucine residue found in LHVS. These compounds were used for preliminary screening of effects on T. gondii invasion as well as microneme protein secretion and processing. 3,4-DCI, a serine protease inhibitor previously shown to impair Toxoplasma invasion, was included as a positive control, whereas the solvent vehicle (DMSO) was used as a negative control. The inhibitory effect on invasion was evaluated using the β-galactosidase-expressing parasite 2F1 (8) and a modified procedure based on McFadden et al. (22). Compounds were screened at 10 μM and 50 μM. Whereas none of the compounds inhibited invasion at 10 μM (data not shown), several compounds significantly impaired invasion when administered at 50 μM (Fig. 1). The two most effective compounds were LHVS (morpholinourea-leucyl-homophenolalaninyl-phenyl-vinyl-sulfone), with approximately 70% inhibition compared to the DMSO control, and ZL3VS [N-benzoxycarbonyl-(leucyl)3-phenyl-vinyl-sulfone], which also inhibited parasite invasion by approximately 50%. These vinyl sulfone-based compounds were more effective than the epoxide-based general cysteine protease inhibitors E-64 and E-64d but were similar in effectiveness to 3,4-DCI.
TABLE 1.
Protease inhibitor characteristics
| Compound name | Target | Class | Source or reference |
|---|---|---|---|
| LHVS-PhOH | Papain family | Vinyl sulfone | 3 |
| YL3-VS | Proteasome | Vinyl sulfone | 2 |
| ZL3-VS | Proteasome | Vinyl sulfone | 1 |
| NLVS | Proteasome | Vinyl sulfone | 1 |
| LHVS | Papain family | Vinyl sulfone | 3 |
| Ac-PRKN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-PRLN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-PKLN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-PKRN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-PKKN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-YRKN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-YRLN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-YKKN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-YKLN-VS | Proteasome | Vinyl sulfone | 23 |
| Ac-YKRN-VS | Proteasome | Vinyl sulfone | 23 |
| G-AOMK | General cysteine | Acyloxymethyl ketone | 20 |
| L-AOMK | General cysteine | Acyloxymethyl ketone | 20 |
| N-AOMK | General cysteine | Acyloxymethyl ketone | 20 |
| R-AOMK | General cysteine | Acyloxymethyl ketone | 20 |
| JPM-OEt | Papain family | Epoxysuccinyl | 3 |
| Z-YA-CHN2 | Papain family | Diazomethyl ketone | Enzyme Systems Products |
| MB-074 | Papain family | Epoxysuccinyl | 3 |
| K777 | Papain family | Vinyl sulfone | J. McKerrow |
| E-64 | Papain family | Epoxysuccinyl | Roche |
| E-64d | Papain family | Epoxysuccinyl | Roche |
| 3,4-DCI | Serine protease | Isocoumarin | Roche |
FIG. 1.
Cysteine protease inhibitor screen for Toxoplasma gondii invasion. (A) A representative assay plate showing color development from β-gal activity as an indicator of parasite attachment and invasion. Wells showing minimal attachment/invasion are yellow, whereas those with higher attachment/invasion are orange to red. Parasites were treated with 50 μM of each inhibitor prior to being added to HFF monolayers. (B) Quantification of attachment/invasion. The data are mean values ± standard errors of the means of two independent experiments, each with duplicate samples, and are expressed as a percentage of the solvent control (DMSO). The peptidyl vinyl sulfone inhibitors LHVS and ZL3VS were the most effective inhibitors tested. DMSO and 3,4-DCI were included as negative and positive controls, respectively.
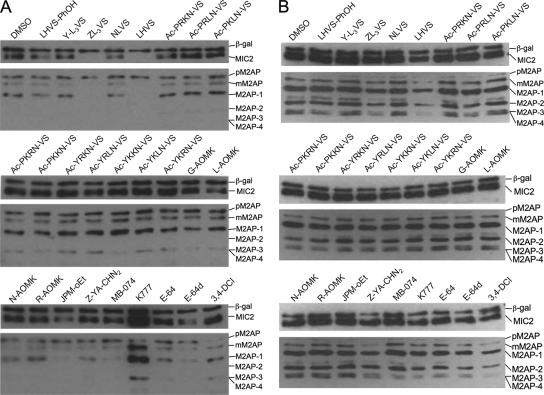
Proteins released from the micronemal pathway are subjected to extensive proteolytic processing both before and after exocytosis (11). The correlation between microneme secretion and a successful host cell invasion is well established (5, 15). To determine whether inhibition of invasion was linked to a deficiency in the micronemal secretion pathway, both basal and induced secretion of MIC2 and M2AP in ESA fractions was assessed by immunoblotting. The level of β-galactosidase detected in the ESA was monitored for inadvertent parasite lysis during manipulation. Consistent with the results from invasion screening, LHVS and ZL3VS were also the most effective inhibitors of microneme secretion among the collection of compounds tested (Fig. 2). Moreover, we observed a differential inhibition between the basal and induced secretions in which a better inhibitory effect was repeatedly seen during basal secretion. Interestingly, these compounds appeared to reduce the total amount of MIC2 and M2AP without markedly affecting the profile of the processed species (note that pM2AP is not a processed species). This observation suggests that both compounds impair invasion by blocking microneme protein secretion, not surface processing. Also, secretion of pM2AP was unaffected, or perhaps slightly increased, as a result of treatment, implying that the inhibition is selective for the micronemal pathway.
FIG. 2.
Effect of cysteine protease inhibitors on microneme protein secretion and processing. Protein levels of MIC2 and M2AP in ESA fractions were examined by immunoblotting after (A) basal secretion or (B) induced secretion, as described in Materials and Methods. β-Gal levels were monitored as an indicator of general toxicity and inadvertent parasite lysis. LHVS and ZL3VS partially blocked the release of MIC2 and the processed forms of M2AP into the ESA, an effect that was more prominent for basal secretion.
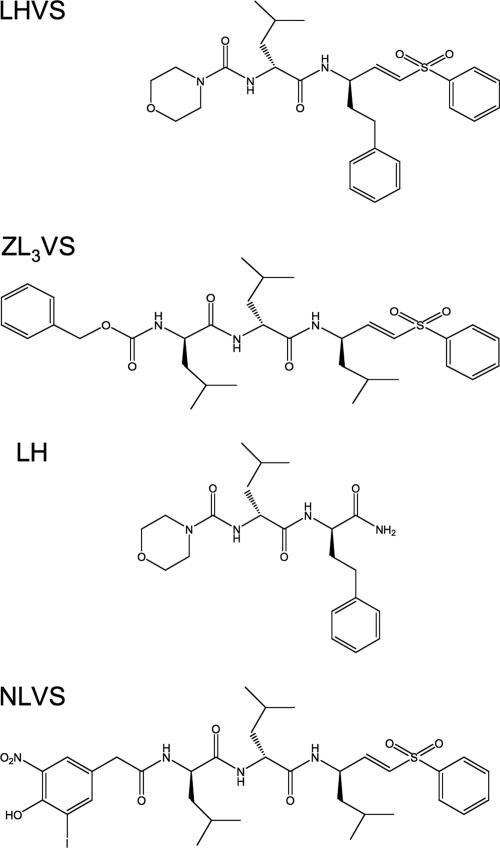
Because LHVS and ZL3VS were two of the most effective inhibitors emerging from the screens, we focused on these compounds for further investigation. Two chemically related compounds, LH (morpholinourea-leucyl-homophenolalanine) and NLVS [4-hydroxy-5-iodo-3-nitrophenylacetyl-(leucyl)3-phenyl-vinyl-sulfone], were synthesized (Fig. 3). LH is a truncated version of LHVS synthesized without the thioreactive vinyl sulfone moiety. NLVS is structurally similar to ZL3VS but with a distinct N-terminal capping moiety, and it was included as a negative control because it showed minimal activity in the invasion assay (Fig. 1).
FIG. 3.
Structures of LHVS, ZL3VS, LH, and NLVS.
Inhibition occurs primarily at the attachment step during invasion.
As part of its active entry strategy, T. gondii utilizes microneme proteins to initiate attachment to the host cell via its apical end. We used a sequential staining method (red/green assay) and fluorescence microscopy to distinguish T. gondii tachyzoites that were attached to host cells from those parasites that had actively invaded the monolayers. BAPTA-AM (B-AM), a calcium chelator that can inhibit microneme protein secretion, thereby precluding attachment, was used as a positive control along with 3,4-DCI. Cytochalasin D (CytD), an actin polymerization inhibitor that blocks parasite penetration without affecting attachment, was also included.
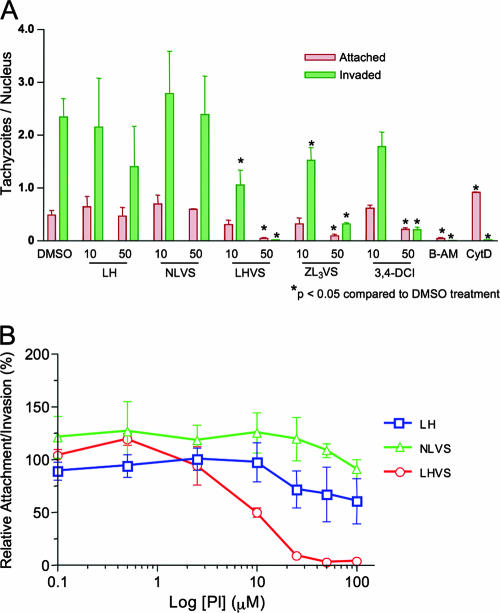
As shown in Fig. 4A, LHVS and ZL3VS effectively blocked both attachment and penetration during invasion, leading to an overall decrease of parasite association with host cells. On the contrary, parasites treated with LH or NLVS exhibited a similar invasion capacity compared to that of the solvent control. Also, the red/green assay appears to be more sensitive than the β-galactosidase assay since, for example, LHVS showed no inhibitory activity at 10 μM in the β-gal invasion assay yet exhibited ∼50% inhibition at the same concentration in the red/green assay. This may be due to the more extensive washes performed in the red/green assay. As established by previous studies (17), an overall decrease in both attached and invaded parasites strongly indicates an effect on parasite attachment, since attachment is an essential prerequisite for penetration. Nonetheless, we cannot rule out a secondary effect on penetration that is masked by the attachment deficit. Collectively, the above findings indicate that both LHVS and ZL3VS impair tachyzoite attachment by blocking the release of at least two key invasion proteins, MIC2 and M2AP, from the micronemes.
FIG. 4.
LHVS and ZL3VS impair T. gondii invasion by blocking parasite attachment and penetration. (A) Attached (red) and invaded (green) parasites in an invasion assay were distinguished by a sequential staining method as described in Materials and Methods. Data are expressed as the number of tachyzoites per host cell nucleus, visualized by DAPI staining. Both attached and invaded parasites from LHVS and ZL3VS treatments were significantly decreased (*) compared to that of the DMSO solvent control, whereas LH and NLVS treatment did not substantially inhibit parasite invasion. 3,4-DCI, B-AM, and CytD were included as positive control treatments that impair parasite invasion. Data are mean values ± standard errors of the means of three independent experiments. (B) To determine the dose-response effect of LHVS treatment on T. gondii invasion, parasites associated (regardless attached or invaded) per host nuclei were enumerated and normalized as a percentage of solvent control (DMSO)-treated parasites. LHVS inhibited T. gondii invasion with an IC50 of 10 μM. A similar trend was observed using the β-gal invasion assay (data not shown). Data are mean values ± standard errors of the means of three independent experiments. Log [PI], log10 concentration of protease inhibitor.
To determine the dose-response relationship for invasion, the parasites were treated with various concentrations (0.1 to 100 μM) of compounds, and the inhibition was evaluated by the red/green assay. The total number of associated parasites counted (regardless of attached or invaded parasites) per host cell nuclei was normalized to that of the solvent control (being 100%). LHVS inhibited T. gondii invasion with a 50% inhibitory concentration (IC50) of 10 μM, whereas the IC50 of ZL3VS and 3,4-DCI was approximately 12.5 μM (Fig. 4B). A gradual decrease in the number of successfully attached/invaded parasites was also observed in LH and NLVS treatments as higher concentrations were tested, but the decrease did not reach statistical significance, even at 100 μM. Similar trends were also observed using the β-gal invasion assay (data not shown).
Inhibition of LHVS and ZL3VS selectively impairs microneme protein secretion.
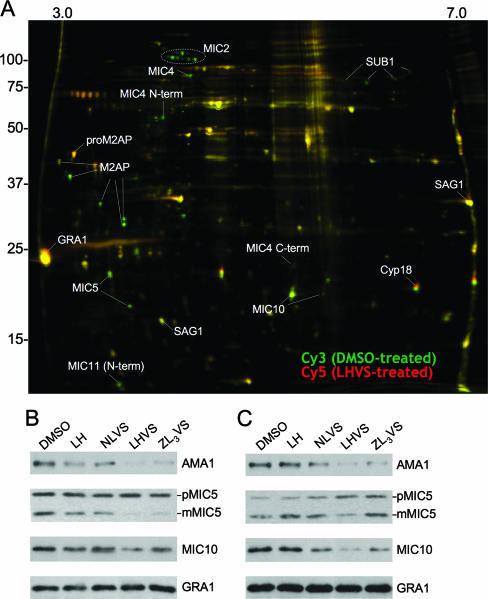
To further investigate whether the invasion blockage was due specifically to an effect on microneme protein release, especially from basal secretion, we performed a differential gel electrophoresis (DIGE) experiment. DMSO- and LHVS-treated large-scale ESA fractions were collected under basal secretion conditions, and proteins therein were covalently modified with Cy3 (pseudocolored green) and Cy5 (pseudocolored red), respectively, mixed together, and resolved by two-dimensional electrophoresis. In this scheme, proteins that show no change in abundance are yellow, whereas those that are lower in abundance are green. As shown in Fig. 5A, all of the green spots that were successfully identified were derived from micronemes (MIC2, MIC4, MIC5, MIC10, MIC11, M2AP, and SUB1), while the levels of proteins released from other sites (GRA1, a dense granule protein; Cyp18, an endoplasmic reticulum protein; and SAG1, a glycosylphosphatidylinositol-linked surface protein) did not change appreciably, as indicated by their yellow appearance.
FIG. 5.
LHVS selectively impairs microneme protein secretion. (A) For DIGE analysis, proteins from DMSO or 50 μM LHVS-treated large-scale ESA were modified with Cy3 (pseudocolored green) and Cy5 (pseudocolored red) fluorescent dyes, respectively. Yellow spots indicate no change in abundance, whereas green spots correspond to proteins diminished by LHVS treatment. Spots were identified by comparing the mobility of proteins on two-dimensional gel electrophoresis to previously resolved DIGE data (28, 29). The inhibitory effect of LHVS and ZL3VS on secretion of AMA1, MIC5, and MIC10 was verified by immunoblotting ESA from (B) basal secretion or (C) induced secretion. Little or no effect was seen for GRA1 secretion.
To confirm the inhibitory activity of compounds in ESA fractions, we examined individual microneme proteins, including AMA1, MIC5, and MIC10, by immunoblotting. As shown in Fig. 5B and C, secretion of these microneme proteins was reduced after treating with LHVS or ZL3VS, while samples treated with LH or NLVS showed levels similar to that of DMSO treatment. Levels of GRA1 were unchanged or slightly upregulated after treatment with LHVS or ZL3VS.
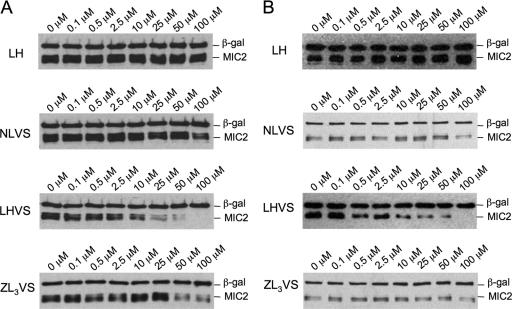
The dose-response relationship was evaluated based on the relative amount of MIC2 in ESA fractions. The results shown in Fig. 6 revealed that the IC50 of LHVS and ZL3VS in blocking microneme protein secretion was ∼10 to 25 μM, i.e., in the same potency range as that for impairing parasite attachment.
FIG. 6.
Dose-response of LHVS and ZL3VS in blocking microneme protein secretion. The potency of LHVS and ZL3VS was examined by immunoblotting MIC2 and M2AP in ESA fractions from (A) basal secretion and (B) induced secretion. Collectively, LHVS inhibited microneme protein secretion with an IC50 of ∼25 μM in both basal and induced secretions, while ZL3VS showed a higher IC50 at a concentration of ∼50 μM.
Treatments of LHVS and ZL3VS diminish parasite gliding motility.
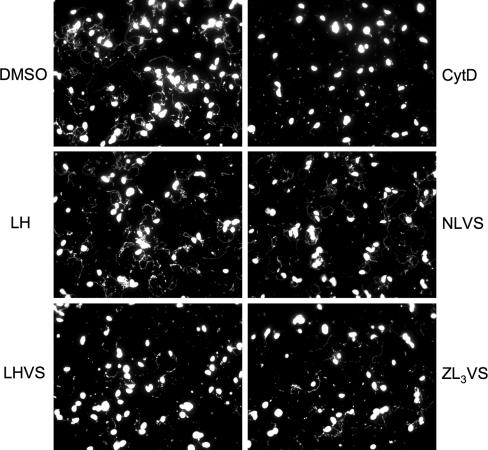
Gliding motility is thought to be a critical factor in driving parasite invasion. MIC2 has previously been shown to serve as a bridge between the internal actin-myosin motor system and external substrates (receptors, surfaces, etc.), thereby serving as a mediator in T. gondii movement (18). From the above experiments, we have established that LHVS and ZL3VS blocked the release of microneme contents that was associated with parasite invasion. Next, we examined whether their effects were also related to parasite motility by performing a gliding assay on plastic chamber slides. The trails, containing SAG1 and produced by parasite movements, were visualized by immunofluorescence staining.
LH or NLVS had little or no effect on gliding, as indicated by the abundant presence of trails, including some of considerable length, compared to DMSO solvent control (Fig. 7). Although few of the LHVS- and ZL3VS-treated parasites were still able to deposit relatively long and normal trails, the overall trail deposition was greatly diminished in a manner similar to CytD treatment. Collectively, these observations suggest that both LHVS and ZL3VS effectively disrupt parasite gliding motility, presumably as a consequence of their effects on microneme secretion.
FIG. 7.
LHVS and ZL3VS treatment impedes parasite gliding motility. DMSO or 50 μM of LH-, NLVS-, LHVS-, and ZL3VS-treated parasites were allowed to glide on precoated slides, and trails were stained and visualized by fluorescence microscopy. Unlike DMSO, LH, and NLVS treatments, in which parasites were frequently observed to associate with long trails, most of the LHVS- or ZL3VS-treated parasites failed to produce trails.
LHVS and ZL3VS do not affect MIC protein shedding.
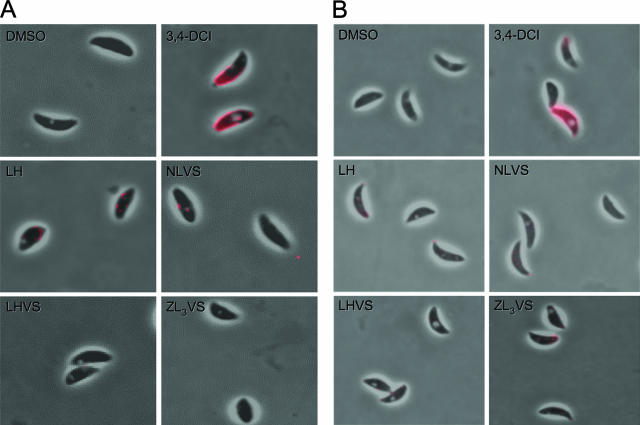
Based on the immunoblotting data from the above experiments, we concluded that the peptidyl vinyl sulfones did not inhibit the surface-trimming process. However, our results do not readily distinguish whether the effect was due to an effect on surface shedding of MIC products. Although surface shedding occurs through intramembrane proteolysis by serine proteases of the rhomboid family (4, 12), a role for other types of proteases in shedding has not been ruled out. To determine if LHVS and ZL3VS affect MIC shedding, we looked for accumulation of MIC2 on the surface of inhibitor-treated tachyzoites.
As a known sheddase inhibitor, 3,4-DCI treatment of extracellular parasites resulted in a significantly stronger surface staining due to the retention of MIC2 under basal (Fig. 8A) or induced (Fig. 8B) secretion conditions. By contrast, no accumulation of MIC2 was seen after treatment with LHVS, ZL3VS, or any of the other control compounds. This result strongly indicates that LHVS and ZL3VS do not target the shedding step. Therefore, we conclude that these compounds in some way block the secretion of MIC proteins from the micronemes.
FIG. 8.
LHVS and ZL3VS do not affect MIC protein shedding. Distribution of surface MIC was detected after treating with 50 μM of LH, NLVS, LHVS, and ZL3VS. DMSO was a solvent control, whereas 3,4-DCI was an inhibitor specific to the shedding process. Shown is (A) basal secretion or (B) induced secretion.
DISCUSSION
We report herein that two vinyl sulfone cysteine protease inhibitors, LHVS and ZL3VS, effectively block T. gondii microneme protein secretion, gliding motility, and cell invasion. The anti-invasion activity of LHVS was found to slightly exceed that of the serine protease inhibitor 3,4-DCI, whereas ZL3VS was as effective as 3,4-DCI. No significant inhibition was observed when general cysteine protease inhibitors, such as E-64 and E-64d, were tested, suggesting that peptidyl vinyl-based compounds may be more potent than these epoxide-based compounds. In addition, LH and NLVS, which were included as controls for the thiol-reactive moiety of LHVS and the N-terminal group in ZL3VS, respectively, had little or no effect on invasion, microneme protein secretion, or gliding motility. Lack of inhibition by LH demonstrates a key role for the vinyl-sulfone warhead, presumably due to its ability to covalently modify the active-site sulfhydryl of a target cysteine protease. The failure of NLVS to affect cell invasion may be due to insufficient membrane penetration, since its N-terminal 4-hydroxy-5-iodo-3-nitrophenylacetyl group is more hydrophilic that the N-benzoxycarbonyl group on ZL3VS. Alternatively, the N-terminal group may contribute significantly to the binding in the active-site cleft of the target cysteine protease.
Unexpectedly, instead of interrupting the postexocytic proteolysis steps, LHVS and ZL3VS treatments led to an overall ablation of MICs released into ESA fractions. This phenomenon does not correlate with the previous reports in which two speculated cysteine proteases, MPP2 and MPP3, trim microneme proteins after they are released onto the parasite surface (7, 28). Instead, our data are consistent with the possibility that these inhibitors act upon an earlier step associated with the secretion of microneme contents onto the parasite surface. No difference in the distribution or staining intensity of micronemes was seen after treatment (data not shown), suggesting that the effect is not due to overt disruption of micronemes. Since secretion is dependent upon the fusion of micronemes with the apical plasma membrane, a cysteine protease might be involved in this process. Alternatively, it is possible that the protease targeted by peptidyl vinyl sulfone inhibitors is contained within the micronemes where its activity is required to aid the efficient flow of contents from the micronemes onto the parasite's apical surface. It should also be noted that we cannot rule out the possibility that the inhibitors are targeting a step that is unrelated to proteolysis. Further studies will be necessary to clarify the specificity of LHVS and ZL3VS and to identify the target responsible for their inhibitory effects.
Carey et al. recently used a high-throughput invasion assay to screen a structurally diverse library of small molecules (4a). Strikingly, the majority of the inhibitory compounds identified in this screen appear to work by selectively blocking microneme protein secretion, with effective concentrations in the 10 to 50 μM range. Differential effects on basal and induced secretions similar to what we observed in LHVS and ZL3VS were also seen for several of the compounds. The mechanism of the basal and induced secretion and their contributions to T. gondii invasion have yet to be clarified. Although it is widely accepted that the pool of microneme proteins released under induced conditions governs the parasite's apical-oriented attachment during invasion, Lovett et al. (21) has elegantly, albeit unexpectedly, demonstrated that the intracellular calcium in the parasite is sharply down-regulated during the initial stage of host cell invasion, precisely the opposite of what was expected. Combined with our results herein, this implies that basal secretion may serve a more prominent role in cell invasion than previously thought. Although both pathways are calcium dependent, based on their sensitivity to the calcium agonist BAPTA-AM, basal secretion likely has a lower concentration threshold for calcium than induced secretion. Therefore, the drop in intracellular calcium during apical attachment presumably limits secretion to a basal rate that may provide an optimal supply of surface ligands to direct penetration into the target cell.
For those microneme proteins, such as M2AP and MIC5, which require en route proteolysis to remove their propeptides during transportation to microneme compartments, the levels of their proforms were found to be more abundant in basal secretion than those of induced secretion. There are two possible explanations for this observation. First, since pM2AP and pMIC5 are released in a calcium independent manner (16) (S. D. Brydges and V. B. Carruthers, unpublished), these proproteins are likely discharged into the ESA via the dense granules (DG), which represents the default secretory pathway in T. gondii. DG secretion (based on detection of GRA1) appears to be unaffected or slightly elevated by inhibitor treatment, therefore the increased levels of pM2AP and pMIC5 may be due to activation of the DG pathway. Furthermore, treatments that suppress microneme secretion have been observed to activate DG secretion (6, 8), thus, our results are consistent with this inverse regulatory relationship between the two pathways.
A second factor potentially contributing to elevated release of pM2AP and pMIC5 is that the proteolytic maturation of these precursor proteins may be inhibited by both LHVS and ZL3VS treatments. An ongoing study by our group characterizing a candidate micronemal maturase, TgCPL (cathepsin proteinase L), has found that LHVS can selectively bind this parasite-derived cathepsin L-like protease both in vivo and in vitro (F. Parussini, C. I. Phillips, M. Bogyo, and V. B. Carruthers, unpublished data). Preliminary localization of TgCPL suggests that this protease is largely confined to a post-Golgi compartment that is distinct from the micronemes. Unlabeled LHVS can compete for labeling of this compartment by a fluorescent conjugate of LHVS (bodipy-LHVS) in extracellular parasites (but not intracellular parasites), suggesting that LHVS is at least somewhat membrane permeable (C.-F. Teo, F. Parussini, and V. B. Carruthers, unpublished data). Since presumably the bulk of the microneme material is processed and trafficked during parasite replication, it remains unclear how a brief blockage of propeptide processing in extracellular parasites would impair microneme secretion. It is possible that some TgCPL is trafficked to the micronemes, where it could play a role in facilitating the release of microneme contents upon fusion with the parasite apical membrane. Additional studies will be required to clarify the role of TgCPL in microneme secretion, and further work will also be necessary to determine whether LHVS acts upon alternative targets that are responsible for the observed effects.
Cysteine protease inhibitors, especially peptidyl vinyl sulfones, have gained interest in pharmaceutical research during the past decades. Importantly, peptidyl vinyl sulfones have been demonstrated to be potent anti-parasitic agents in Trypanosoma cruzi and Plasmodium species (14, 24). In both cases, the target enzymes have been localized to digestive compartments: the lysosomes/reservasomes of T. cruzi (cruzain) and the food vacuole of P. falciparum (falcipain-2a, falcipain-2b, and falcipain-3). Interestingly, digestive compartments have not been described in Toxoplasma. Therefore, with the discovery of both LHVS and ZL3VS as effective cysteine protease inhibitors, future work may not only unveil chemotherapeutic candidates for treating Toxoplasma infections but may also provide a useful tool in identifying the target enzyme(s) and unravel how and where it fulfills its key role in microneme secretion, gliding motility, and cell invasion.
Acknowledgments
We thank Claudia Bordon and Björn Kafsack for help with experiments, Marie-France Cesbron-Delauw and Lloyd Kasper for providing antibodies, and James McKerrow for providing K777.
This study was supported by a Stanley Medical Research Institute grant (to V.B.C.) and U.S. National Institutes of Health National Technology Center for Networks and Pathways grant U54 RR020843 (to M.B.).
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Bogyo, M., J. S. McMaster, M. Gaczynska, D. Tortorella, A. L. Goldberg, and H. Ploegh. 1997. Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc. Natl. Acad. Sci. USA 94:6629-6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogyo, M., S. Shin, J. S. McMaster, and H. L. Ploegh. 1998. Substrate binding and sequence preference of the proteasome revealed by active-site-directed affinity probes. Chem. Biol. 5:307-320. [DOI] [PubMed] [Google Scholar]
- 3.Bogyo, M., S. Verhelst, V. Bellingard-Dubouchaud, S. Toba, and D. Greenbaum. 2000. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem. Biol. 7:27-38. [DOI] [PubMed] [Google Scholar]
- 4.Brossier, F., T. J. Jewett, L. D. Sibley, and S. Urban. 2005. A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc. Natl. Acad. Sci. USA 102:4146-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Carey, K. L., N. J. Westwood, T. J. Mitcheson, and G. E. Ward. 2004. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 101:7433-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers, V. B., O. K. Giddings, and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers, V. B., S. N. J. Moreno, and L. D. Sibley. 1999. Ethanol and acetaldehyde elevate intracellular calcium and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 342:379-386. [PMC free article] [PubMed] [Google Scholar]
- 7.Carruthers, V. B., G. D. Sherman, and L. D. Sibley. 2000. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275:14346-14353. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers, V. B., and L. D. Sibley. 1999. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31:421-428. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. [PubMed] [Google Scholar]
- 10.Conseil, V., M. Soete, and J. F. Dubremetz. 1999. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob. Agents Chemother. 43:1358-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowse, T., and D. Soldati. 2004. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr. Opin. Microbiol. 7:388-396. [DOI] [PubMed] [Google Scholar]
- 12.Dowse, T. J., J. C. Pascall, K. D. Brown, and D. Soldati. 2005. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Int. J. Parasitol. 35:747-756. [DOI] [PubMed] [Google Scholar]
- 13.Dowse, T. J., and D. Soldati. 2005. Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parasitol. 21:254-258. [DOI] [PubMed] [Google Scholar]
- 14.Engel, J. C., P. S. Doyle, I. Hsieh, and J. H. McKerrow. 1998. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J. Exp. Med. 188:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Réguet, N., M. Lebrun, M.-N. Fourmaux, O. Mercereau-Puijalon, T. Mann, C. J. M. Beckers, B. Samyn, J. Van Beeumen, D. Bout, and J.-F. Dubremetz. 2000. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell Microbiol. 2:353-364. [DOI] [PubMed] [Google Scholar]
- 16.Harper, J. M., M. H. Huynh, I. Coppens, F. Parussini, S. Moreno, and V. B. Carruthers. 2006. A cleavable propeptide influences Toxoplasma Infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol. Biol. Cell 17:4551-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh, M. H., K. E. Rabenau, J. M. Harper, W. L. Beatty, L. D. Sibley, and V. B. Carruthers. 2003. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22:2082-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewett, T. J., and L. D. Sibley. 2003. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell 11:885-894. [DOI] [PubMed] [Google Scholar]
- 19.Jewett, T. J., and L. D. Sibley. 2003. The Toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J. Biol. Chem. 279:9362-9369. [DOI] [PubMed] [Google Scholar]
- 20.Kato, D., S. H. Verhelst, K. B. Sexton, and M. Bogyo. 2005. A general solid phase method for the preparation of diverse azapeptide probes directed against cysteine proteases. Org. Lett. 7:5649-5652. [DOI] [PubMed] [Google Scholar]
- 21.Lovett, J. L., N. Marchesini, S. N. Moreno, and L. D. Sibley. 2002. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP[3])/ryanodine-sensitive stores. J. Biol. Chem. 277:25870-25876. [DOI] [PubMed] [Google Scholar]
- 22.McFadden, D. C., F. Seeber, and J. C. Boothroyd. 1997. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 41:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazif, T., and M. Bogyo. 2001. Global analysis of proteasomal substrate specificity using positional-scanning libraries of covalent inhibitors. Proc. Natl. Acad. Sci. USA 98:2967-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson, J. E., G. K. Lee, A. Semenov, and P. J. Rosenthal. 1999. Antimalarial effects in mice of orally administered peptidyl cysteine protease inhibitors. Bioorg. Med. Chem. 7:633-638. [DOI] [PubMed] [Google Scholar]
- 25.Opitz, C., M. Di Cristina, M. Reiss, T. Ruppert, A. Crisanti, and D. Soldati. 2002. Intramembrane cleavage of microneme proteins at the surface of the apicomplexan parasite Toxoplasma gondii. EMBO J. 21:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Ven, A. J., P. P. Koopmans, T. B. Vree, and J. W. van der Meer. 1991. Adverse reactions to co-trimoxazole in HIV infection. Lancet 338:431-433. [DOI] [PubMed] [Google Scholar]
- 27.Vieira, M. C., and S. N. Moreno. 2000. Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol. Biochem. Parasitol. 106:157-162. [DOI] [PubMed] [Google Scholar]
- 28.Zhou, X. W., M. J. Blackman, S. A. Howell, and V. B. Carruthers. 2004. Proteomic analysis of cleavage events reveals a dynamic two-step mechanism for proteolysis of a key parasite adhesive complex. Mol. Cell Proteomics 3:565-576. [DOI] [PubMed] [Google Scholar]
- 29.Zhou, X. W., B. F. Kafsack, R. N. Cole, P. Beckett, R. F. Shen, and V. B. Carruthers. 2005. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. J. Biol. Chem. 280:34233-34244. [DOI] [PMC free article] [PubMed] [Google Scholar]