Abstract
Human immunodeficiency virus type 2 (HIV-2) contains numerous natural polymorphisms in its protease (PR) gene that are implicated in drug resistance in the case of HIV-1. This study evaluated emergent PR resistance in HIV-2. Three HIV-2 isolates were selected for resistance to amprenavir (APV), nelfinavir (NFV), indinavir (IDV), and tipranavir (TPV) in cell culture. Genotypic analysis determined the time to the appearance of protease inhibitor (PI)-associated mutations compared to HIV-1. Phenotypic drug susceptibility assays were used to determine the levels of drug resistance. Within 10 to 15 weeks of serial passage, three major mutations—I54M, I82F, and L90M—arose in HIV-2 viral cultures exposed to APV, NFV, and IDV, whereas I82L was selected with TPV. After 25 weeks, other cultures had developed I50V and I84V mutations. In contrast, no major PI mutations were selected in HIV-1 over this period except for D30N in the context of NFV selective pressure. The baseline phenotypes of wild-type HIV-2 isolates were in the range observed for HIV-1, except for APV and NFV for which a lower degree of sensitivity was seen. The acquisition of the I54M, I84V, L90M, and L99F mutations resulted in multi-PI-resistant viruses, conferring 10-fold to more than 100-fold resistance. Of note, we observed a 62A/99F mutational motif that conferred high-level resistance to PIs, as well as novel secondary mutations, including 6F, 12A, and 21K. Thus, natural polymorphisms in HIV-2 may facilitate the selection of PI resistance. The increasing incidence of such polymorphisms in drug-naive HIV-1- and HIV-2-infected persons is of concern.
Human immunodeficiency virus type 2 (HIV-2) is a second causative agent of AIDS (6, 13) and is mainly endemic in west Africa (23, 32). In terms of pathogenesis, HIV-2-infected individuals exhibit slower disease progression, lower rates of vertical transmission, and lower viral loads and are often asymptomatic compared to individuals infected with HIV-1 (10, 28, 41).
The protease (PR) genes of HIV-1 and HIV-2 possess ca. 50% sequence homology, and the structures of the protease enzymes are very similar (20). Patients infected with HIV-2 or coinfected with HIV-1 and HIV-2 are routinely treated with protease inhibitors (PIs). No database or algorithm exists for the interpretation of mutations selected during treatment of HIV-2 disease. In contrast, a wealth of information is available on drug resistance to PIs in HIV-1 (5, 17, 30, 38, 43, 44).
Although several studies have reported the selection of mutations associated with PIs in HIV-1 in patients infected with HIV-2 (1, 7, 9, 33), the biological significance of these genotypic changes in phenotypic susceptibility to PIs remains unclear. In addition, it has been suggested that different patterns of mutations associated with resistance to PIs may emerge in HIV-2 compared to HIV-1 (7). It is clear that relevant mutations in the PR of HIV-2 can affect phenotypic susceptibility to PIs (36, 37, 46). Despite the overall sensitivity of HIV-2 to PIs (42, 45), some authors suggest that HIV-2 may be naturally resistant to some PI drugs (1, 22).
The present study assessed the role of natural polymorphisms in the PR gene on the time to the development of resistance to PIs using an HIV-2 tissue culture model. Furthermore, we investigated the role of drug-related genotypic changes in the PR gene on phenotypic susceptibility to PIs.
(This study was performed by Michel Ntemgwa in partial fulfillment of the requirements for a Ph.D. degree from the Faculty of Graduate Studies and Research, McGill University, Montreal, Quebec, Canada).
MATERIALS AND METHODS
Virus isolates and cells.
Three HIV-2 subtype A isolates—CBL-20, CBL-23, and MVP-15132—were obtained from the AIDS Research and Reference Reagent Program of the National Institutes of Health, Bethesda, MD. CBL-20 and CBL-23 were isolated from symptomatic patients from The Gambia and have been previously described (40), whereas MVP-15132 was from a German woman who had been infected by a Senegalese man (14). No information is available on whether the patient donors had been exposed to antiretroviral drugs. However, PIs were not available when these isolates were obtained in 1988. The HIV-1 subtype B clinical isolate 5512 was obtained with informed consent from a drug-naive individual at our clinics in Montreal, Canada. Viral strains were amplified as described earlier by coculture of peripheral blood mononuclear cells from infected patients with uninfected cord blood mononuclear cells (CBMCs) (24, 39). The HIV-2 isolates were amplified in CBMCs according to specifications provided by the NIH (2).
Drugs.
We received amprenavir (APV), nelfinavir (NFV), indinavir (IDV), tipranavir (TPV), and lopinavir (LPV) as gifts from Glaxo SmithKline (Research Triangle Park, NC); Pfizer, Inc. (San Diego, CA); Merck & Co., Inc. (Albany, GA); Boehringer Ingelheim (Laval, Quebec, Canada); and Abbott Laboratories (North Chicago, IL), respectively.
Selection of resistance mutations to PIs in HIV-1 and HIV-2.
The three HIV-2 subtype A (14, 40) and the HIV-1 subtype B wild-type isolates were used to select for mutations conferring resistance to the PIs APV, NFV, IDV, and TPV in CBMCs at a multiplicity of infection of 0.1 as previously described (15). Selection for resistance was performed by standard procedures using increasing concentrations of drugs that were introduced weekly based on reverse transcriptase (RT) values in culture fluids at the previous round of replication. Selections were initiated with suboptimal drug concentrations of 0.05 μM and were increased to concentrations ranging from 0.25 to 10 μM. Culture fluids were analyzed by RT enzyme assays to monitor the levels of viral replication (15, 27, 31). At each passage, culture fluids were harvested and kept at −80°C for subsequent genetic analysis by sequencing.
Nucleic acid extraction and PCR amplification.
Viral RNA was extracted from culture supernatants by using the QIAGEN QIAamp viral extraction kit (Mississauga, Ontario, Canada). For amplification of HIV-2, RNA in culture supernatants was reverse transcribed and the PR and RT genes were amplified by PCR using specific primer pairs, i.e., RT2-reverse and PR1-forward and nested PR3-forward and RT4-reverse (13, 35). The PR and RT genomic regions of the HIV-2 pol gene were amplified in the same PCR products, which were analyzed in 1% agarose gels with ethidium bromide. The resulting PCR-amplified DNA fragments were purified by using the QIAquick PCR purification kit. The PCR products were used as templates for nucleotide sequencing analysis of the protease gene. Amplification of HIV-1 was performed by using the TruGene HIV-1 genotyping assay kit (Bayer, Inc., Toronto, Ontario, Canada). Oligonucleotides were chemically synthesized and purchased from Invitrogen (Burlington, Ontario, Canada).
DNA sequencing analysis.
The PR gene of HIV-2 samples was directly sequenced to analyze for PI-associated major and minor mutations using primer pairs PR3 and PR2 (35). Cycle sequencing of both strands was performed on the GeneAmp PCR 9700 instrument with the BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA). Excess dye-labeled terminators were removed from the extension products and purified products were sequenced on the ABI Prism 3100 genetic analyzer (Applied Biosystems). The nucleotide sequences of the PR genes were then assembled using GeneTool 2.0 auto-assembler software. The nucleotides were aligned and translated to amino acids using Bioedit version 7.0 software (21) and references to HIV-2ROD (GenBank accession number M15390). Differences in amino acids at sites conferring resistance to PIs were then analyzed. The HIV-1 sample was sequenced in both directions using the OpenGene automated DNA system (Bayer, Inc., Toronto, Ontario, Canada) according to manufacturer's instructions (18, 26). The sequence for each sample was compared to databases of known drug resistance mutations.
Phenotypic susceptibility of HIV-1/HIV-2 to PIs.
Wild-type HIV-1 and HIV-2 viral isolates and viral variants selected with APV, NFV, IDV, and TPV were phenotyped against APV, NFV, IDV, TPV, and LPV. Briefly, fivefold dilutions of drugs were made from 0.00064 to 10 μM and plated onto 96-well plates. CBMCs were infected over 2 h with wild-type or selected viruses. The infected CBMCs were then plated on 96-well plates containing drug dilutions. After 3 days of incubation at 37°C under 5% CO2, cells were fed with fresh medium containing appropriate drug dilutions. On day 7, RT enzyme assays were performed to determine the 50% drug inhibitory concentration (IC50) of the above-named PIs, using Prism analytic software (GraphPad, Inc.). The IC50 values of select viruses were then compared to those of the wild-type viruses to determine the fold change in drug susceptibility (27, 39).
Replicative ability of HIV-2 and HIV-1.
Wild-type or serially passaged drug-resistant variants were used to infect CBMCs over 2 h. Infected CBMCs were plated onto 96-well plates containing medium supplemented with interleukin-2 (IL-2). After 3 days of incubation, cells were refed with fresh medium containing IL-2. At day 7, RT assays were performed to determine the growth rates relative to wild-type virus as described previously (4). In addition, p24 antigen levels were measured in these cultures using the Vironostika HIV-1 Antigen MicroELISA kit (Biomerieux bv, Boxtel, The Netherlands).
GenBank accession numbers.
The sequences obtained in the present study have been deposited in GenBank under the following accession numbers: CBL-20 (DQ854623), CBL-23 (DQ854624), MVP-15132 (DQ854625), and 5512 (DQ917283).
RESULTS
Phylogenetic analysis.
After sequencing, HIV-2 samples were aligned with 43 other HIV-2 drug-naive samples from the Los Alamos database (http://www.hiv.lanl.gov) using Bioedit version 7.0 (21), and gaps were stripped manually. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 3.0 (25). Phylogenetic trees were generated by using the neighbor-joining method with nucleotide distance datum sets calculated by Kimura's two-parameter approach with 500 bootstrap replicates. Bootstrap values of >70% were considered significant. Trees were rooted at HIV-1 HXB2 (GenBank accession no. K03455). From the phylogenetic analyses, all three HIV-2 isolates clustered with other subtype A HIV-2 viruses from the database (data not shown).
Baseline genotype for HIV-1 and HIV-2 samples.
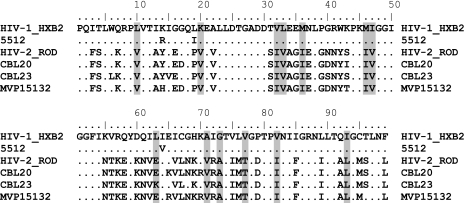
At baseline, the HIV-2 samples had amino acid changes at 56 of 99 positions in PR (Fig. 1) compared to the HIV-1 subtype B reference HXB2. Thirteen of these positions are known to be implicated in HIV-1 resistance to PIs (10I/V, 20V, 32I, 33V, 36I, 46I, I47V, 63E/K, 71V, 73A, 77T, 82I, and 93L). The HIV-1 sample 5,512 had polymorphisms at three positions (K14R, L19I, and I64V) in the PR.
FIG. 1.
Baseline polymorphisms in HIV-2 PR compared to HIV-1 subtype B. Amino acid alignment of PR sequences of HIV-2 and HIV-1 used in the present study. The positions highlighted are those involved in HIV-1 drug resistance (10I/V, 20V, 32I, 33V, 36I, 46I, I47V, 63E/K, 71V, 73A, 77T, 82I, and 93L). Dots indicate identity with HIV-1 subtype B HXB2. The HIV-1 subtype B sample 5512 possessed three known baseline polymorphisms: K14R, L19I, and I64V. The HIV-2 viruses studied included CBL-20, CBL-23, and MVP-15132. The HIV-1 virus studied was isolate 5512.
Time to development of resistance mutations in HIV-1 and HIV-2.
Within 10 to 15 weeks in passage, three major mutations—I54M, I82F, and L90M—had been selected in the HIV-2 viral cultures. I54M was selected in viruses exposed to APV, NFV, or IDV, whereas I82F and L90M were selected only in cultures exposed to NFV. The I82L mutation was selected in all HIV-2 viruses exposed to TPV (Table 1) . By 25 weeks in culture, some of the HIV-2 isolates also developed I50V and I84V under APV pressure. During the 10- to 15-week replication period, no major PI mutations in HIV-1 were selected, except for D30N in the context of NFV selective pressure. By week 20, this HIV-1 isolate also developed the V32I and M46L mutations and the M46I mutation under APV and NFV selective pressure, respectively. By 38 weeks in culture, most of the mutations were maintained except for HIV-2 CBL-23, which selected for an additional V71I mutation, and HIV-1 5512, which acquired V77I in addition to D30N and M46I under NFV selective pressure (Table 1).
TABLE 1.
Time to development of mutations associated with resistance to PIs in HIV-1 and HIV-2a
| Drug | Wk | Mutation(s)
|
|||
|---|---|---|---|---|---|
| HIV-2 isolates
|
HIV-1 isolate 5512 | ||||
| CBL-20 | CBL-23 | MVP-15132 | |||
| APV | 1-10 | WT | WT | WT | WT |
| 15 | WT | I54M | I54M | WT | |
| 20 | I50V | I54M | I54M | V32I, M46L | |
| 25 | I50V | E21K, I54M | I54M, I84V | V32I, M46L | |
| 38 | T12A, I50V, I64V | E21K, I54M | I54M, I84V | V32I, M46L | |
| NFV | 1-10 | WT | WT | WT | WT |
| 15 | I82I/F | V62V/A, L90L/M | I54M | D30N | |
| 20 | I82F | I54M, V62V/A L90M | I54M | D30N, M46I | |
| 25 | I82F | I54M, L90M | I54M, L99F | D30N, M46I | |
| 38 | I82F | W6F, I54M, L90M, V71I | I54M, L99F | D30N, M46I, V77I | |
| IDV | 1-10 | WT | WT | WT | WT |
| 15 | I54M | V62A, L99F | I54M | WT | |
| 20 | I54M | V62A, L99F | I54M | WT | |
| 25 | I54M | V62A, L99F | I54M | WT | |
| 38 | I54M | V62A, L99F | I54M | WT | |
| TPV | 1-10 | WT | WT | WT | WT |
| 15 | I82L | I82L | I82L | WT | |
| 20 | I82L | I82L | I82L | WT | |
| 25 | I82L | I82L | I82L | WT | |
| 38 | I82L | I82L | I82L | M36I/M, V82T | |
Viral isolates were serially passaged in increasing doses of APV, NFV, IDV, or TPV. Genotypic analysis ascertained the time to first appearance of resistance-associated mutations. At baseline, all wild-type HIV-2 isolates harbored the following polymorphisms and minor mutations known to be implicated in emergent resistance to PIs in HIV-1: 10I/V, 20V, 32I, 33V, 36I, 46I, I47V, 63E/K, 71V, 73A, 77T, 82I, and 93L. WT, wild type.
HIV-2 viruses under APV selective pressure followed different pathways. CBL-20 selected for the I50V mutation, CBL-23 selected for I54M and a novel E21K mutation, while MVP-15132 selected for I54M and I84V (Table 1). Under NFV selective pressure, all three HIV-2 isolates followed different pathways. While CBL-20 selected I82F, CBL-23 selected I54M, L90M, and V77I, and MVP-15132 selected I54M and L99F (Table 1). HIV-2 isolates never selected for the D30N mutation under NFV drug selective pressure. When viruses were exposed to IDV, CBL-23 developed the V62A and L99F mutations whereas the other HIV-2 isolates selected I54M (Table 1). All HIV-2 isolates under TPV selective pressure went through the I82L pathway (Table 1). Some mutations, e.g., Y14H, S43I, E65K, and S96T, which were selected by some HIV-2 isolates under drug selection pressure occurred as natural polymorphisms in the other isolates (data not shown).
Phenotypic susceptibility of HIV-2 to PIs.
Selected viruses were phenotyped at week 38 after they had acquired the mutations shown in Table 1. At baseline, the mean IC50 values of the HIV-2 wild-type isolates were 0.44 μM for APV, 0.016 μM for IDV, 0.064 μM for NFV, 0.160 μM for TPV, and 0.004 μM for LPV. Viruses containing only the I54M mutation showed a >10-fold increase in IC50 for APV, IDV, NFV, and LPV, and a sevenfold increase in IC50 for TPV (Table 2). Viruses with the I54M and I84V mutations showed a >22.7-fold increase in IC50 for APV, IDV, NFV, and LPV and a 12.7-fold increase for TPV compared to the wild type. Viruses with the I54M and L99F mutations showed the highest increase in IC50: >22.7-fold for APV, 18.2-fold for TPV, and >35-fold for all other PIs. Viruses with the I50V mutation showed a >22.7-fold increase in IC50 for APV and LPV. The I82F mutation selected by NFV conferred a 34-fold increase in IC50 for IDV, a 4-fold increase in IC50 for NFV, and a 36.3-fold increase in IC50 for LPV compared to wild-type mean IC50 values. The I82L mutation conferred a 27.7-fold resistance to TPV but less resistance to other PIs except for LPV, against which it conferred an 18.8-fold increase in IC50 (Table 2). The I54M, V71I, and L90M mutations conferred high-level cross-resistance to all of the PIs tested (22.7- to 131-fold) except for TPV. The V62A and L99F mutations conferred cross-resistance to IDV (9-fold) and NFV (15-fold) and high-level resistance to LPV (124-fold) but less resistance to APV and TPV.
TABLE 2.
Phenotypic drug susceptibility of HIV-2 and HIV-1 to PIs
| Viral isolate (no. of isolates) | PI mutation(s) | Mean IC50 (μM) ± SDa
|
||||
|---|---|---|---|---|---|---|
| APV | IDV | NFV | TPV | LPV | ||
| HIV-2 WT (3) | None | 0.440 ± 0.369 | 0.016 ± 0.011 | 0.064 ± 0.074 | 0.160 ± 0.075 | 0.004 ± 0.003 |
| I82L | 0.528 ± 0.300 (1.2) | 0.064 ± 0.021 (4) | 0.106 ± 0.050 (1.7) | 4.436 ± 2.867 (27.7) | 0.075 ± 0.074 (18.8) | |
| I82F | 0.965 ± 0.166 (2.2) | 0.544 ± 0.021 (34) | 0.244 ± 0.063 (4) | 0.306 ± 0.069 (2) | 0.145 ± 0.050 (36.3) | |
| V62A, L99F | 0.098 ± 0.071 | 0.143 ± 0.022 (9) | 0.961 ± 0.228 (15) | 0.524 ± 0.302 (3.3) | 0.496 ± 0.031 (124) | |
| I50V | >10 (>22.7) | 0.072 ± 0.001 (4.5) | 0.132 ± 0.013 (2.1) | 0.316 ± 0.014 (2) | 0.203 ± 0.139 (50.8) | |
| I54M | >10 (>22.7) | 0.236 ± 0.051 (15) | 0.624 ± 0.0.368 (10) | 1.106 ± 0.468 (7) | 0.481 ± 0.275 (120.3) | |
| I54M, L90M | >10 (>22.7) | 1.112 ± 0.447 (70) | 2.564 ± 0.390 (40) | 0.145 ± 0.001 | 0.524 ± 0.004 (131) | |
| I54M, I84V | >10 (>22.7) | 0.388 ± 0.011 (24.3) | 1.747 ± 0.074 (27.3) | 2.028 ± 0.144 (12.7) | 0.375 ± 0.006 (93.8) | |
| I54M, L99F | >10 (>22.7) | 0.825 ± 0.377 (52) | 2.241 ± 0.303 (35) | 2.908 ± 0.544 (18.2) | 0.414 ± 0.010 (103.5) | |
| HIV-1 WT (1) | None | 0.026 ± 0.003 | 0.027 ± 0.001 | 0.014 ± 0.007 | 0.092 ± 0.013 | 0.002 ± 0.001 |
| V32I, M46L | 0.116 ± 0.012 (4.5) | 0.045 ± 0.005 (1.7) | 0.031 ± 0.004 (2.2) | 0.042 ± 0.003 | 0.001 ± 0.001 | |
| D30N, M46I, V77I | 0.019 ± 0.004 | 0.025 ± 0.006 | 0.677 ± 0.221 (48.4) | 0.001 ± 0.001 | 0.002 ± 0.001 | |
| M36I/M, V82T | 0.003 ± 0.002 | 0.014 ± 0.003 | 0.010 ± 0.001 | 0.192 ± 0.013 (2.1) | 0.007 ± 0.002 (3.5) | |
The values represent the means of at least two independent experiments, each performed in duplicate. The values in parentheses are the fold resistance compared to that of wild-type (WT) HIV-2 or HIV-1. The mean IC50s of the HIV-2 wild-type isolates were 0.440 ± 0.369 μM for APV, 0.016 ± 0.011 μM for IDV, 0.064 ± 0.074 μM for NFV, 0.160 ± 0.075 μM for TPV, and 0.004 ± 0.003 μM for LPV, while those of HIV-1 were 0.026 ± 0.003 for APV, 0.027 ± 0.001 for IDV, 0.014 ± 0.007 for NFV, 0.092 ± 0.013 for TPV, and 0.002 ± 0.001 for LPV. The HIV-2 isolates included CBL-20, CBL-23, and MVP-15132; the HIV-1 isolate was 5512.
Phenotypic susceptibility of HIV-1 to PIs.
At baseline, the mean IC50 values of the HIV-1 wild-type isolate were 0.026 ± 0.003 μM for APV, 0.027 ± 0.001 μM for IDV, 0.014 ± 0.007 μM for NFV, 0.092 ± 0.013 μM for TPV, and 0.002 ± 0.001 μM for LPV. The V32I and M46L mutations selected under APV drug pressure did not result in significant increases in the IC50s for the drugs tested compared to wild-type mean values. The D30N, M46I, and V77I mutations yielded a 47-fold increase in IC50 for NFV compared to the wild-type mean values (Table 2).
Effects of drugs and mutations on viral replication.
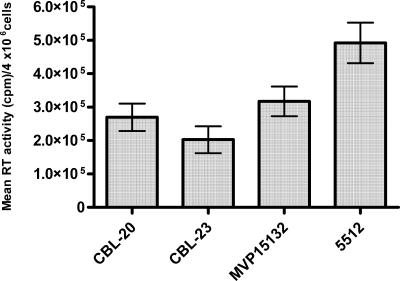
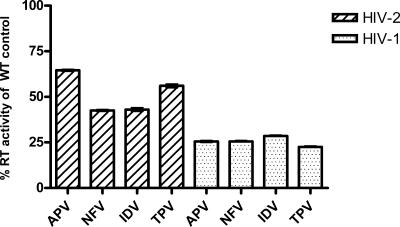
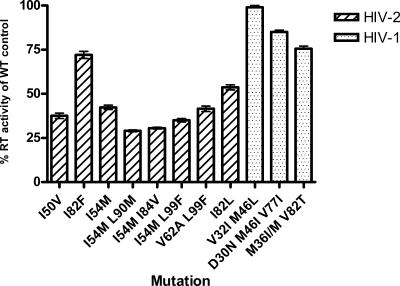
Figure 2 shows differences in RT activities per 4 × 106 cells per round of infection for HIV-1 and HIV-2 isolates. As shown, the RT activities of the HIV-2 isolates were below those of HIV-1. RT activities observed for isolate 5512 are representative of the HIV-1 wild-type isolates (data not shown). The RT levels of drug-selected variants were 23 to 29% of those of the wild-type controls, measured at weekly selections prior to demonstration of resistance mutations (Fig. 3). In contrast, values for HIV-2 ranged between 43 and 65% of the values for wild-type controls (Fig. 3), suggesting that the HIV-2 isolates were less sensitive to the PI drugs.
FIG. 2.
RT activities of wild-type HIV-1 and HIV-2 isolates. The errors bars represent the mean ± the standard error of the mean (SEM) of maximal RT activity of WT viruses averaged per round of infection measured each week for 38 weeks. HIV-2 viruses studied included CBL-20, CBL-23, and MVP-15132. The HIV-1 virus studied was isolate 5512.
FIG. 3.
Relative levels of RT activity, compared to the wild type, of HIV-2 and HIV-1 variants serially passaged in the presence of PIs prior to the selection of resistance mutations. The results are expressed as the percentage of wild-type controls for HIV-1 and HIV-2, which, after 12 weeks, were 422,601 ± 50,410 cpm and 217,146 ± 20,008 cpm, respectively. Error bars represent the mean ± the SEM of the maximal RT activity of PI-selected viruses averaged per round of infection measured each week for 12 weeks. The HIV-2 values represent the means of all of the HIV-2 isolates in the present study (CBL-20, CBL-23, and MVP-15132), while those for HIV-1 represent isolate 5512.
All of the resistance-associated mutations selected in HIV-2 caused a reduction in RT activity in culture fluids (Fig. 4). The RT activities of HIV-1 isolates containing the V32I and M46L mutations were within the range of those of the wild type (Fig. 4). The mutations also caused a reduction in the p24 antigen levels in culture fluids in HIV-1 and HIV-2 (results not shown). No significant differences were observed when replicative activity was expressed based on RT or p24 measurements. The mutated viruses are currently being cultured in the absence of drugs; the monitoring of reversions to the wild type over time will provide an indication of the extent to which the various mutations may have impacted on viral fitness.
FIG. 4.
Effects of mutations on RT activity in HIV-2 and HIV-1. Wild-type and mutated viruses were cultured as described in Materials and Methods. Histograms represent the maximal RT activity of mutated viruses expressed as a percentage of the values for wild-type controls. Error bars represent the mean ± the SEM of two independent experiments performed in duplicate. The HIV-2 viruses studied included CBL-20, CBL-23, and MVP-15132. The HIV-1 virus studied was isolate 5512.
DISCUSSION
HIV-2 expresses natural polymorphisms in the PR (10I/V, 20V, 32I, 33V, 36I, 46I, I47V, 63E/K, 71V, 73A, 77T, 82I, and 93L) that may be implicated in emergent drug resistance.
In the present study, 13 mutations in the PR of HIV-2 (W6F, T12A, E21K, I50V, I54M, V62A, I64V, V71I, I82F, I82L, I84V, L90M, and L99F) were selected with different PIs. Three of these mutations have not been previously associated with either HIV-2 or HIV-1 drug resistance (W6F, T12A, and E21K).
In previous studies (7, 9, 33), treatment-associated changes occurred in the HIV-2 PR gene at sites corresponding to some of those that confer drug resistance in HIV-1. However, these changes could not be directly associated with a particular PI (9). One study described the selection of I82F, I54M, and V71I in one HIV-2-infected patient who received IDV for 12 months (35). In our study, we now show that several mutational pathways can be associated with resistance to different PIs.
We have observed a novel mutational motif involving V62A and L99F that was selected in several instances under IDV and NFV pressure that confers resistance to PIs. Other HIV-2 isolates selected for I54M, I82F, L90M, and V71I under NFV selective pressure, while one HIV-1 isolate selected for D30N with NFV as expected. The L99F mutation was selected by NFV and IDV, showing the potential for cross-resistance to these drugs as previously reported (7). The E21K and L99F mutations, selected under drug pressure in the present study, were also present in some HIV-2 subtype B drug-naive patients (4 to 13%), suggesting that natural resistance to PIs might occur in HIV-2 subtypes (7, 33).
We observed a novel mutational motif, including 62A and 99F, with or without 54 M under selective pressure with NFV and IDV. Of note, 99F is a resistance mutation, conferring cross-resistance to multiple PIs. L99F has been reported to be selected under PI pressure in HIV-2-infected patients (7), but the effect of L99F on phenotypic susceptibility of HIV-2 to PIs has not been elucidated. Indeed, two HIV-2 isolates developed resistance to NFV via the 90M and 54M/99F pathways, although one isolate used the 82F pathway. This contrasts with emergent resistance to NFV by a D30N pathway in HIV-1 in cell culture.
In our study, none of three HIV-2 isolates selected the D30N mutation under NFV selective pressure after 38 weeks in culture. Although both HIV-1 and HIV-2 have a D at position 30 in PR, it is encoded by GAT in HIV-1 and by GAC in HIV-2. The transition from D to N at position 30 involves a change from GAT to AAT. In HIV-2, a G→A transition from GAC to AAC should also encode N. Alternatively, two nucleotide changes from GAC to AAT should code for N, but these fail to occur. It is also possible that the L90M change in HIV-2 is more advantageous and/or better tolerated than a change at residue 30 (9). However, our results suggest that the pathway for resistance to NFV in HIV-2 can also involve I82F and I54M substitutions. The failure to select 30N in HIV-2 may be dictated by fitness constraints (19). In addition, we observed 12A and 21K as compensatory mutations after the acquisition of major mutations.
Our study has shown that the I50V mutation is specific for APV, while I82L is specific for TPV in HIV-2. It is known that these two mutations are selected both in vitro and in vivo in HIV-1 clinical isolates exposed to these drugs (12, 44). Moreover, our study showed a more rapid development of 82L in HIV-2 compared to HIV-1. The HIV-2 isolates developed 82L by week 15, whereas the 82T mutation did not arise until week 38 in HIV-1.
The I54M mutation was the most frequently selected mutation in HIV-2 isolates, suggesting its association with broad cross-resistance to PIs. This mutation was selected by APV, NFV, and IDV. I54M is also selected in HIV-1 subtype B viruses under APV drug pressure (11). Confirmation that I54M confers major resistance to APV in HIV-1 has been obtained by site-directed mutagenesis (unpublished data).
Some natural polymorphisms in HIV-2 may confer baseline natural resistance to APV. HIV-2 naturally harbors 46I and 47V, which are associated with resistance to APV in HIV-1. In our study, wild-type HIV-2 isolates had reduced susceptibilities to APV and NFV of 16.9- and 4.6-fold, respectively, a finding consistent with the work of other groups (1, 22, 36, 46).
It has also been reported that the acquisition of the 54M and 82F mutations in HIV-2-infected patients may result in cross-resistance to PIs and high-level resistance (33- to >1,000-fold) to LPV (36). However, in our study, the 54M and 82F mutations were never found together, perhaps for reasons of diminished viral fitness, and 54M yielded more cross-resistance than 82F. LPV was not used in our drug selection study but was included in our phenotyping panel. All mutations selected showed resistance (18- to 131-fold) to LPV. Our results suggest that the acquisition of 54M alone or together with 84V, 90M, or 99F resulted in multi-PI-resistant viruses (10- to >100-fold).
Almost all HIV-1 protease mutations have been shown to decrease viral fitness (34). Enzymatic as well as viral competition assays have shown reduced catalytic activity and fitness, respectively, of PI-resistant viruses (3, 8, 29). Although there are no published studies on the impact of these resistance mutations in HIV-2, an analogy with HIV-1 makes it likely that decreased viral fitness occurs in PI-resistant HIV-2.
Multidrug-resistant HIV-1 may have reduced pathogenicity. Indeed, many patients with such viruses who remain on treatment seem to recover some CD4 cells, despite detectable viral load (16). It is not known whether similar findings will be obtained in the case of HIV-2.
The baseline phenotypes of HIV-2 isolates are generally in the range of HIV-1, suggesting that the 13 polymorphisms described in HIV-2 PR are secondary mutations. Although they do not confer resistance to PIs, they may facilitate the time to development of resistance in HIV-2 compared to HIV-1. Moreover, the viral backbone of HIV-2 may lead to novel mutational motifs, including 99F. These findings underscore that HIV-2 and non-B subtypes of HIV-1 may show differential responses to PIs. With extended exposure to PIs in HIV-1-infected persons, there is a rising incidence of mutations at codons 10, 33, 36, 63, 71, and 77 in drug-naive persons. The effect of these mutations in facilitating the development of resistance to PIs is a matter of public health concern.
Acknowledgments
This study was sponsored by each of the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for AIDS Research, and the Reseau SIDA of the Fonds de la Recherche en Santé du Québec. M.N. is the recipient of a CIHR doctoral fellowship award.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Adje-Toure, C. A., R. Cheingsong, J. G. Garcia-Lerma, S. Eholie, M. Y. Borget, J. M. Bouchez, R. A. Otten, C. Maurice, M. Sassan-Morokro, R. E. Ekpini, M. Nolan, T. Chorba, W. Heneine, and J. N. Nkengasong. 2003. Antiretroviral therapy in HIV-2-infected patients: changes in plasma viral load, CD4+ cell counts, and drug resistance profiles of patients treated in Abidjan, Cote d'Ivoire. AIDS 17(Suppl. 3):S49-S54. [PubMed] [Google Scholar]
- 2.Beatty, C., M. Bradley, D. Brambilla, F. Breakenridge, J. W. Bremer, J. Dragavon, B. Ladd, D. Livnat, C. Michels, C. Mundy, V. Price, T. Ramacciotti, P. Reichelderfer, B. Staes, C. Starkey, and M. Winters. 1997, posting date. DAIDS Virology Manual for HIV Laboratories. Publication NIH-97-3939. U.S. Department of Health and Human Services, Washington, DC. [Online.] http://www.niaid.nih.gov/daids/vir_manual/full_vir_manual.pdf.
- 3.Borman, A. M., S. Paulous, and F. Clavel. 1996. Resistance of human immunodeficiency virus type 1 to protease inhibitors: selection of resistance mutations in the presence or absence of the drug. J. Gen. Virol. 77(Pt. 3):419-426. [DOI] [PubMed] [Google Scholar]
- 4.Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner, B. G., D. Turner, and M. A. Wainberg. 2002. HIV-1 drug resistance: can we overcome? Expert Opin. Biol. Ther. 2:751-761. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., A. Luckay, D. L. Sodora, P. Telfer, P. Reed, A. Gettie, J. M. Kanu, R. F. Sadek, J. Yee, D. D. Ho, L. Zhang, and P. A. Marx. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson, P., M. Henry, C. Tourres, D. Lozachmeur, H. Gallais, J. A. Gastaut, J. Moreau, and C. Tamalet. 2004. Polymorphism and drug-selected mutations in the protease gene of human immunodeficiency virus type 2 from patients living in Southern France. J. Clin. Microbiol. 42:570-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croteau, G., L. Doyon, D. Thibeault, G. McKercher, L. Pilote, and D. Lamarre. 1997. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J. Virol. 71:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damond, F., F. Brun-Vezinet, S. Matheron, G. Peytavin, P. Campa, S. Pueyo, F. Mammano, S. Lastere, I. Farfara, F. Simon, G. Chene, and D. Descamps. 2005. Polymorphism of the human immunodeficiency virus type 2 (HIV-2) protease gene and selection of drug resistance mutations in HIV-2-infected patients treated with protease inhibitors. J. Clin. Microbiol. 43:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Cock, K. M., G. Adjorlolo, E. Ekpini, T. Sibailly, J. Kouadio, M. Maran, K. Brattegaard, K. M. Vetter, R. Doorly, and H. D. Gayle. 1993. Epidemiology and transmission of HIV-2: why there is no HIV-2 pandemic. JAMA 270:2083-2086. [DOI] [PubMed] [Google Scholar]
- 11.Diallo, K., B. Brenner, M. Oliveira, D. Moisi, M. Detorio, M. Gotte, and M. A. Wainberg. 2003. The M184V substitution in human immunodeficiency virus type 1 reverse transcriptase delays the development of resistance to amprenavir and efavirenz in subtype B and C clinical isolates. Antimicrob. Agents Chemother. 47:2376-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyon, L., S. Tremblay, L. Bourgon, E. Wardrop, and M. G. Cordingley. 2005. Selection and characterization of HIV-1 showing reduced susceptibility to the non-peptidic protease inhibitor tipranavir. Antivir. Res. 68:27-35. [DOI] [PubMed] [Google Scholar]
- 13.Gao, F., L. Yue, D. L. Robertson, S. C. Hill, H. Hui, R. J. Biggar, A. E. Neequaye, T. M. Whelan, D. D. Ho, G. M. Shaw, et al. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433-7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, F., L. Yue, P. M. Sharp, and B. H. Hahn. 1993. Genetic typing of HIV-2 from a Senegalese/German heterosexual transmission. AIDS Res. Hum. Retrovir. 9:703-704. [DOI] [PubMed] [Google Scholar]
- 15.Gao, Q., Z. Gu, M. A. Parniak, J. Cameron, N. Cammack, C. Boucher, and M. A. Wainberg. 1993. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 37:1390-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geretti, A. M. 2005. The clinical significance of viral fitness. J. HIV Ther. 10:6-10. [PubMed] [Google Scholar]
- 17.Gonzalez, L. M., R. M. Brindeiro, M. Tarin, A. Calazans, M. A. Soares, S. Cassol, and A. Tanuri. 2003. In vitro hypersusceptibility of human immunodeficiency virus type 1 subtype C protease to lopinavir. Antimicrob. Agents Chemother. 47:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant, R. M., D. R. Kuritzkes, V. A. Johnson, J. W. Mellors, J. L. Sullivan, R. Swanstrom, R. T. D'Aquila, M. Van Gorder, M. Holodniy, R. M. Lloyd, Jr., C. Reid, G. F. Morgan, and D. L. Winslow. 2003. Accuracy of the TRUGENE HIV-1 genotyping kit. J. Clin. Microbiol. 41:1586-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman, Z., E. E. Paxinos, D. Averbuch, S. Maayan, N. T. Parkin, D. Engelhard, M. Lorber, V. Istomin, Y. Shaked, E. Mendelson, D. Ram, C. J. Petropoulos, and J. M. Schapiro. 2004. Mutation D30N is not preferentially selected by human immunodeficiency virus type 1 subtype C in the development of resistance to nelfinavir. Antimicrob. Agents Chemother. 48:2159-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustchina, A., and I. T. Weber. 1991. Comparative analysis of the sequences and structures of HIV-1 and HIV-2 proteases. Proteins 10:325-339. [DOI] [PubMed] [Google Scholar]
- 21.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids. Symp. Ser. 41:95-98. [Google Scholar]
- 22.Goncalves, J. S. C., F. Antunes, and J. Moniz-Pereira. 2002. Phenotypic identification of HIV-2 protease resistance mutations by recombinant viral assay. 11th International Workshop on HIV Drug Resistance: Basic Principles and Clinical Implications, Seville, Spain.
- 23.Kanki, P. J., M. Peeters, and A. Gueye-Ndiaye. 1997. Virology of HIV-1 and HIV-2: implications for Africa. AIDS 11(Suppl. B):S33-S42. [PubMed] [Google Scholar]
- 24.Keulen, W., N. K. Back, A. van Wijk, C. A. Boucher, and B. Berkhout. 1997. Initial appearance of the 184Ile variant in lamivudine-treated patients is caused by the mutational bias of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 71:3346-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 26.Kuritzkes, D. R., R. M. Grant, P. Feorino, M. Griswold, M. Hoover, R. Young, S. Day, R. M. Lloyd, Jr., C. Reid, G. F. Morgan, and D. L. Winslow. 2003. Performance characteristics of the TRUGENE HIV-1 genotyping kit and the Opengene DNA sequencing system. J. Clin. Microbiol. 41:1594-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loemba, H., B. Brenner, M. A. Parniak, S. Ma'ayan, B. Spira, D. Moisi, M. Oliveira, M. Detorio, and M. A. Wainberg. 2002. Genetic divergence of human immunodeficiency virus type 1 Ethiopian clade C reverse transcriptase (RT) and rapid development of resistance against nonnucleoside inhibitors of RT. Antimicrob. Agents Chemother. 46:2087-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marlink, R., P. Kanki, I. Thior, K. Travers, G. Eisen, T. Siby, I. Traore, C. C. Hsieh, M. C. Dia, E. H. Gueye, et al. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587-1590. [DOI] [PubMed] [Google Scholar]
- 29.Miller, V. 2001. International perspectives on antiretroviral resistance: resistance to protease inhibitors. J. Acquir. Immune Defic. Syndr. 26(Suppl. 1):S34-S50. [DOI] [PubMed] [Google Scholar]
- 30.Paolucci, S., F. Baldanti, M. Zavattoni, G. Comolli, N. Labo, S. Menzo, M. Clementi, and G. Gerna. 2003. Comparison of levels of HIV-1 resistance to protease inhibitors by recombinant versus conventional virus phenotypic assay and two genotypic interpretation procedures in treatment-naive and HAART-experienced HIV-infected patients. J. Antimicrob. Chemother. 51:135-139. [DOI] [PubMed] [Google Scholar]
- 31.Petrella, M., M. Oliveira, D. Moisi, M. Detorio, B. G. Brenner, and M. A. Wainberg. 2004. Differential maintenance of the M184V substitution in the reverse transcriptase of human immunodeficiency virus type 1 by various nucleoside antiretroviral agents in tissue culture. Antimicrob. Agents Chemother. 48:4189-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieniazek, D., D. Ellenberger, L. M. Janini, A. C. Ramos, J. Nkengasong, M. Sassan-Morokro, D. J. Hu, I. M. Coulibally, E. Ekpini, C. Bandea, A. Tanuri, A. E. Greenberg, S. Z. Wiktor, and M. A. Rayfield. 1999. Predominance of human immunodeficiency virus type 2 subtype B in Abidjan, Ivory Coast. AIDS Res. Hum. Retrovir. 15:603-608. [DOI] [PubMed] [Google Scholar]
- 33.Pieniazek, D., M. Rayfield, D. J. Hu, J. N. Nkengasong, V. Soriano, W. Heneine, C. Zeh, S. M. Agwale, C. Wambebe, L. Odama, and S. Z. Wiktor. 2004. HIV-2 protease sequences of subtypes A and B harbor multiple mutations associated with protease inhibitor resistance in HIV-1. AIDS 18:495-502. [DOI] [PubMed] [Google Scholar]
- 34.Quinones-Mateu, M., and E. Arts. 2001. HIV-1 fitness: implications for drug resistance, disease progression, and global epidemic evolution, p. 134-170. In C. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. Korber (ed.), HIV sequence compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM.
- 35.Rodes, B., A. Holguin, V. Soriano, M. Dourana, K. Mansinho, F. Antunes, and J. Gonzalez-Lahoz. 2000. Emergence of drug resistance mutations in human immunodeficiency virus type 2-infected subjects undergoing antiretroviral therapy. J. Clin. Microbiol. 38:1370-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodes, B., J. Sheldon, C. Toro, V. Jimenez, M. A. Alvarez, and V. Soriano. 2006. Susceptibility to protease inhibitors in HIV-2 primary isolates from patients failing antiretroviral therapy. J. Antimicrob. Chemother. 57:709-713. [DOI] [PubMed] [Google Scholar]
- 37.Rodes, B., C. Toro, J. A. Sheldon, V. Jimenez, K. Mansinho, and V. Soriano. 2006. High rate of proV47A selection in HIV-2 patients failing lopinavir-based HAART. AIDS 20:127-129. [DOI] [PubMed] [Google Scholar]
- 38.Rusconi, S., S. La Seta Catamancio, P. Citterio, S. Kurtagic, M. Violin, C. Balotta, M. Moroni, M. Galli, and A. d'Arminio-Monforte. 2000. Susceptibility to PNU-140690 (Tipranavir) of human immunodeficiency virus type 1 isolates derived from patients with multidrug resistance to other protease inhibitors. Antimicrob. Agents Chemother. 44:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomon, H., A. Belmonte, K. Nguyen, Z. Gu, M. Gelfand, and M. A. Wainberg. 1994. Comparison of cord blood and peripheral blood mononuclear cells as targets for viral isolation and drug sensitivity studies involving human immunodeficiency virus type 1. J. Clin. Microbiol. 32:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz, T. F., D. Whitby, J. G. Hoad, T. Corrah, H. Whittle, and R. A. Weiss. 1990. Biological and molecular variability of human immunodeficiency virus type 2 isolates from The Gambia. J. Virol. 64:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon, F., S. Matheron, C. Tamalet, I. Loussert-Ajaka, S. Bartczak, J. M. Pepin, C. Dhiver, E. Gamba, C. Elbim, J. A. Gastaut, et al. 1993. Cellular and plasma viral load in patients infected with HIV-2. AIDS 7:1411-1417. [DOI] [PubMed] [Google Scholar]
- 42.Tomasselli, A. G., J. O. Hui, T. K. Sawyer, D. J. Staples, C. Bannow, I. M. Reardon, W. J. Howe, D. L. DeCamp, C. S. Craik, and R. L. Heinrikson. 1990. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J. Biol. Chem. 265:14675-14683. [PubMed] [Google Scholar]
- 43.Turner, D., B. Brenner, D. Mosis, C. Liang, and M. A. Wainberg. 2005. Substitutions in the reverse transcriptase and protease genes of HIV-1 subtype B in untreated individuals and patients treated with antiretroviral drugs. MedGenMed 7:69. [PMC free article] [PubMed] [Google Scholar]
- 44.Turner, D., J. M. Schapiro, B. G. Brenner, and M. A. Wainberg. 2004. The influence of protease inhibitor resistance profiles on selection of HIV therapy in treatment-naive patients. Antivir. Ther. 9:301-314. [PubMed] [Google Scholar]
- 45.Vacca, J. P., B. D. Dorsey, W. A. Schleif, R. B. Levin, S. L. McDaniel, P. L. Darke, J. Zugay, J. C. Quintero, O. M. Blahy, E. Roth, et al. 1994. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc. Natl. Acad. Sci. USA 91:4096-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witvrouw, M., C. Pannecouque, W. M. Switzer, T. M. Folks, E. De Clercq, and W. Heneine. 2004. Susceptibility of HIV-2, SIV, and SHIV to various anti-HIV-1 compounds: implications for treatment and post-exposure prophylaxis. Antivir. Ther. 9:57-65. [PubMed] [Google Scholar]