Abstract
GS-7340 and GS-9131 {9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]-propyl]adenine and 9-(R)-4′-(R)-[[[(S)-1-[(ethoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]-2′-fluoro-1′-furanyladenine, respectively} are novel alkylalaninyl phenyl ester prodrugs of tenofovir {9-R-[(2-phosphonomethoxy)propyl]adenine} (TFV) and a cyclic nucleotide analog, GS-9148 (phosphonomethoxy-2′-fluoro-2′, 3′-dideoxydidehydroadenosine), respectively. Both prodrugs exhibit potent antiretroviral activity against both wild-type and drug-resistant human immunodeficiency virus type 1 strains and excellent in vivo pharmacokinetic properties. In this study, the main enzymatic activity responsible for the initial step in the intracellular activation of GS-7340 and GS-9131 was isolated from human peripheral blood mononuclear cells and identified as lysosomal carboxypeptidase A (cathepsin A [CatA]; EC 3.4.16.5). Biochemical properties of the purified hydrolase (native complex and catalytic subunit molecular masses of 100 and 29 kDa, respectively; isoelectric point [pI] of 5.5) matched those of CatA. Recombinant CatA and the isolated prodrug hydrolase displayed identical susceptibilities to inhibitors and identical substrate preferences towards a panel of tenofovir phosphonoamidate prodrugs. Incubation of both enzymes with 14C-labeled GS-7340 or [3H]difluorophosphonate resulted in the covalent labeling of identical 29-kDa catalytic subunits. Finally, following a 4-h incubation with GS-7340 and GS-9131, the intracellular concentrations of prodrug metabolites detected in CatA-negative fibroblasts were approximately 7.5- and 3-fold lower, respectively, than those detected in normal control fibroblasts. Collectively, these data demonstrate the key role of CatA in the intracellular activation of nucleotide phosphonoamidate prodrugs and open new possibilities for further improvement of this important class of antiviral prodrugs.
Tenofovir {9-R-[(2-phosphonomethoxy)propyl]adenine} (TFV), an acyclic nucleotide analog of dAMP, is a potent in vitro and in vivo inhibitor of human immunodeficiency virus type 1 (HIV-1) replication (2). TFV is sequentially phosphorylated in the cell by AMP kinase and nucleoside diphosphate kinase to the active species, tenofovir diphosphate (23, 39), which acts as a potent inhibitor of HIV-1 reverse transcriptase (7, 49). The presence of a nonhydrolyzable phosphonic acid moiety in tenofovir circumvents an initial phosphorylation step which can be rate limiting for the activation of nucleoside analog inhibitors of HIV reverse transcriptase (3, 4).
In order to increase the cellular permeability and oral bioavailability of TFV, which is a dianion at physiological pH, neutral prodrugs of TFV have been synthesized. Tenofovir disoproxil fumarate (TDF) is a bis-isopropoxycarbonyloxymethyl ester prodrug of TFV approved for the treatment of HIV. TDF is well tolerated, with infrequent development of resistance and a favorable long-term toxicity profile (2, 8, 16). The oral administration of TDF results in high systemic levels of TFV (5); however, the rapid systemic degradation of TDF to TFV limits its uptake into target cells.
Investigations to develop plasma-stable prodrugs which would be selectively hydrolyzed inside cells to antiviral nucleotides led to the design of GS-7340 and GS-9131{9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]methoxy]propyl]adenine and 9-(R)-4′-(R)-[[[(S)-1-[(ethoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]-2′-fluoro-1′-furanyladenine, respectively}, alkylalaninyl amidate phenyl ester prodrugs of TFV and a cyclic nucleotide analog, GS-9148 (phosphonomethoxy-2′-fluoro-2′,3′-dideoxydidehydroadenosine), respectively. Both GS-7340 and GS-9131 exhibit potent in vitro anti-HIV-1 activities, favorable resistance profiles, and low cytotoxicities (9, 25). Compared to TDF, GS-7340 is significantly more stable in plasma and delivers ∼30-fold-greater levels of active diphosphate metabolites into peripheral blood mononuclear cells (PBMCs) in vitro and in vivo (12). Compared to what was seen for TDF, oral administration of an equal dose of GS-7340 resulted in significantly higher levels of TFV accumulation both in lymphatic tissues and in PBMCs (24). While GS-7340 and other amidate prodrugs of tenofovir successfully validated the concept of enhanced in vivo intracellular delivery of parent nucleotide, GS-9131 has been selected as a clinical development candidate based on its unique activity against HIV-1 strains resistant to approved antiretroviral nucleosides and its favorable in vivo pharmacological properties, including the ability to effectively deliver the active GS-9148 diphosphate metabolite into PBMCs (9, 36).
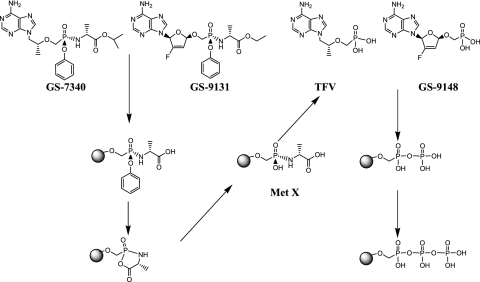
The prodrug moieties of GS-7340 and GS-9131 are structurally similar to a class of nucleoside monophosphate prodrugs referred to as phosphoramidate pronucleotides (27, 28, 43, 47, 51). The initial step in the proposed activation mechanism for the amidate prodrugs is the hydrolysis of the carboxyester bond by an unidentified cellular hydrolase (Fig. 1) (27, 28, 43, 47, 51). Hydrolysis of the ester is followed by a putative nucleophilic attack of the phosphorus by the free carboxyl group, resulting in the spontaneous elimination of phenol, producing metabolite X, which is further deaminated to the parent nucleotide analog (27, 28, 43, 47, 51). The identification and thorough characterization of the enzymes involved in the intracellular activation of this class of prodrugs are essential for a more rational design of compounds with desirable pharmacological and biological properties. Therefore, we focused in this study on the purification and characterization of the intracellular hydrolase from human PBMCs, which is responsible for the initial step in the activation of the phosphonoamidate prodrugs.
FIG. 1.
GS-7340 and GS-9131 metabolic activation pathway. Met X, metabolite X.
MATERIALS AND METHODS
Prodrug hydrolase activity assay.
The activity of the prodrug hydrolase was determined by measuring the rate of production of metabolite X. Various amounts of enzyme fractions were incubated with either 14C-labeled GS-7340 or nonradioactive prodrugs. In separate experiments, the enzymatic activities of purified prodrug hydrolase (∼1.4 μg/ml), recombinant CatA (18 ng/ml), and recombinant cathepsin Z/X (2 μg/ml) were compared using different substrates. In assays using 14C-labeled GS-7340 as the substrate, the production of 14C-labeled metabolite X was monitored by measuring the amount of radioactivity (14C-labeled metabolite X) retained on an anion-exchange filter (DE-81). The final reaction conditions were as follows: 25 mM MES [2-(N-morpholino)ethanesulfonic acid], pH 6.5, 100 mM NaCl, 1 mM dithiothreitol (DTT), 30 μM 14C-labeled GS-7340, 0.1% NP-40, and various amounts of enzyme in a final volume of 60 μl. The reaction mixture was incubated at 37°C for 10-, 30-, and 90-min intervals, and 17 μl of the reaction mixture was spotted onto a DE-81 filter. The filter was washed three times with 25 mM Tris, pH 7.5, and the amount of 14C-labeled metabolite X was determined (LS 6500; Beckman, Fullerton, CA).
In reactions using nonradioactive substrates, incubations were performed as described above and extracted with 180 μl of ice-cold methanol. Extracts were centrifuged at 13,000 × g, and the supernatants were evaporated, resolubilized in 100 μl of buffer A (25 mM potassium phosphate, pH 6.0, 5 mM tetrabutylammonium bromide), and injected onto a C18 reverse-phase column [Prodigy 5u ODS(2); Phenomenex, Torrance, CA] equilibrated with buffer A. Bound substrate and metabolites (metabolite X, TFV, or GS-9148) were resolved using a linear gradient of acetonitrile (0 to 70%, 11 min, 0.25 ml/min) with buffer B (25 mM KPO4, pH 6.0, 5 mM tetrabutylammonium bromide, 70% acetonitrile). Metabolites were quantitated by the integration of peak area. In both assays, activity was expressed as picomoles of metabolites produced per minute per volume of enzyme sample. Prodrug hydrolase-specific activity was expressed as picomoles of metabolites produced per minute per μg protein.
Isolation of hydrolase.
Fresh human PBMCs were obtained from patients undergoing leukopheresis; cells were shipped in plasma and processed within 26 h of draw. PBMCs were harvested by centrifugation at 1,200 × g for 5 minutes and washed three times by resuspension in red blood cell lysis buffer (155 mM NH4Cl, 0.1 mM EDTA, 10 mM KHCO3). Washed cells (29 × 109) were resuspended in 150 ml of lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 20 mM CaCl2, 1 mM DTT, and 1% NP-40) and incubated on ice for 20 min. The PBMC crude extract was centrifuged at 1,000 × g for 30 min to remove unlysed cells, and the supernatant was recentrifuged at 100,000 × g for 1 h. The 100,000 × g supernatant (PBMC extract; P0) was harvested (165 ml), and the pellets (1,000 × g and 100,000 × g pellets) were resuspended in 10 mM Tris, pH 7.4, 150 mM NaCl, 20 mM CaCl2, 1 mM DTT and assayed for prodrug hydrolase activity. Assays showed that <2% of the prodrug hydrolase activity was present in the pellets. The cell extract was snap-frozen in liquid nitrogen and stored at −70°C.
The PBMC extract was diluted 1:10 (vol/vol) with 25 mM Tris, pH 7.5, 10% glycerol, 1 mM DTT (Q15 buffer A) and loaded onto an anion-exchange column (Source Q15; GE Healthcare) previously equilibrated with Q15 buffer A. Bound protein was eluted with a linear NaCl gradient (30 column volumes [CV]) from 0 to 0.5 M NaCl, and fractions were assayed for prodrug hydrolase activity.
The Q15 pool was diluted 1:1 (vol/vol) with butyl Sepharose hydrophobic interaction chromatography (BS-HIC) buffer (25 mM Tris, pH 8.0, 1 mM DTT, 10% glycerol) containing 1.0 M (NH4)2SO4, and the sample was loaded onto a butyl Sepharose HIC column (5-ml HiTrap column; GE Healthcare) previously equilibrated with BS-HIC buffer, 0.5 M (NH4)2SO4. Bound protein was eluted with a linear gradient (15 CV) decreasing to 25 mM Tris, pH 8.0, 1 mM DTT, 10% glycerol, and fractions were assayed as described above.
The BS-HIC pool was diluted (1:1, vol/vol) with 20 mM Tris, pH 7.5, 0.5 M NaCl, 1 mM MnCl2, 1 mM CaCl2 (concanavalin [ConA] buffer A) and loaded onto a ConA column (1.0 ml) previously equilibrated with ConA buffer A. Bound protein was eluted with ConA buffer B (ConA buffer A + 1 M methyl-α1-mannopyranoside), and fractions were assayed.
Identification of proteins in ConA pool.
The ConA pool containing purified prodrug hydrolase was concentrated to ∼60 μl, and proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4 to 20% acrylamide gradient, NUPAGE; Invitrogen, Inc., Carlsbad, CA). Protein bands were visualized using silver staining, and gel slices in an adjacent lane (unstained, loaded with 50 μl of the sample) corresponding to stained protein bands were excised. In-gel digestion of proteins was performed in 50 mM ammonium bicarbonate containing excess trypsin at 37°C overnight. The resultant peptides were purified by passage through a C18 ZipTip column and analyzed by positive electrospray ionization-mass spectrometry (ESI-MS) using a Sciex Q-Star/Pulsar mass spectrometer (ABI Biotechnologies, Foster City, CA). Peptides were sequenced using tandem MS (MS-MS) fragmentation. Proteins were identified from the generated sequences by BLAST analysis of the NCBI nonredundant protein/peptide database.
Biochemical characterization of prodrug hydrolase.
An aliquot of the BS-HIC pool was loaded onto a gel filtration column (1- by 30-cm Superdex S200; GE Healthcare, Piscataway, NJ) previously equilibrated with 25 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 20 mM CaCl2, 1 mM DTT. Similarly, an aliquot was buffer exchanged into 25 mM ethanolamine-iminodiacetic acid, pH 7.8, 10% glycerol (Mono P buffer A) and loaded onto a chromatofocusing column (5- by 5-mm HR Mono P column; GE Healthcare, Piscataway, NJ) previously equilibrated with Mono P buffer A. Bound protein was eluted with a 20-CV gradient to pH 3.6 with 10 ml/100 ml Polybuffer 74 (GE Healthcare, Piscataway, NJ) adjusted to pH 4.0 with iminodiacetic acid.
Inhibition of prodrug hydrolase.
Purified prodrug hydrolase (1.4 μg/ml), recombinant CatA (180 ng/ml) (1049-SE; R&D Systems, Minneapolis, MN), crude PBMC extract (120 μg/ml), and control enzymes (10 μg/ml human leukocyte elastase [huLE] and 40 μg/ml porcine liver carboxylesterase [PLCE]; both from Sigma, St. Louis, MO) were incubated with 100 μM 14C-labeled GS-7340, 25 mM MES, pH 6.5, 100 mM NaCl, 1 mM DTT, 0.1% NP-40 in the presence and absence of various amounts of known serine hydrolase inhibitors (di-isopropylfluorophosphate [DFP] and 3,4-dichloroisocoumarin [3,4-DCI]) at 37°C for 10 to 90 min. A known cysteine protease inhibitor [trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane (E64); Sigma] was also used as a negative control. The production of 14C-labeled metabolite X was monitored by measuring the amount of radioactivity retained on an anion-exchange filter (DE-81) as described above. Inhibition of prodrug hydrolase and control hydrolases are expressed as the concentration of inhibitor required to reduce the rate of product (metabolite X) production by 50% (50% inhibitory concentration [IC50]).
Labeling of prodrug hydrolase and recombinant CatA.
Labeling of prodrug hydrolase (5 μg/ml), recombinant CatA (10 μg/ml), and ConA (75 μg/ml) with 14C-labeled GS-7340 and [3H]DFP was performed with both native and denatured enzymes. Denatured (80°C, 3 min) and native enzymes and ConA (60 μl each) were preincubated separately for 2 min at 37°C and reacted with either 14C-labeled GS-7340 (1.0 mM; 0.04 mCi/ml; 37°C/2 min) or [3H]DFP (25 μM; 0.1mCi/ml; 37°C/30 min) as described above. Labeling was quenched with 0.3% SDS, and proteins were precipitated with ice-cold 10% trichloroacetic acid, harvested by centrifugation, and washed with ice-cold 100% acetone. Proteins were denatured for 10 min at 70°C in SDS-PAGE sample buffer (Novex) prior to electrophoresis. The gels were fixed in anisopropanol:water:acetic acid solution (25:65:10), soaked in Amplify fluorographic reagent (GE Healthcare, Piscataway, NJ), dried, and exposed to preflashed X-ray film (HyperFilm MP; GE Healthcare, Piscataway, NJ) for 2 weeks. Radioactively labeled bands were visualized after development according to the manufacturer's instructions.
Western blot analysis.
Aliquots of recombinant CatA (50 ng), human PBMC extract (7.5 μg), and 1 μg each of the Q-Source, HIC, and ConA pools were resolved by SDS-PAGE (4 to 12% Bis-Tris, NuPAGE; Novex, La Jolla, CA) and transferred to a polyvinylidene difluoride (PVDF) membrane by use of a modification of the manufacturer's protocol. The PVDF membrane was blocked with a 3% bovine serum albumin-Tris-buffered saline (TBS)-0.1% Tween solution overnight at 4°C and incubated with polyclonal anti-human cathepsin A antibody (goat immunoglobulin G, 250 ng/ml; R&D Systems, Minneapolis, MN) diluted in 1% bovine serum albumin-TBS-0.1% Tween. Proteins were visualized by labeling bound CatA antibodies with a fluorescently labeled secondary antibody at 20 μg/ml (Alexa Fluor 647 chicken anti-goat immunoglobulin G; Molecular Probes, Eugene, OR) and detected using STORM860 according to the manufacturer's protocol (Molecular Dynamics, Piscataway, NJ).
Determination of kinetic constants for GS-7340 hydrolase.
Prodrug hydrolase (CatA) kinetic constants (Km and kcat) were determined by measuring the initial rates at various concentrations (0.062 mM to 4.0 mM) of GS-7340 or GS-9131.
Metabolism of prodrugs in CatA+/− fibroblasts.
CatA-deficient (CatA−) (GM05076 and GM02348) and CatA+ (GM00409, GM5400, and GM05757B) fibroblasts (Coriell Institute for Medical Research, Camden, NJ) were cultivated in minimum essential medium (MEM) (Eagle-Earle; Cambrex Biosciences, Baltimore, MD) supplemented with 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1× vitamins, and 15% fetal bovine serum. HEL fibroblasts (CatA+) (ATCC, Manassas, VA) were maintained in MEM (Eagle) medium as described previously and containing 1.0 mM sodium pyruvate, 10% fetal bovine serum.
Flasks were seeded (4,000 to 12,000 cells per cm2), cultured overnight, and incubated in fresh medium in the presence of 14C-labeled GS-7340 or 14C-labeled GS-9131 (10 μM, 0.521 μCi/ml) at 37°C for 30 to 240 min. Medium was removed at selected time points (0, 30, 60, 120 and 240 min), and cells were washed with ice-cold phosphate-buffered saline (PBS) and detached from the flask with trypsin (1.5 ml 0.5 mg/ml in PBS, 1 mM EDTA for 3 min at 37°C). Detachment was stopped by adding 5 ml of MEM, and the cells were harvested by centrifugation, washed with PBS, and extracted with 1 ml of 80% methanol. Cell extracts containing solubilized 14C-labeled metabolites were evaporated and resolubilized in 200 μl of water, and aliquots (40 μl) were analyzed to determine the amount of radioactivity (14C-labeled metabolites) present in the cells (Beckman LS 6500). The total amount of 14C-labeled metabolites per million cells was determined based on the known specific activity (cpm/pmol) of 14C-labeled GS-7340 or 14C-labeled GS-9131 and the number of cells in the sample. Cell volume measurements were used to determine metabolite concentration (μM). The metabolites were resolved by high-pressure liquid chromatography according to previously described conditions (12).
Cell volume measurement.
Fibroblasts were washed with PBS and detached by incubation with trypsin (0.5 g/ml in PBS, 1 μΜ EDTA) for 5 minutes at 37°C. Thereafter, the cells were incubated for 30 min in the culture medium to regain their spherical shape. Pictures of cells were taken and their diameters determined using a hematocytometer grid. Cell volume was calculated using the mean diameter obtained from 150 cells.
RESULTS
Isolation of prodrug ester hydrolase.
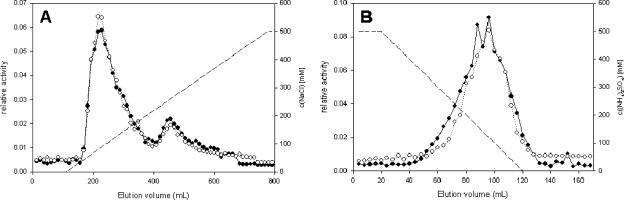
The activity of prodrug hydrolase was monitored by measuring the rate of the formation of monoalaninyl amidates of tenofovir and GS-9148 (respective metabolites X) (Fig. 1). The reaction rate was constant for at least 40 min, and the amount of product observed was proportional to the amount of hydrolase fraction in the reaction mixture. Initial experiments demonstrated that a large proportion of the prodrug hydrolase activity (>97%) present in purified human PBMCs could be extracted with buffers containing 1% NP-40. The activity present in the PBMC extract was retained on anion-exchange column (Source Q15) and eluted as a single major peak at 50 to 75 mM NaCl (Fig. 2A and Table 1). A minor peak, representing approximately 20% of the hydrolase activity, eluted at 200 mM NaCl (Fig. 2A). Recovery of total activity in the eluted fractions was 50 to 65% with an increase in specific activity of approximately sixfold (Table 1). In the following step, prodrug hydrolase activity bound to a butyl Sepharose column and eluted as a single peak at 75 to 200 mM (NH4)2SO4 with a relatively high recovery (50 to 65%) and a sixfold-increased specific activity (Fig. 2B and Table 1). Prodrug hydrolase activity present in the butyl Sepharose pool bound quantitatively to the ConA affinity column and was eluted with 1 M methyl-α1-mannopyranoside (Table 1). This affinity step increased the specific activity of the hydrolase by >50-fold, resulting in an overall purification of approximately 2,000-fold. The total recovery of prodrug hydrolase from the initial PBMC extracts was approximately 5 to 6%.
FIG. 2.
Purification of prodrug hydrolase. (A) Anion-exchange chromatography. PBMC extract from 15 × 109 cells (75 to 85 ml) was diluted 1:10 (vol/vol) with Q15 buffer A and loaded onto an anion-exchange column. Bound protein was eluted and assayed for both GS-7340 and GS-9131 hydrolase activity as described in the text. (B) Hydrophobic interaction chromatography. The Q15 pool was processed as described in the text and loaded onto a butyl Sepharose HIC column. Bound protein was eluted and assayed for prodrug hydrolase activity. Symbols: •, GS-7340 hydrolase activity; ○, GS-9131 hydrolase activity; ---, salt concentration gradient. c, concentration.
TABLE 1.
Summary of prodrug hydrolase purificationa
| Purification step | Protein concn (mg/ml) | Protein amt (mg) | Specific activity (pmol/min · μg) for:
|
% Recovery | |
|---|---|---|---|---|---|
| GS-7340 | GS-91631 | ||||
| P0 PBMC | 5.0 | 1,000 | 16 | 5 | |
| Q15 pool | 0.116-0.167 | 42.5 | 112 | 23 | ∼50 |
| BS-HIC pool | 0.02-0.035 | 2.75 | 730 | 145 | ∼50 |
| ConA pool | 0.002 | 0.02 | 35,000 | 10,000 | ∼25 |
Fresh human PBMCs (30 × 109) were extracted and the prodrug hydrolase was purified as described in Results. Protein concentration was measured using the Bradford Coomassie blue assay. Specific activity of CatA in the ConA pool was determined based on an estimation of the percentage of the 29-kDa catalytic subunit present in the sample.
Identification of proteins in the ConA pool.
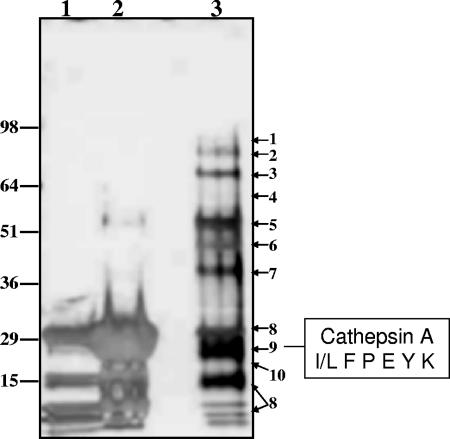
Proteins present in the ConA pool containing purified prodrug hydrolase were resolved by SDS-PAGE and visualized using silver staining (Fig. 3). Seven major and two minor protein bands were identified using in-gel tryptic digestion, mass spectrometry analysis of peptides, and sequencing MS-MS fragmentation. A prominent protein band migrating with an apparent molecular mass of 29 kDa yielded the sequence I/L F P E Y K (Fig. 3). This band represented approximately 5 to 10% of the total protein present in the ConA pool and based on the fragment sequence was tentatively identified as human CatA (NCBI accession number gi:12653639). In addition to its function as a protective protein for beta-galactosidase, CatA is a serine hydrolase with both protease and carboxyesterase activity. CatA has been shown to form a homodimer composed of two identical 50,000-Da subunits (10). Each subunit contains disulfide-linked 30- and 20-kDa polypeptide chains (29, 41, 52). Cathepsin Z/X (NCBI accession number gi:329458; EC 3.428.2), a cysteine carboxypeptidase (30), was identified among the proteins present in a sample eluted from the ConA column (Fig. 3).
FIG. 3.
SDS-PAGE resolution and identification of proteins in the ConA pool. Proteins present in the ConA pool were concentrated to 60 μl and resolved using SDS-PAGE (4 to 20% acrylamide gradient gel, NUPAGE; Invitrogen, Inc., Carlsbad, CA). Lanes: 1, ConA beads after elution; 2, ConA beads eluted with 8 M urea; 3, concentrated ConA pool (10 μl). Protein bands in the 10-μl lane were visualized using silver staining. Adjacent areas of the gel in an unstained lane (50 μl aliquot loaded) corresponding to a stained protein band were excised and identified by MS-MS sequencing of tryptic peptides generated by digestion. The resultant peptides were purified by passage through a C18 ZipTip column, analyzed by positive ESI-MS, and sequenced using MS-MS fragmentation. Proteins were identified from the generated sequences by BLAST analysis of the NCBI nonredundant protein/peptide database (1). Proteins indicated to the right: 1, fibrinogen; 2, N-acetylglucosamidase; 3, acid α-glucosidase; 4, sulfamidase; 5, fibrinogen; 6, α-fucosidase; 7, iduronate sulfatase; 8, concanavalin A; 9, CatA; 10, cathepsin Z/X.
Biochemical characterization of prodrug hydrolase.
Gel filtration chromatography indicated that prodrug hydrolase eluted as a single major peak (data not shown) corresponding to an apparent molecular mass of 70 to 100 kDa. Chromatofocusing demonstrated that the purified enzyme has a pI of ∼5.5 (data not shown). Both of these characteristics were identical to the corresponding ones for recombinant CatA. In addition, the specific activities of the purified enzyme and the recombinant CatA towards GS-7340 were virtually identical (35 nmol · min−1 · μg). DFP and 3,4-DCI are effective inhibitors of serine hydrolases, including CatA (6, 11, 17, 22, 26, 32). Purified prodrug hydrolase and the recombinant CatA were both inhibited by 3,4-DCI and DFP with IC50s of ∼0.5 μM and 5 to 10 μM, respectively (Table 2). E64, a specific inhibitor of cysteine hydrolases, inhibited neither prodrug hydrolase nor recombinant CatA. These data demonstrate that the biochemical and enzymatic properties of the isolated hydrolase are identical to those of human CatA.
TABLE 2.
Inhibition of prodrug hydrolase, CatA, human PBMC extract, and control enzymes by serine and cysteine protease inhibitorsa
| Inhibitor | IC50 (μM) for:
|
||||
|---|---|---|---|---|---|
| Prodrug hydrolase | CatA | PBMC extract | PLCE | huLE | |
| 3,4-DCI | 0.5 | 0.6 | 5 | >250 | 3.0 |
| DFP | 5.0 | 10.0 | 20 | 0.05 | |
| E64 | >300 | >300 | >300 | >300 | >300 |
Purified prodrug hydrolase (1.4 μg/ml), recombinant CatA (180 ng/ml), crude PBMC extract (120 μg/ml), and control enzymes huLE (10 μg/ml) and PLCE (40 μg/ml) were incubated with 100 μM 14C-labeled GS-7340 in the presence and absence of varying amounts of known serine hydrolase inhibitors. Inhibitions of prodrug hydrolase and control hydrolases are expressed as IC50 values.
Labeling of prodrug hydrolase.
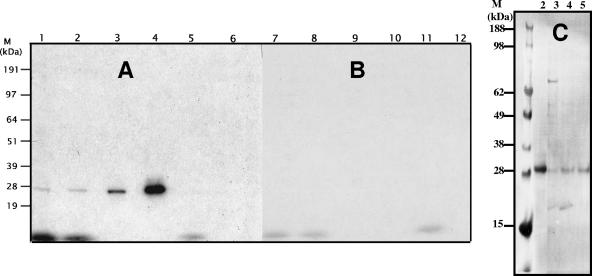
The reaction mechanism of serine hydrolases involves the formation of an acyl-enzyme complex between the serine residue in the active site and the carboxyl in the substrate cleavage site (45). Denaturing the enzyme during the course of the reaction allowed for capturing this acyl-enzyme complex. SDS-PAGE analysis of reactions containing either the prodrug hydrolase or CatA and 14C-labeled GS-7340 (Fig. 4A) showed the presence of a single labeled band at 29 kDa. The substrate failed to label the heat-denatured prodrug hydrolase, CatA, or concanavalin A protein, a major contaminant from the affinity chromatography step (Fig. 4B).
FIG. 4.
Labeling of prodrug hydrolase and recombinant CatA with 14C-labeled GS-7340 and [3H]DFP. Labeling of prodrug hydrolase, recombinant CatA, and concanavalin A with 14C-labeled GS-7340 and [3H]DFP was performed with both native (A) and denatured (B) enzymes as described in the text. Proteins were analyzed by SDS-PAGE and visualized by exposure to X-ray film. Incubation mixtures contained 1.0 mM 14C-labeled GS-7340 and prodrug hydrolase (lanes 1 and 7), recombinant CatA (lanes 2 and 8) or concanavalin A (lanes 5 and 11); 25 μM [3H]DFP or prodrug hydrolase (lanes 3 and 9), and recombinant CatA (lanes 4 and 10) or concanavalin A (lanes 6 and 12). (C) Aliquots of recombinant CatA (50 ng; lane 2) and 1 μg each of the Q-Source (lane 3), HIC (lane 4), and ConA (lane 5) pools were resolved using SDS-PAGE, transferred to a PVDF membrane, incubated with human cathepsin-A antibody, and visualized with a fluorescently labeled secondary antibody. M, molecular mass marker.
Unlike transient formation of an acyl-enzyme complex, [3H]DFP forms a stable covalent bond with the hydroxy group of serine in the hydrolase active site (31, 42). Figure 4A shows that [3H]DFP specifically labeled the 29-kDa catalytic subunits of both prodrug hydrolase and CatA. Western blot analysis using polyclonal goat antibody against human CatA confirmed the presence of CatA in all pools of fractions showing the prodrug hydrolase activity (Fig. 4C).
Substrate specificity of prodrug hydrolase and recombinant CatA.
The relative rates of cleavage of the carboxyester bond present in different monoamidate prodrugs of tenofovir were determined in the presence of the native isolated prodrug hydrolase and recombinant CatA (Table 3). Notably, both enzymes exhibit the same substrate specificity and have similar specific activities against the tested series of TFV prodrugs. Prodrugs containing Phe, Gly, and α-aminobutyric acid are cleaved approximately 5- to 10-fold less efficiently than GS-7340 (prodrug containing Ala) by both CatA and the purified prodrug hydrolase (Table 3). Neither of the two enzymes is capable of hydrolyzing prodrugs containing branched hydrophobic amino acid residues (Val, Leu, Ile). Cathepsin Z/X, found in the ConA pool (Fig. 3), was not capable of hydrolyzing GS-9131, GS-7340, or any other tenofovir amidate prodrug.
TABLE 3.
Relative activities of prodrug hydrolase and recombinant CatA towards various TFV amidate prodrugsa
| Compound | Phosphonoamidate structure | Relative rate of hydrolysisb
|
|
|---|---|---|---|
| CatA | Prodrug hydrolase | ||
| GS-7340 | Ala-isopropyl | 1.0 | 1.0 |
| GS-7003 | Phe-methyl | 0.143 | 0.18 |
| GS-7119 | Gly-ethyl | 0.079 | 0.075 |
| GS-7120 | α-ABA-ethyl | 0.094 | 0.082 |
| GS-7095 | Leu-methyl | 0 | 0 |
| GS-7096 | Val-methyl | 0 | 0 |
| GS-7098 | Ile-methyl | 0 | 0 |
The enzymatic activities of purified prodrug hydrolase and recombinant CatA against a series of phosphonoamidate prodrugs were determined. Reactions were performed as described in the text and the production of metabolite X was monitored using high-pressure liquid chromatography. The rate of each prodrug hydrolysis is expressed relative to that of GS-7340. Recombinant cathepsin Z/X failed to hydrolyze any of these prodrugs.
Specific activities of CatA and purified prodrug hydrolase used in the calculations were 31,000 pmol/min · μg and 26,600 pmol/min · μg, respectively (see Table 1).
Kinetic constants of nucleotide prodrug hydrolysis.
The kinetic constants (Km and kcat) for the hydrolysis of GS-7340 and GS-9131 by the isolated prodrug hydrolase were determined by measuring the initial reaction rates at various substrate concentrations (0.062 to 4 mM). kcat values were calculated from Vmax values with the assumption that all enzyme molecules are catalytically active. The amount of CatA in the sample was estimated based on the Western blot. The observed Km and kcat values were 610 μΜ and 1.77 × 103 s−1 for GS-7340 and 363 μΜ and 0.24 × 103 s−1 for GS-9131, indicating a slightly higher affinity but a lower rate of hydrolysis for the latter prodrug (Table 4).
TABLE 4.
Kinetics of GS-7340 and GS-9131 hydrolysis by CatA
| Compound | Km (μM) | kcat (s−1)a | kcat/Km (s−1 · M−1) |
|---|---|---|---|
| GS-7340 | 610 ± 190 | 1,777 ± 290 | 2.9 × 106 |
| GS-9131 | 363 ± 35 | 237 ± 12 | 0.6 × 106 |
kcat values were calculated from Vmax values with the assumption that all enzyme molecules are catalytically active. The amount of CatA in the sample was estimated based on the Western blot.
Metabolism of GS-7340 and GS-9131 in CatA-deficient fibroblasts.
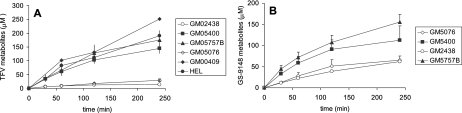
Human primary fibroblasts obtained from patients exhibiting galactosialidosis are deficient in the expression of CatA due to mutations present in the protein that prevent its proper folding (46, 50). When incubated in the presence of 14C-labeled GS-7340 or 14C-labeled GS-9131, both control CatA+ and hydrolase-deficient CatA− fibroblasts show a linear accumulation of the prodrug metabolites for up to 2 h. However, the concentrations of TFV and GS-9148 metabolites observed for control fibroblasts were six to nine and two to four times greater, respectively, than those observed for CatA− fibroblasts (Fig. 5).
FIG. 5.
Metabolism of GS-7340 and GS-9131 in CatA+/− fibroblasts. Fibroblasts deficient in CatA (CatA−) (GM05076 and GM02438) and control fibroblasts (Cat A+) (GM00409, GM5400, GM05757B, and HEL) were cultivated and incubated with 14C-labeled GS7340 or 14C-labeled GS9131 (10 μM; 0.521 μCi/ml) for 30 to 240 min. At selected time points, medium was removed and cells were processed as described in the text. Associated 14C-labeled metabolites were extracted from the cells and expressed as picomoles/106 cells. Cell volume was used to calculate metabolite concentration (μM).
DISCUSSION
A single major enzyme capable of hydrolyzing GS-7340 and GS-9131 was isolated from human PBMCs. In addition, a minor hydrolase peak, representing <20% of total prodrug hydrolase activity, was detected during the isolation procedure and has yet to be identified. The activities of GS-7340 and GS-9131 hydrolases coeluted during all purification steps. The hydrolase activity was purified >2,000-fold, yielding specific activities of 35 (GS-7340) and 10 (GS-9131) nmoles/min · μg protein. SDS-PAGE resolved 10 proteins in the final isolate, all of which were identified by in-gel digestion and MS-MS peptide sequencing. A prominent protein migrating at 29 kDa was identified as a subunit of CatA (EC 3.4.16.5). Several independent lines of evidence indicate that the isolated enzyme capable of hydrolyzing nucleotide amidate prodrugs is CatA. First, the biochemical properties of prodrug hydrolase, including its apparent molecular weight and pI value, matched those of human CatA. In addition, both the prodrug hydrolase and recombinant CatA showed identical sensitivities to a set of serine hydrolase inhibitors. Further experiments demonstrated that the identified 29-kDa protein was able to form a covalent acyl-enzyme complex with both 14C-labeled GS-7340 and [3H]DFP and was recognized by polyclonal antibodies specific for human CatA. The catalytic subunit of recombinant CatA comigrated with the 29-kDa prodrug hydrolase and formed an identical covalent acyl intermediate complex with 14C-labeled GS-7340. Furthermore, the substrate specificity of isolated prodrug hydrolase towards a series of TFV monoamidate prodrugs was identical with that of recombinant human CatA. Finally, primary fibroblasts from two different patients expressing nonfunctional mutants of CatA accumulated levels of tenofovir and GS-9148 metabolites about 7.5-fold and 3-fold lower, respectively, than those accumulated by normal control fibroblasts. Taken together, the presented data strongly indicate that the isolated prodrug hydrolase is CatA. In addition, the results of intracellular metabolism experiments demonstrate that CatA plays an important role in the cellular activation pathway for both GS-7340 and GS-9131.
Interestingly, the Km value for the hydrolysis of GS-7340 and GS-9131 by CatA is in the submillimolar range, suggesting that under in vivo conditions the conversion of GS-7340 and GS-9131 is likely to occur well below saturating substrate concentrations. In fact, intracellular levels of tenofovir metabolites achieved in dog PBMCs incubated at concentrations of GS-7340 of up to 20 μM are directly proportional to the extracellular concentration of the prodrug (12, 25). However, the kcat/Km values for GS-7340 and GS-9131 cleavage by cathepsin A (2.9 × 106 and 0.6 × 106 s−1 · M−1, respectively) are comparable to those obtained for synthetic oligopeptides (0.7 × 106 to 0.19 × 106 s−1 · M−1) (35), suggesting that the nucleotide prodrug hydrolysis is likely to occur with a reasonable efficiency even in the presence of natural substrates.
CatA was discovered as a component of a high-molecular-weight complex of lysosomal β-galactosidase and N-acetyl neuraminidase (10). An important function of CatA in this multienzyme complex is to protect both glycosidases from rapid proteolysis in the lysosomes (10, 33, 34, 52). Further studies revealed that CatA is a multifunctional enzyme possessing deaminase and esterase activities at neutral pH and carboxypeptidase activity at pH 5.0 to 5.5 (18-21, 50). Peptide substrates with hydrophobic amino acid residues in the P1′ position are cleaved with high efficiency; however, CatA also shows high affinity for charged residues in this position (13, 35, 37, 38). The substrate specificity of CatA towards peptidic substrates is predictive of its activity against phosphonoamidate prodrugs of tenofovir. CatA efficiently cleaves GS-7340 containing alanine and isopropanol moieties analogous to the P1 and P1′ positions, respectively. The rate of hydrolysis of a prodrug containing phenylalanine at the P1 position is approximately twofold greater than the cleavage rate of prodrugs containing glycine or α-aminobutyric acid at the same position. Prodrugs with branched amino acids at P1 are not cleaved by CatA. Modeling of the S1′ binding site and crystallographic analysis of CatA have suggested that Tyr247 provides the majority of the substrate binding energy for hydrophobic P1′ residues, whereas positively charged residues at P1′ interact with Asp64 (13, 40, 41). This structural information combined with the results from the present study should facilitate the rational design of novel prodrugs that are even more effective substrates for CatA. Such prodrugs may exhibit further improved ability for the intracellular delivery of antiviral nucleotides. In addition, CatA is expressed in a broad range of human tissues, including kidney, liver, macrophages, platelets, and testis (18, 44, 48) and thus might be a useful target enzyme for prodrug activation for various nucleotides active against pathogens that exhibit a wide range of tissue tropism (e.g., herpes and hepatitis viruses).
Despite the fact that CatA is not exclusively expressed in various immune cell types, TFV and GS-9148 metabolites accumulate rapidly and to high levels in both lymphatic tissues and PBMCs following in vivo oral administration of GS-7340 and GS-9131, respectively (25, 36). The high membrane permeability of these prodrugs and their efficient intracellular hydrolysis by CatA allow for enhanced delivery of TFV and GS-9148 into cells, where they are efficiently phosphorylated to their active diphosphate metabolites (39). Overall, this results in >500- and >200-fold improvement in the in vitro antiviral activities of GS-7340 and GS-9131, respectively, compared to those of the corresponding parent nucleotides (9, 25). In addition, as previously described for phosphoramidate nucleoside monophosphate prodrugs (27, 28, 43, 47, 51), GS-7340 and GS-9131 are relatively stable in human plasma (24, 25). Together, these enhanced pharmacokinetic and metabolic properties allow for efficient intracellular delivery of nucleotide metabolites in vitro and in vivo (9, 12, 25).
Phosphonoamidate prodrugs of nucleotides provide significant pharmacodynamic and therapeutic advantages over other types of nucleotide prodrugs and their parent nucleosides/nucleotides. The identification and characterization of cathepsin A as a key enzyme for the intracellular activation of nucleotide amidates should facilitate further optimization and broader application of this novel prodrug technology.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniou, T., L. Y. Park-Wyllie, and A. L. Tseng. 2003. Tenofovir: a nucleotide analog for the management of human immunodeficiency virus infection. Pharmacotherapy 23:29-43. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J. 1994. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113-126. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., A. Karlsson, S. Aquaro, C. F. Perno, D. Cahard, L. Naesens, E. De Clercq, and C. McGuigan. 1996. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc. Natl. Acad. Sci. USA 93:7295-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullock, T. L., K. Breddam, and S. J. Remington. 1996. Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity. J. Mol. Biol. 255:714-725. [DOI] [PubMed] [Google Scholar]
- 7.Cherrington, J. M., S. J. Allen, B. H. McKee, and M. S. Chen. 1994. Kinetic analysis of the interaction between the diphosphate of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, ddCTP, AZTTP, and FIAUTP with human DNA polymerases beta and gamma. Biochem. Pharmacol. 48:1986-1988. [DOI] [PubMed] [Google Scholar]
- 8.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antivir. Res. 54:37-45. [DOI] [PubMed] [Google Scholar]
- 9.Cihlar, T., A. Ray, D. Boojamra, L. Zhang, H. Hui, D. Grant, K. White, M. Desai, N. Parkin, and R. Mackman. 2006. GS9148: a novel nucleotide active against HIV-1 variants with drug resistance mutations in reverse transcriptase. 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO.
- 10.D'Azzo, A., A. Hoogeveen, A. J. Reuser, D. Robinson, and H. Galjaard. 1982. Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc. Natl. Acad. Sci. USA 79:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delbaere, L. T., and G. D. Brayer. 1985. The 1.8 A structure of the complex between chymostatin and Streptomyces griseus protease A. A model for serine protease catalytic tetrahedral intermediates. J. Mol. Biol. 183:89-103. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg, E. J., G. X. He, and W. A. Lee. 2001. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids 20:1091-1098. [DOI] [PubMed] [Google Scholar]
- 13.Elsliger, M. A., A. V. Pshezhetsky, M. V. Vinogradova, V. K. Svedas, and M. Potier. 1996. Comparative modeling of substrate binding in the S1′ subsite of serine carboxypeptidases from yeast, wheat, and human. Biochemistry 35:14899-14909. [DOI] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Reference deleted.
- 16.Grim, S., and F. Romanelli. 2003. Tenofovir disoproxil fumarate. Ann. Pharmacother. 37:849-859. [DOI] [PubMed] [Google Scholar]
- 17.Hiraiwa, M. 1999. Cathepsin A/protective protein: an unusual lysosomal multifunctional protein. Cell. Mol. Life Sci. 56:894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackman, H. L., P. W. Morris, P. A. Deddish, R. A. Skidgel, and E. G. Erdos. 1992. Inactivation of endothelin I by deamidase (lysosomal protective protein). J. Biol. Chem. 267:2872-2875. [PubMed] [Google Scholar]
- 19.Jackman, H. L., P. W. Morris, S. F. Rabito, G. B. Johansson, R. A. Skidgel, and E. G. Erdos. 1993. Inactivation of endothelin-1 by an enzyme of the vascular endothelial cells. Hypertension 21:925-928. [DOI] [PubMed] [Google Scholar]
- 20.Jackman, H. L., F. Tan, D. Schraufnagel, T. Dragovic, B. Dezso, R. P. Becker, and E. G. Erdos. 1995. Plasma membrane-bound and lysosomal peptidases in human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 13:196-204. [DOI] [PubMed] [Google Scholar]
- 21.Jackman, H. L., F. L. Tan, H. Tamei, C. Beurling-Harbury, X. Y. Li, R. A. Skidgel, and E. G. Erdos. 1990. A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal “protective protein”. J. Biol. Chem. 265:11265-11272. [PubMed] [Google Scholar]
- 22.Kam, C. M., A. S. Abuelyaman, Z. Li, D. Hudig, and J. C. Powers. 1993. Biotinylated isocoumarins, new inhibitors and reagents for detection, localization, and isolation of serine proteases. Bioconjug. Chem. 4:560-567. [DOI] [PubMed] [Google Scholar]
- 23.Krejcova, R., K. Horska, I. Votruba, and A. Holy. 2000. Phosphorylation of purine (phosphonomethoxy)alkyl derivatives by mitochondrial Amp kinase (Ak2 type) from L1210 cells. Collect. Czech. Chem. Comm. 65:1653-1668. [Google Scholar]
- 24.Lee, W., G. He, A. Mulato, W. Delaney, E. Eisenberg, T. Cihlar, S. Xiong, M. Miller, S. Gill, R. Shibata, and C. Gibbs. 2002. In vivo and in vitro characterization of GS 7340, an isopropylalaninyl phenyl ester prodrug of tenofovir: selective intracellular activation of GS 7340 leads to preferential distribution in lymphatic tissues, p. 384-T. Ninth Conference on Retroviruses and Opportunistic Infections, Seattle, WA.
- 25.Lee, W. A., G. He, E. Eisenberg, T. Cihlar, S. Swaminathan, A. Mulato, and K. Cundy. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, Y., M. P. Patricelli, and B. F. Cravatt. 1999. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96:14694-14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuigan, C., P. W. Sutton, D. Cahard, K. Turner, G. O'Leary, Y. Wang, M. Gumbleton, E. De Clercq, and J. Balzarini. 1998. Synthesis, anti-human immunodeficiency virus activity and esterase lability of some novel carboxylic ester-modified phosphoramidate derivatives of stavudine (d4T). Antivir. Chem. Chemother. 9:473-479. [DOI] [PubMed] [Google Scholar]
- 28.McGuigan, C., H. W. Tsang, P. W. Sutton, E. De Clercq, and J. Balzarini. 1998. Synthesis and anti-HIV activity of some novel chain-extended phosphoramidate derivatives of d4T (stavudine): esterase hydrolysis as a rapid predictive test for antiviral potency. Antivir. Chem. Chemother. 9:109-115. [DOI] [PubMed] [Google Scholar]
- 29.Morreau, H., N. J. Galjart, R. Willemsen, N. Gillemans, X. Y. Zhou, and A. d'Azzo. 1992. Human lysosomal protective protein. Glycosylation, intracellular transport, and association with beta-galactosidase in the endoplasmic reticulum. J. Biol. Chem. 267:17949-17956. [PubMed] [Google Scholar]
- 30.Nagler, D. K., and R. Menard. 1998. Human cathepsin X: a novel cysteine protease of the papain family with a very short proregion and unique insertions. FEBS Lett. 434:135-139. [DOI] [PubMed] [Google Scholar]
- 31.Pasternack, M., and H. Eisen. 1985. A novel serine esterase expressed by cytotoxic T lymphocytes. Nature 314:743-745. [DOI] [PubMed] [Google Scholar]
- 32.Powers, J. C., and Z. H. Bengali. 1986. Elastase inhibitors for treatment of emphysema. Approaches to synthesis and biological evaluation. Am. Rev. Respir. Dis. 134:1097-1100. [DOI] [PubMed] [Google Scholar]
- 33.Pshezhetsky, A. V., and M. Ashmarina. 2001. Lysosomal multienzyme complex: biochemistry, genetics, and molecular pathophysiology. Prog. Nucleic Acid Res. Mol. Biol. 69:81-114. [DOI] [PubMed] [Google Scholar]
- 34.Pshezhetsky, A. V., M. A. Elsliger, M. V. Vinogradova, and M. Potier. 1995. Human lysosomal beta-galactosidase-cathepsin A complex: definition of the beta-galactosidase-binding interface on cathepsin A. Biochemistry 34:2431-2440. [DOI] [PubMed] [Google Scholar]
- 35.Pshezhetsky, A. V., M. V. Vinogradova, M. A. Elsliger, F. el-Zein, V. K. Svedas, and M. Potier. 1995. Continuous spectrophotometric assay of human lysosomal cathepsin A/protective protein in normal and galactosialidosis cells. Anal. Biochem. 230:303-307. [DOI] [PubMed] [Google Scholar]
- 36.Ray, A., J. Vela, R. Mackman, L. Zhang, H. Hui, R. Pakdaman, A. Carey, M. Wright, G. Rhodes, and T. Cihlar. 2006. Amidate prodrugs of a nucleotide analog GS9148 enhances the in vivo intracellular delivery of the active disphosphate metabolite: potential for clinical efficacy, abstr. 498. 16th Conference on Retroviruses and Opportunistic Infections, Denver, CO.
- 37.Remington, S. J. 1993. Serine carboxypeptidases: a new and versatile family of enzymes. Curr. Opin. Biotechnol. 4:462-468. [DOI] [PubMed] [Google Scholar]
- 38.Remington, S. J., and K. Breddam. 1994. Carboxypeptidases C and D. Methods Enzymol. 244:231-248. [DOI] [PubMed] [Google Scholar]
- 39.Robbins, B. L., J. Greenhaw, M. C. Connelly, and A. Fridland. 1995. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob. Agents Chemother. 39:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudenko, G. 1996. Structure determination of the human protective protein: twofold averaging reveals the three-dimensional structure of a domain which was entirely absent in the initial model. Acta Crystallogr. Sect. D 52:923-936. [DOI] [PubMed] [Google Scholar]
- 41.Rudenko, G., E. Bonten, A. d'Azzo, and W. G. Hol. 1995. Three-dimensional structure of the human ‘protective protein’: structure of the precursor form suggests a complex activation mechanism. Structure 3:1249-1259. [DOI] [PubMed] [Google Scholar]
- 42.Saboori, A. M., and D. S. Newcombe. 1990. Human monocyte carboxylesterase. Purification and kinetics. J. Biol. Chem. 265:19792-19799. [PubMed] [Google Scholar]
- 43.Saboulard, D., L. Naesens, D. Cahard, A. Salgado, R. Pathirana, S. Velazquez, C. McGuigan, E. De Clercq, and J. Balzarini. 1999. Characterization of the activation pathway of phosphoramidate triester prodrugs of stavudine and zidovudine. Mol. Pharmacol. 56:693-704. [PubMed] [Google Scholar]
- 44.Satake, A., K. Itoh, M. Shimmoto, T. C. Saido, H. Sakuraba, and Y. Suzuki. 1994. Distribution of lysosomal protective protein in human tissues. Biochem. Biophys. Res. Commun. 205:38-43. [DOI] [PubMed] [Google Scholar]
- 45.Satoh, T., and M. Hosokawa. 1998. The mammalian carboxylesterases: from molecules to functions. Annu. Rev. Pharmacol. Toxicol. 38:257-288. [DOI] [PubMed] [Google Scholar]
- 46.Shimmoto, M., Y. Fukuhara, K. Itoh, A. Oshima, H. Sakuraba, and Y. Suzuki. 1993. Protective protein gene mutations in galactosialidosis. J. Clin. Investig. 91:2393-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui, A. Q., C. McGuigan, C. Ballatore, F. Zuccotto, I. H. Gilbert, E. De Clercq, and J. Balzarini. 1999. Design and synthesis of lipophilic phosphoramidate d4T-MP prodrugs expressing high potency against HIV in cell culture: structural determinants for in vitro activity and QSAR. J. Med. Chem. 42:4122-4128. [DOI] [PubMed] [Google Scholar]
- 48.Sohma, O., M. Mizuguchi, S. Takashima, A. Satake, K. Itoh, H. Sakuraba, Y. Suzuki, and K. Oyanagi. 1999. Expression of protective protein in human tissue. Pediatr. Neurol. 20:210-214. [DOI] [PubMed] [Google Scholar]
- 49.Suo, Z., and K. A. Johnson. 1998. Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-phosphonylmethoxypropyl)adenine. J. Biol. Chem. 273:27250-27258. [DOI] [PubMed] [Google Scholar]
- 50.Tranchemontagne, J., L. Michaud, and M. Potier. 1990. Deficient lysosomal carboxypeptidase activity in galactosialidosis. Biochem. Biophys. Res. Commun. 168:22-29. [DOI] [PubMed] [Google Scholar]
- 51.Valette, G., A. Pompon, J. L. Girardet, L. Cappellacci, P. Franchetti, M. Grifantini, P. La Colla, A. G. Loi, C. Perigaud, G. Gosselin, and J. L. Imbach. 1996. Decomposition pathways and in vitro HIV inhibitory effects of isoddA pronucleotides: toward a rational approach for intracellular delivery of nucleoside 5′-monophosphates. J. Med. Chem. 39:1981-1990. [DOI] [PubMed] [Google Scholar]
- 52.Verheijen, F. W., S. Palmeri, A. T. Hoogeveen, and H. Galjaard. 1985. Human placental neuraminidase. Activation, stabilization and association with beta-galactosidase and its protective protein. Eur. J. Biochem. 149:315-321. [DOI] [PubMed] [Google Scholar]