Abstract
Encephalitozoon cuniculi, a spore-forming obligate intracellular parasitic pathogen belonging to the phylum Microsporidia, has a unique and highly specialized organelle called the polar tube. Using an enzyme immunostaining assay in which germinated E. cuniculi spores were coated onto plastic surfaces, we tested healthy and human immunodeficiency virus (HIV)-infected individuals in Japan for anti-polar tube antibodies of each immunoglobulin (Ig) class. Anti-polar tube IgG was detected in just 4 of 380 healthy individuals; no anti-polar tube IgA was detected in any individuals; however, unexpectedly, anti-polar tube IgM antibodies were detected in 138 individuals (36%). When the healthy individuals were grouped by age, the highest rate of positivity to anti-polar tube IgM antibodies was seen in individuals aged 20 years old or younger. Fifty-nine percent (24/41) of the individuals aged 20 years or younger were anti-polar tube IgM antibody positive. This rate tended to decrease among individuals in older age groups. However, no anti-polar tube IgM antibodies were detected in 21 HIV-infected persons who were younger than 30 years of age and who had CD4 cell levels below 250/μl. These seroepidemiological results clearly indicate that circulating anti-polar tube IgM antibodies that are capable of strongly reacting with filaments extruded from geminated spores exist and suggest that such antibodies may play a part in protective immunity.
Encephalitozoon cuniculi is a microsporidian parasitic pathogen listed in a 1996 WHO report as an emerging infectious agent (17). The pathogen is also considered a zoonotic parasite (4). Various animals can be naturally infected by E. cuniculi, and its geographical distribution is worldwide (3). In Japan, E. cuniculi infection in rabbits and in squirrel monkeys in zoos is of current concern (1, 5). The rate of infection is considered to increase each year, and the infection has now spread throughout Japan. However, to the best of our knowledge, the only case of human microsporidiosis reported in Japan was in a 9-year-old boy in 1958 (10). Although immunological conditions of the Japanese case were not recorded, almost all other patients infected with this pathogen in other nations have been immunocompromised groups of human immunodeficiency virus (HIV)-infected patients (16). A few cases have also been found among renal transplant recipients (6, 11). E. cuniculi can thus be regarded as an opportunistic pathogen (2). Cases of HIV-associated infections with E. cuniculi are increasingly reported, although they remain less common than those due to Encephalitozoon bieneusi and Encephalitozoon intestinalis (2). Many reports on the seroprevalence of human E. cuniculi infection have been published (3, 8). However, the reported rates of microsporidial seropositivity vary greatly, depending on the serological technique used, probably due to the use of antigens unsuitable for measurement of specific antibodies and the use of secondary antibodies without differential specificities.
Recently, specific immunoglobulin G (IgG) antibodies against the polar tube (PT) of E. cuniculi were demonstrated in a healthy laboratory worker accidentally infected with E. cuniculi (14). The PT is a typical microsporidian spore structure with an extrusion that is essential for invasion of a host cell, as sporoplasm flows through the discharged PT and into the host cell (13).
We have recently developed an enzyme immunostaining assay (EIA) for measuring anti-E. cuniculi PT antibodies using 96-well microplates coated with germinated spores. This method allows us to screen human sera for anti-PT antibodies on a large scale for seroepidemiological analysis. This study reports on the screening of sera from 380 healthy persons and 78 HIV-infected persons seroepidemiologically analyzed by this particular EIA, which is capable of measuring anti-PT antibodies of each Ig class, that is, IgM, IgG, and IgA.
MATERIALS AND METHODS
Serum samples.
For this study we used serum samples from 380 healthy people living in Hokkaido Prefecture, Japan; serum samples from 180 residents who underwent a serological test for parasitosis in 2000 but who showed negative results; and serum samples from 200 blood donors collected in 2005.
Serum samples from 78 HIV-infected persons, collected in 1999 from the Kanto region of Japan, were also provided for this study. These included sera from 51 persons with CD4 cell levels below 250/μl and sera from 27 persons with CD4 cell levels between 251 and 900/μl. The 51 persons in the former group were of various ages, while the 27 persons in the latter group were younger than 30 years of age. Tests for HIV infection and determination of CD4 lymphocyte counts were performed by standard laboratory protocols.
E. cuniculi spores.
For this study we used the E. cuniculi HF strain, isolated from a rabbit with encephalitozoonosis. Strain HF was then cultivated in RK-13 cells (ATCC CCL-37) (5). Culture supernatants of HF-infected RK-13 cells were collected, centrifuged, and used for serological tests.
Strain HF was genetically analyzed beforehand by PCR, followed by direct DNA sequencing (1). The internal transcribed spacer gene sequence revealed that strain HF was classified into genotype I, since it contained three GTTT repeats. Sequence analysis of the spore wall protein I gene revealed that the strain belonged to genotype Ia because of the amplification of a 399-bp PCR product.
Microplate enzyme immunostaining assay.
Sediments containing germinated spores, nongerminated spores, and heavily infected cells detached from cell sheets, were suspended in Gibco minimal essential medium including Earle's salts and glutamine (Invitrogen Corporation, Grand Island, NY) and supplemented with 1,000 U/ml penicillin G, 1,000 μg/ml streptomycin, and 10% fetal bovine serum; this medium was also used for cultures of RK-13 and BS-C-1 (ATCC CCL-26) cells, as described below. Approximately 4 × 106 free spores (containing detached cells) were inoculated into each well of a 96-well flat bottom microplate (high-binding polystyrene; Corning Incorporated, NY) and cultured for 3 days at 35°C in an incubator with 5% CO2. Subsequently, the wells were washed once with phosphate-buffered saline (PBS; pH 7.2) and fixed with 10% formalin in PBS for 1 h at room temperature (RT). The wells were then washed twice with PBS, treated with PBS containing 1% Tween 20 for 1 h at RT, and washed twice with PBS. The wells were further treated with blocking buffer (SuperBlock; Pierce, Rockford, IL) for 1 h at RT and were finally washed twice with PBS. The plates with wells coated with more than 100 germinated spores per well were then studied.
Twofold dilutions of each serum sample were made by using PBS containing 0.05% Tween 20 (PBS-T), starting from a 1:50 dilution; 100 μl of each of the dilutions was added to each coated well. The wells were incubated for 90 min at RT and then washed five times with 200 μl PBS-T. Subsequently, the wells were incubated with 100 μl of the secondary antibody or protein A/G, incubated for 60 min at RT, and washed five times with PBS-T. A 1:3,000 dilution of protein A/G labeled with peroxidase (PO) (Prozyme Inc., San Leardro, CA), a 1:5,000 dilution of anti-human IgM (μ-chain specific) labeled with PO (QED Bioscience Inc., San Diego, CA), and a 1:3,000 dilution of anti-human IgA (Fc specific) labeled with PO (Nordic Immunology, The Netherlands) were used to capture IgG, IgM, and IgA antibodies, respectively. Finally, the signals of PO bound to human Ig antibodies were visualized by using the substrate aminoethyl carbazole (Zymed Laboratories Inc., San Francisco, CA), according to the manufacturer's instructions. After the wells were washed with pure water, the results were observed with a light microscope. In this assay, only judgments on the serological reactions to filaments extruded from germinated spores (i.e., PTs) were made, while the reactions to the spore walls and the host cells were recorded as reference data.
The concentrations of each secondary antibody, noted above, were determined beforehand by using a dot immunoassay and the corresponding purified Ig. Sera from rabbits with encephalitozoonosis were used as positive controls (5). PO-labeled protein A/G was used for detection of rabbit IgG antibodies as the secondary antibody.
BS-C-1 cells were infected with E. intestinalis (ATCC 5057) and E. hellem (ATCC 50451) spores. The resultant germinated spores were examined by the procedures mentioned above.
Ethical considerations.
The protocol for this study was approved by the Committee for Research on Human Subjects of the National Institute of Infectious Diseases, Tokyo, Japan. Written informed consent was obtained from the HIV-positive subjects. The use of healthy residents' sera and the use of blood donors' sera were approved by the Institutional Review Board of the Hokkaido Institute of Public Health and the Institutional Review Board of the Hokkaido Red Cross Blood Center, respectively. All serum samples included in this study were processed to protect personal information. For all serum samples, the only specific clinical information available was the sex, age, and health condition.
Statistical analysis.
The sera of the healthy residents and blood donors were each divided into six groups according to age. The relationship between anti-PT IgM prevalence in each age group and the year of blood collection (2000 for healthy residents and 2005 for blood donors) was analyzed by the Mantel-Haenszel method. The relationship between increasing age and decreasing rate of positivity for anti-PT IgM antibodies in healthy subjects (healthy residents plus blood donors) was analyzed by the Cochran-Armitage test. The statistical significance of the presence of anti-PT IgM antibodies by gender was determined by the chi-square test. P values of <0.01 were considered statistically significant. Excel Statistics 2006 software for Windows (release 6.7.1; Social Survey Research Information Co. Ltd., Tokyo, Japan) was used for statistical analysis.
RESULTS
Table 1 summarizes the results of the EIA for the detection of anti-E. cuniculi PT antibodies in samples from healthy residents, blood donors, and HIV-infected persons. Anti-PT IgG antibodies were detected in only 3 of 180 serum samples from healthy individuals; the titers were 1:50, which was significantly lower than those for the controls (naturally infected rabbit sera), which showed titers of 1:6,400 to 1:102,400. When the 200 donor serum samples were examined by EIA, anti-E. cuniculi PT IgG antibodies were detected in only 1 serum sample; the titer was 1:1,600, but the positive signals were very weak even at the lower dilutions.
TABLE 1.
Results of serological detection of anti-E. cuniculi PT antibodies in healthy residents, blood donors, and HIV-infected persons
| Subject group and antibody | No. (%a) of individuals with antibody titer of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <1/50 | 1/50 | 1/100 | 1/200 | 1/400 | 1/800 | 1/1,600 | Total | |
| Healthy residents | ||||||||
| IgM | 114 (63.3) | 26 (14.4) | 16 (8.9) | 16 (8.9) | 5 (2.8) | 3 (1.7) | 0 | 180 |
| IgG | 177 | 3 | 0 | 0 | 0 | 0 | 0 | 180 |
| IgA | 180 | 0 | 0 | 0 | 0 | 0 | 0 | 180 |
| Blood donors | ||||||||
| IgM | 128 (64) | 29 (14.5) | 27 (13.5) | 11 (5.5) | 2 (1.0) | 2 (1.0) | 1 (0.5) | 200 |
| IgG | 199 | 0 | 0 | 0 | 0 | 0 | 1 | 200 |
| IgA | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 200 |
| HIV-infected persons with CD4 cell counts below 250/μl | ||||||||
| IgM | 47 | 2 | 1 | 1 | 0 | 0 | 0 | 51 |
| IgG | 49 | 0 | 1 | 1 | 0 | 0 | 0 | 51 |
| IgA | 51 | 0 | 0 | 0 | 0 | 0 | 0 | 51 |
Rates of positivity for anti-PT IgM antibodies.
No anti-PT IgA antibody was detected in any of the 380 serum samples with titers below 1:50. Furthermore, 2 of the 51 HIV-infected persons with CD4 cell levels below 250/μl had anti-PT IgG antibodies, and anti-PT IgA antibodies were not detected in any of the 51 HIV-infected persons.
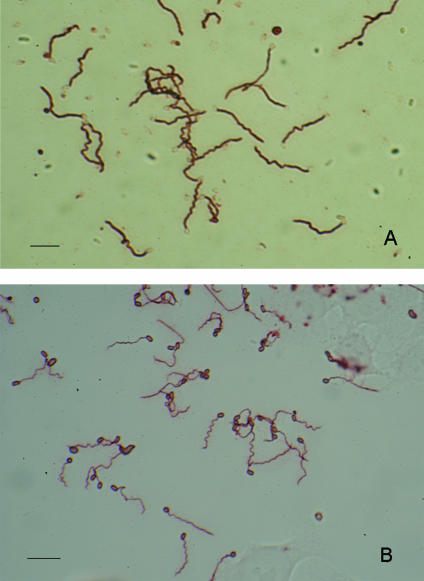
On the other hand, when the same serum samples described above were examined for the presence or absence of anti-PT IgM antibodies, the results were quite different from those for anti-PT IgG and IgA antibodies. Anti-PT IgM antibodies were detected in 66 (36.7%) of the 180 serum samples from healthy persons, which showed titers of 1:50 to 1:800; and in 72 (36%) of the 200 serum samples from the blood donors, which showed titers of 1:50 to 1:1,600. The reactivities of the IgM antibodies with filaments were considered to be typical of IgM, because of the low titers (below 1:1,600), but the IgM antibodies had stronger reactivities than the rabbit anti-E. cuniculi PT IgG antibodies (Fig. 1A and B). The four serum samples from healthy persons and donors with anti-PT IgG activities also had anti-PT IgM activities.
FIG. 1.
Immunostaining of filaments (PTs) extruded from E. cuniculi-geminated spores with human IgM antibodies or rabbit IgG antibodies. (A) Positive results obtained from the serum sample from donor 197 diluted 1:200. Note the strongly positive signals on the filaments extruded from the germinated spores but the unstained spore walls. (B) Positive results obtained from a serum sample from a symptomatic rabbit with natural E cuniculi infection diluted 1:400. Note the positive signals on the spore wall and the filament. Bars, 10 μm.
By using almost the same procedures used for the EIA used for detection of anti-E. cuniculi PT IgM antibodies, sera with titers of more than 1:200 were examined for cross-reactions with PTs of E. hellem and E. intestinalis; however, no antibody activity against the filaments was detected (data not shown).
A decreasing trend in positivity rates for anti-PT IgM antibodies was observed when subjects (healthy residents plus blood donors) were grouped according to age. As seen from Table 2, the rate of positivity for anti-PT IgM antibodies was the highest among people aged 19 years or younger: 59% of healthy subjects. The seropositivity rates clearly tended to decrease among the older subjects (P < 0.01). The rates of positivity for anti-PT IgM antibodies in each age group were irrelevant to the year that the serum samples were collected, i.e., in 2000 (healthy residents) and 2005 (blood donors). The rate of positivity for anti-PT IgM antibodies among females was a little higher than that among males: 43.8% (39/89) for females and 29.7% (27/91) for males. A total of 41.1% (39/95) of the female donors and 31.4% (33/105) of the male donors showed anti-PT IgM antibody titers of 1:50 or more.
TABLE 2.
Age distribution of cases with anti-E. cuniculi PT IgM antibodies of healthy and HIV-infected persons
| Subject group | Value for age group
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 yr or younger
|
20-29 yr
|
30-39 yr
|
40-49 yr
|
50-59 yr
|
60 yr or older
|
|||||||
| No. of individuals tested | No. (%) positive | No. of individuals tested | No. (%) positive | No. of individuals tested | No. (%) positive | No. of individuals tested | No. (%) positive | No. of individuals tested | No. (%) positive | No. of individuals tested | No. (%) positive | |
| Healthy residentsa | 31 | 18 (58.1) | 29 | 13 (44.8) | 26 | 12 (46.2) | 31 | 4 (12.9) | 32 | 10 (31.3) | 31 | 9 (29.0) |
| Blood donorsa | 10 | 6 (60.0) | 46 | 19 (41.3) | 54 | 19 (35.2) | 33 | 7 (21.2) | 44 | 18 (40.9) | 13 | 3 (23.1) |
| Healthy residents and blood donorsb | 41 | 24 (58.5) | 75 | 32 (42.7) | 80 | 31 (38.8) | 64 | 11 (17.2) | 76 | 28 (36.8) | 44 | 12 (27.3) |
| HIV-infected persons with CD4 cell counts below 250/μl | 4 | 0 (0) | 17 | 0 (0) | 16 | 2 (12.5) | 10 | 1 (10.0) | 2 | 1 (50.0) | 2 | 0 (0) |
Serum samples were collected in 2000 from healthy residents and in 2005 from blood donors. The relationship between anti-PT IgM prevalence in each age group and the year of blood collection was analyzed by the Mantel-Haenszel method, which did not give statistically significantly different values.
An association between increasing age and a decreasing rate of positivity for anti-PT IgM was analyzed by the Cochran-Armitage test, which gave statistically significant P values of <0.01.
Anti-E. cuniculi PT IgM antibodies were detected in only 4 of 51 samples from HIV-positive individuals with CD4 cell levels below 250/μl (Table 1). In particular, anti-PT IgM antibodies were not detected in persons younger than age 30 years and with <250 CD4 cells/μl (Table 2). Interestingly, a high rate of positivity for anti-PT IgM antibodies was observed among the 27 HIV-positive persons with CD4 cell counts between 251 and 900/μl; i.e., 25% (3/12) of people with CD4 cell counts between 251 and 399/μl and in 60% (9/15) of people with CD4 cell counts between 400 and 900/μl ( Table 3); furthermore, all these individuals were younger than 30 years of age.
TABLE 3.
Relationship between number of CD4 cells and rate of positivity for anti-E. cuniculi PT IgM antibodies among HIV-infected persons younger than 30 years of age
| No. of CD4 cells/μl | No. of persons examined | No. (%) of persons with anti-E. cuniculi PT IgM antibodies |
|---|---|---|
| <250 | 21 | 0 (0) |
| 251-399 | 12 | 3 (25) |
| 400-900 | 15 | 9 (60) |
DISCUSSION
Our present results indicate that anti-E. cuniculi PT antibodies could be detected in 36% of the people, healthy residents and blood donors, who should be considered immunocompetent. In respect to antibodies against Encephalitozoon PT among immunocompetent persons, it has been reported that anti-E. intestinalis PT was demonstrated in 8% of Dutch blood donors and 5% of pregnant French women (15). Our anti-E. cuniculi PT antibodies were detected by EIA, while the anti-E. intestinalis PT antibodies were detected by an indirect fluorescent-antibody test (15). The sensitivities of enzyme immunoassays are generally believed to be fairly higher than those of immunofluorescence assays.
No cross-reactive relationship between human anti-E. cuniculi PT and human anti-E. intestinalis PT has been proved. We clearly showed that our human sera containing anti-PT IgM antibodies did not cross-react with filaments extruded from germinated spores of E. intestinalis and E. hellem. Our finding is in agreement with previous ones that human sera containing anti-E. intestinalis PT antibody activity did not immunostain the filaments extruded from E. cuniculi (13).
Anti-E. cuniculi PT antibodies, unlike the immunoglobulin class of anti-E. intestinalis PT antibodies, which was shown to be IgG (13, 15), were not of the IgG class but were of the IgM class. Anti-E. cuniculi PT IgG antibodies were detected in the sera of just four persons (one had a titer of 1:1,600, and the others showed low titers of 1:50) (Table 1). Their reactivities probably resulted from anti-E. cuniculi PT IgM antibodies. In our experiments, we used protein A/G instead of anti-human IgG to capture IgG antibodies. It is known that protein A strongly binds to IgG molecules but also weakly binds to some IgM molecules (9). In fact, the four serum samples all exhibited elevated titers of anti-PT IgM antibodies, but their ability to stain extruded filaments was not so strong.
There was no significant difference in the rate of positivity for anti-PT IgM antibodies by gender, although the rate of positivity for anti-PT IgM antibodies was slightly higher among females than among males. However, some relationship between the prevalence of anti-PT IgM antibodies and age would be expected. The rate of positivity for anti-PT IgM antibodies was significantly higher among people <20 years of age than among people in older groups (Table 2). However, this activity for anti-PT IgM antibodies was not found among the 21 HIV-positive persons younger than 30 years of age (Table 2), all of whom had CD4 lymphocyte levels below 250/μl, indicating that they were severely immunocompromised. However, when CD4 counts were between 400 and 900 cells/μl, anti-PT IgM antibodies were detectable in 60% (9/15) of HIV-positive persons younger than 30 years of age (Table 3). Thus, we were surprised to find that increasing age and decreasing numbers of CD4 lymphocytes, factors that can induce immunosuppression, influence the production of anti-PT IgM antibodies.
Anti-PT IgG antibodies were not detectable in most of our subjects, as noted above. The only human case demonstrating elevated anti-PT IgG antibodies involved an accidental E. cuniculi infection (14). In respect to the specific immune responses to the E. cuniculi infection, anti-spore wall IgG was observed to precede anti-PT IgG (14). Most of our subjects were negative for anti-spore wall IgM and IgG (data not shown). Additionally, the rate of positivity for anti-PT IgM antibodies for the serum samples collected from blood donors in 2005 was almost the same as that for the serum samples collected from healthy residents in 2000 (Table 1). These findings suggest that most of our anti-PT IgM antibodies do not belong to the class of early IgM antibodies found after infection by E. cuniculi. Although anti-PT IgM reacted only with the E. cuniculi PTs of the Encephalitozoon sp. tested, as described above, further research concerning the specificities and immunoreactivities of human anti-E. cuniculi PT IgM antibodies needs to be undertaken.
E. cuniculi infection in immunocompromised patients results in disseminated disease that is clinically manifested in symptoms such as keratoconjunctivitis, hepatitis, and peritonitis (16). However, no symptomatic cases of infection with E. cuniculi among immunocompetent persons have been described (16), apart from the accidentally infected French individual, who had severe keratoconjunctivitis (14). A Japanese child with encephalitozoonosis due to E. cuniculi infection in 1958 is considered the only case due to natural infection, but unfortunately, the patient's immune status was not recorded (10). A few cases of E. cuniculi infection have also been reported in transplant patients (6, 11). Thus, apart from some extremely rare situations, it is most unlikely that E. cuniculi causes microsporidiosis in immunocompetent persons (12, 16). Considering that almost all human encephalitozoonosis cases occurred in immunocompromised patients infected with HIV (16), one can speculate that protective immunity plays a very important role against E. cuniculi infection. In experimental models, the protective immune response against E. cuniculi is noted to be mediated by cytotoxic CD8+ T cells (7). Also, the in vitro infectivity of microsporidia has been observed to be reduced by treatment with monoclonal and polyclonal antibodies to the polar tube protein (7), suggesting that anti-PT antibody may constitute a first line of defense against infection by E. cuniculi. Our study clearly indicates that there are circulating IgM antibodies that are capable of strongly reacting with the filaments that extrude from germinated E. cuniculi spores. We believe that this is the first study to provide seroepidemiological data on human anti-PT IgM antibodies. Further studies focused on human anti-E. cuniculi PT IgM antibodies need to be performed from the parallel perspectives of protective immunity and preventive medicine.
Acknowledgments
We appreciate the assistance with serological testing and cell culture given by Tokiko Asakura.
This work was supported by grant-in-aid no. 16390177 from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Asakura, T., S. Nakamura, M. Ohta, Y. Une, and K. Furuya. 2006. Genetically unique microsporidian Encephalitozoon cuniculi strain type III isolated from squirrel monkeys. Parasitol. Int. 55:159-162. [DOI] [PubMed] [Google Scholar]
- 2.Bryan, R. T. 1995. Microsporidiosis as an AIDS-related opportunistic infection. Clin. Infect. Dis. 21(Suppl. 1):S62-S65. [DOI] [PubMed] [Google Scholar]
- 3.Canning, E. U., and W. S. Hollister. 1987. Microsporidia of mammals—widespread pathogens or opportunistic curiosities? Parasitol. Today 3:267-273. [DOI] [PubMed] [Google Scholar]
- 4.Deplazes, P., A. Mathis, R. Baumgartner, I. Tanner, and R. Weber. 1996. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin. Infect. Dis. 22:557-559. [DOI] [PubMed] [Google Scholar]
- 5.Furuya, K., D. Fukui, M. Yamaguchi, Y. Nakaoka, G. Bando, and M. Kosuge. 2001. Isolation of Encephalitozoon cuniculi using primary tissue culture techniques from a rabbit in a colony showing encephalitozoonosis. J. Vet. Med. Sci. 63:203-206. [DOI] [PubMed] [Google Scholar]
- 6.Gamboa-Dominguez, A., J. De Anda, J. Donis, F. Ruiz-Maza, G. S. Visvesvara, and H. Diliz. 2003. Disseminated Encephalitozoon cuniculi infection in a Mexican kidney transplant recipient. Transplantation 75:1898-1900. [DOI] [PubMed] [Google Scholar]
- 7.Khan, I. A., M. Moretto, and L. M. Weiss. 2001. Immune response to Encephalitozoon cuniculi infection. Microbes Infect. 3:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucerova-Pospisilova, Z., and O. Ditrich. 1998. The serological surveillance of several groups of patients using antigens of Encephalitozoon hellem and E. cuniculi antibodies to microsporidia in patients. Folia Parasitol. (Praha) 45:108-112. [PubMed] [Google Scholar]
- 9.Langone, J. J. 1982. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv. Immunol. 32:157-252. [PubMed] [Google Scholar]
- 10.Matsubayashi, H., T. Koike, I. Mikata, H. Takei, and S. Hagiwara. 1959. A case of Encephalitozoon-like body infection in man. Arch. Pathol. 67:181-187. [PubMed] [Google Scholar]
- 11.Mohindra, A. R., M. W. Lee, G. Visvesvara, H. Moura, R. Parasuraman, G. J. Leitch, L. Xiao, J. Yee, and R. del Busto. 2002. Disseminated microsporidiosis in a renal transplant recipient. Transplant. Infect. Dis. 4:102-107. [DOI] [PubMed] [Google Scholar]
- 12.Orenstein, J. M., H. P. Gaetz, A. T. Yachnis, S. S. Frankel, R. B. Mertens, and E. S. Didier. 1997. Disseminated microsporidiosis in AIDS: are any organs spared? AIDS 11:385-386. [PubMed] [Google Scholar]
- 13.Peek, R., F. Delbac, D. Speijer, V. Polonais, S. Greve, E. Wentink-Bonnema, J. Ringrose, and T. van Gool. 2005. Carbohydrate moieties of microsporidian polar tube proteins are targeted by immunoglobulin G in immunocompetent individuals. Infect. Immun. 73:7906-7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gool, T., C. Biderre, F. Delbac, E. Wentink-Bonnema, R. Peek, and C. P. Vivares. 2004. Serodiagnostic studies in an immunocompetent individual infected with Encephalitozoon cuniculi. J. Infect. Dis. 189:2243-2249. [DOI] [PubMed] [Google Scholar]
- 15.van Gool, T., J. C. M. Vetter, B. Weinmayr, A. Van Dam, F. Derouin, and J. Dankert. 1997. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J. Infect. Dis. 175:1020-1024. [DOI] [PubMed] [Google Scholar]
- 16.Weber, R., D. A. Schwartz, and P. Deplazes. 1999. Laboratory diagnosis of microsporidiosis, p. 315-362. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, DC.
- 17.World Health Organization. 1996. Fighting disease fostering development, p. 112. In The world health report. World Health Organization, Geneva, Switzerland.