Abstract
In this study, we assessed the effect of administering the antibiotic amoxicillin to rat pups on the immune response to orally fed ovalbumin (OVA). We first established that amoxicillin administration durably altered the gut microbiota of these animals. In parallel, we observed that the induction of the specific humoral response to ovalbumin was impaired when it occurred during antibiotic administration to the rat pups. We also examined the consequences of those observations on further allergic reactions. Amoxicillin administration had no significant impact on subsequent sensitization to OVA, as nonexacerbated systemic allergic responses were induced in antibiotic-treated animals. However, increased rat mast cell protease II levels and higher mast cell numbers were detected in their small intestines, independently of the antigen administration. Globally, our data suggest that antibiotic administration early in life negatively affects the specific immune response to a luminal antigen when it is first introduced during antibiotic administration. The increased mast cell numbers and mediator concentrations in the intestinal mucosae of the antibiotic-treated animals may testify to the early stages of an altered immune system homeostasis.
The intestinal mucosa is particularly exposed to a large variety of microorganisms as well as to protein antigens from foods. The gut-associated lymphoid tissue (GALT) is adapted to this particular environment. It has an important dual role in the maintenance of the intestinal homeostasis, on the one hand providing the host with protective mechanisms against invasion by potential pathogens and on the other hand developing suppressive and/or downregulatory mechanisms against antigens from the commensal microbiota and from the diet. The latter process, which is referred to as “oral tolerance” (OT), prevents the induction of potentially damaging active immunity against innocuous antigens. Both specific humoral immune responses (immunoglobulin G [IgG] and IgE antibody responses) and cellular immune responses are suppressed (for a review, see reference 41), with the IgE response being the most sensitive to suppression (34). This immune unresponsiveness occurs after a first interaction of GALT with either a single high dose or several low doses of the antigen (OT induction) and is maintained long thereafter (OT maintenance). An alteration of OT disrupts the mucosal homeostasis and leads to food hypersensitivity (8), mucosal inflammation (10), or allergic diseases (41).
At birth, GALT is poorly developed but is rapidly confronted with an array of microbial antigens flowing from the bacteria colonizing the gut. Studies with germfree animals have shown that the intestinal microbiota has profound effects on both the development and the function of the intestinal mucosal immune system (6, 29).
Epidemiological data suggest that changes in the composition of the gut microbiota may explain the increasing prevalence of allergic conditions in Western and westernized societies. On this basis, the hygiene hypothesis that was formulated several years ago suggests that atopic diseases may arise from reduced microbial exposure early in life (24, 49). Newborns generally have Th2-skewed patterns of immunity, and early exposure to a certain pattern of bacterial colonization or exposure of the neonatal intestine (39) may be essential to induce regulatory cells which control the immune response and induce tolerance to foreign antigens (49). Consistent with this hypothesis, studies have shown differences in the composition of the gut microbiota between allergic and nonallergic infants (4) and infants who will or who will not develop allergy (18). Furthermore, early supplementation with probiotics has been shown to reduce the risk of later atopic manifestations (19).
The administration of broad-spectrum antibiotics, frequent in pediatric clinical practices, impairs intestinal bacterial colonization during infancy (3). In young mice, antibiotic administration was shown to increase total IgG1 and IgE plasma levels and interleukin-4 secretion after spleen cell stimulation, while it was found to decrease gamma interferon levels (30). Also in mice, Noverr et al. showed that antibiotic treatment combined with fungal microbiota colonization led to increased sensitization to an airway antigen and to pulmonary allergic responses (27). In line with this finding, a number of epidemiological studies have examined the association between antibiotic administration and the risk of allergy and asthma; but the results are still controversial (7, 9, 17, 48).
While some studies show that the absence of gut microbiota has no effect on the induction of OT (12), other studies show the contrary finding and indicate that either the induction of OT (47) or the maintenance of OT (25, 26) is impaired in germfree animals and that colonization with commensal bacteria restores the maintenance of tolerance (23). Interestingly, Sudo et al. showed that OT induction, which was abrogated in germfree mice, was reestablished after intestinal colonization by bifidobacteria only when OT induction occurred during the neonatal period (42).
Altogether, increasing evidence supports the hypothesis that the postnatal development and homeostasis of the intestinal immune system depend on the establishment of a rich and complex commensal microbiota. In this particular context, we aimed to study the effect of antibiotic administration in early life on the immune response to food antigens.
We demonstrate that specific humoral responses were impaired in suckling rats during antibiotic administration. In addition, mast cell mediators and mast cell numbers were significantly increased in the gut mucosa of the antibiotic-treated animals, suggesting a predisposition to inflammatory status.
MATERIALS AND METHODS
Animals.
Sprague-Dawley rats were obtained from Charles River (Lyon, France) and were acclimatized to our animal facility for 2 weeks before they were mated. All the pups were born and housed under conventional conditions. After the pups were weaned, they were fed a nutritionally adapted semisynthetic diet (AIN 93 G) that was free of ovalbumin (OVA). All experiments were carried out in agreement with the Swiss Animal Protection Law and were approved by the district veterinary office (authorization no. 1683; Office Vétérinaire Cantonal, Lausanne, Switzerland).
Antibiotic administration: experimental design.
The pups were weighed and randomly assigned to one of the study groups at postnatal day 6 (PND6). Standardized litters of 6 to 10 pups were randomly assigned for fostering and received a daily dose of 100 mg/kg body weight of either a commercial form of amoxicillin (SmithKline Beecham Pharmaceuticals, Toledo, Spain) (amoxicillin-treated group) or the equivalent volume of saline (saline-treated group) by intragastric gavage from PND7 to PND18 (OT model) or PND25 (sensitization parenteral model).
Induction and maintenance of specific humoral responses.
Three different protocols were used to induce and monitor OT in rats previously treated or not treated with antibiotics. Outlines of the different protocols are presented in Fig. 2A, 3A, and 3C. To induce OT, rats were force-fed a single dose of OVA solution (grade V; 100 mg in 0.4 ml water; Sigma-Aldrich, St. Louis, MO) (tolerized animals) or with water (nontolerized animals) at PND15 (see Fig. 2A and 3A) or PND43 (see Fig. 3C). Then, the rats were challenged subcutaneously once (at PND21 [see Fig. 2A] and PND49 [see Fig. 3C]) or twice (at PND21 and PND41 [see Fig. 3A]) with OVA (100 μg) and keyhole lympet hemocyanin (KLH; 100 μg) in 2.4% in Al(OH)3 (Alhydrogel, 2%; Brenntag Biosector, Frederikssund, Denmark). The rats were killed 15 days after the last challenge by exhaustive bleeding after anesthesia with isoflurane (3.5%). Blood was collected in Li-heparin tubes (Milian SA) and centrifuged to obtain plasma for IgE and IgG analyses. The colon was removed and kept on ice. One fecal pellet was collected from the colon and kept on ice until bacterial analysis.
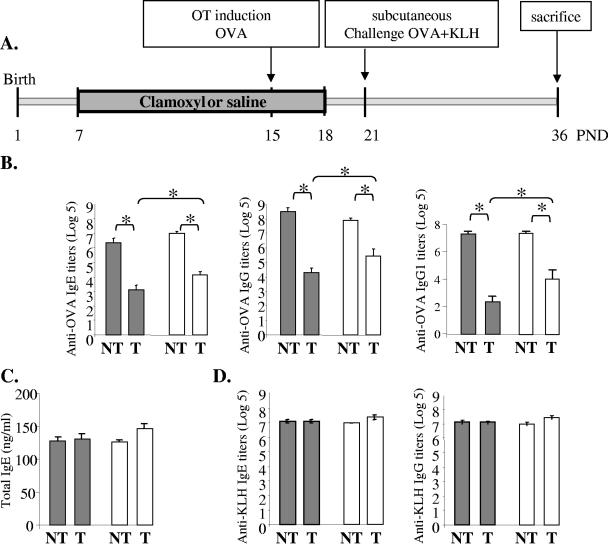
FIG. 2.
Effect of amoxicillin (Clamoxyl) on specific humoral responses induced during antibiotic treatment. (A) Experimental design of OT induction in amoxicillin-treated rats. Amoxicillin was administered to the pups from PND7 to PND18. OT was induced by intragastric administration of one dose of 100 mg OVA at PND15. Challenge was performed by subcutaneous injection of 100 μg OVA and 100 μg of KLH in Al(OH)3 at PND21. The animals were killed at PND36. (B) OVA-specific IgE, IgG, and IgG1 titers in sera of rats at PND36. (C) Total IgE levels (ng/ml) in sera of rats at PND36. (D) KLH-specific IgE and IgG titers in sera of rats at PND36. Rats were treated with saline (gray bars) or amoxicillin (white bars) and force-fed water (nonfed animals, nontolerized [NT]) or OVA (fed animals, tolerized [T]). Data are expressed as the mean log5 titers ± standard errors of the means (n = 8 to 18 rats/group from two independent experiments). Differences were considered significant when P was <0.05 (*).
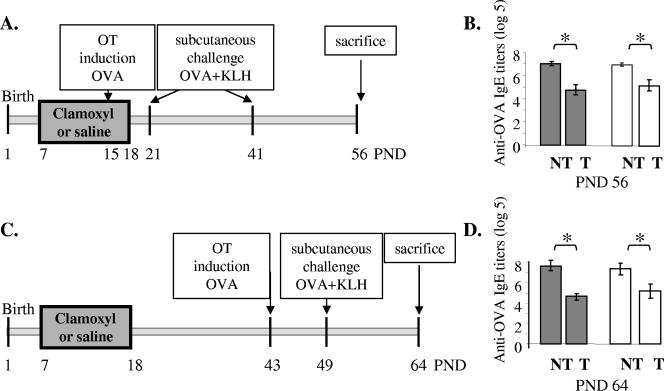
FIG. 3.
Effect of amoxicillin (Clamoxyl) on specific humoral immune responses induced after antibiotic treatment and on OT maintenance. (A and C) Experimental design of OT induction in amoxicillin-treated rats. Amoxicillin was administered to the pups from PND7 to PND18. OT was induced by the intragastric administration of one dose of 100 mg OVA at PND15 (A) or PND43 (C). One challenge (C) or two challenges (A) were performed by subcutaneous injection of 100 μg OVA and 100 μg of KLH in Al(OH)3 at PND21 and PND41 (A) or PND49 (C). The animals were killed at PND56 (A) or PND64 (C). (B and D) OVA-specific IgE titers in the sera of rats at PND56 (B) or PND64 (D). Rats were treated with saline (gray bars) or amoxicillin (white bars) and force-fed water (nontolerized [NT]animals) or OVA (tolerized [T]animals). Data are expressed as the mean log5 titers ± standard errors of the means (one experiment; n = 8 to 10 rats/group). Differences were considered significant when P was <0.05 (*).
Sensitization parenteral rat model.
The protocol of sensitization was similar to that for the rat parenteral model reported by Fritsché et al. (11) (see Fig. 4A). Briefly, at PND11, a group of pups, treated or not treated with amoxicillin from PND7 to PND25, was sensitized with 50 μg OVA (grade V; Sigma-Aldrich) in 2.4% Al(OH)3 (Alhydrogel, 2%; Brenntag Biosector) by subcutaneous injection. At PND25, animals received intragastrically a dose of OVA (100 mg) or saline. Two hours later, the animals were killed by exhaustive bleeding after anesthesia with isoflurane (3.5%). Blood was collected in Li-heparin tubes for analyses for IgE, IgG, and rat mast cell protease II (RMCPII). The small intestine and the colon were removed and kept on ice. The small intestine was divided into two parts of identical length, namely, proximal and distal parts. One-centimeter pieces of both segments were snap-frozen and kept at −80°C until analysis for RMCPII. Half-centimeter pieces of the proximal small intestine were kept in 10% formalin for further histological preparation. One fecal pellet was collected from the colon and kept on ice until bacterial analysis.
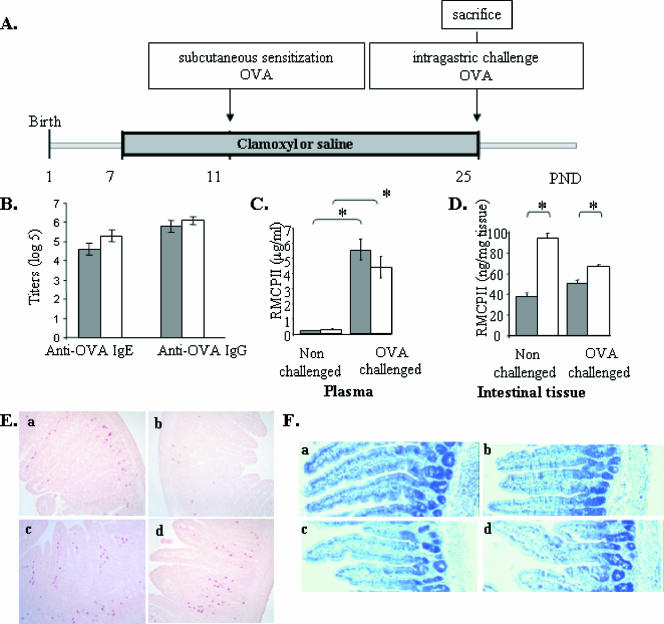
FIG. 4.
Effect of amoxicillin (Clamoxyl) on the severity of the allergic reaction. (A) Experimental design of sensitization in amoxicillin-treated rats. Antibiotics were administered to the pups from PND7 to PND25. Sensitization was performed by subcutaneous injection of 50 μg OVA in Al(OH)3 at PND11. Challenge was performed by intragastric gavage of 100 mg OVA at PND25. The animals were killed 2 h after the challenge. (B) IgE and IgG levels in amoxicillin- and saline-treated rats (log5 titers). (C and D) Plasma (C) and small intestinal (D) RMCPII levels in rats treated with saline (gray bars) or amoxicillin (white bars), sensitized with OVA, and force-fed saline solution (nonchallenged) or OVA (OVA challenged). (E and F) Light micrographs of the small intestine sampled 2 h after challenge and stained either with chloroacetate esterase activity for mast cell visualization (E) or with hematoxylin and eosin (F) (magnification, ×100) in (a) saline-treated nonchallenged, (b) saline-treated challenged, (c) amoxicillin-treated nonchallenged, and (d) amoxicillin-treated challenged animals. Data are expressed as the means ± standard errors of the means (one experiment; n = 12). Differences were considered significant when P was <0.05 (*).
Bacterial counts in colon.
A fresh fecal pellet from the large intestine (n = 5 to 12/group) was collected and processed within 30 min for the enumeration of the endogenous populations of lactobacilli, enterobacteria, and enterococci. Classical culture techniques were used, as described previously (13). Values are expressed as the numbers of CFU/g of pellet.
Plasmatic immunoglobulin quantification.
For detection of anti-OVA IgE, IgG, and IgG1 and anti-KLH IgE and IgG, microtiter plates were coated with OVA or KLH (150 μl at 50 μg/ml) in carbonate buffer and incubated overnight at 4°C. The plates were washed and blocked with 200 μl/well of phosphate-buffered saline-Tween (0.05%)-fish gelatin (0.5%). Plasma samples were diluted 1:25 (for anti-OVA and anti-KLH IgE and IgG detection) and 1:2 (for anti-OVA IgG1 detection) and were then serially diluted 1:5. After 2 h of incubation, the plates were washed and 100 μl of an anti-rat IgE antibody (1/1,000 dilution; MARE-1; Zymed) for anti-OVA IgE measurement or an anti-rat IgG antibody (1/1,000 dilution; R5130; Sigma) for anti-OVA IgG measurement was added for 1 h. After the plates were washed, they were further incubated for 1 h with a second alkaline phosphatase-conjugated antibody (a 1/1,000 dilution of anti-mouse IgG [A-0162; Sigma] for anti-OVA IgE detection, a 1/5,000 dilution of anti-goat IgG [A-4062; Sigma] for anti-OVA IgG detection, and a 1/100 dilution of anti-rat IgG1 [A110-106A; Bethyl Laboratories, Montgomery, AL] for anti-OVA IgG1 detection). Finally, the plates were washed and 100 μl of para-nitrophenyl phosphate (1 mg/ml; Sigma-Aldrich) was added for 30 min. The reaction was stopped with 25 μl of 3 N NaOH, and the plates were read with a Dynex Technologies MRXII instrument. The values are expressed as the log5 of the reciprocal of the cutoff dilution (log5 titers).
Total IgE was quantified with a commercial kit, according to the manufacturer's instructions (Bethyl Laboratories). The results are expressed as ng/ml.
Mast cell mediators in plasma and intestinal tissue.
RMCPII plasma levels were measured by an enzyme-linked immunosorbent assay, according to the manufacturer's instructions (Moredun Scientific Midlothian). Values are expressed in μg/ml. The RMCPII in samples from the small intestine was also analyzed, by use of the Moredum kit, after tissue homogenization (polytron; Kinimatica, Switzerland) in ice-cold saline. The results are expressed as ng/mg tissue.
Mast cell quantification in small intestine.
Segments from the proximal small intestine were fixed in 10% formalin and embedded in paraffin. Five-micrometer tissue sections were stained with chloroacetate esterase activity for the detection of mucosal mast cells, as described elsewhere (22). For each animal (n = 11), at least three sections were processed for mast cell enumerations. Quantification of the number of stained cells per square millimeter of the lamina propria was performed by morphometric analysis (38). Data are expressed as the number of mast cells per mm2.
Histology.
Samples of the jejunum were opened longitudinally 2 h after the OVA challenge (sensitization parenteral rat model) and were immediately fixed in 10% formalin, dehydrated, and embedded in paraffin. Five-micrometer longitudinal sections were cut, rehydrated, and stained with May Grünwald-Giemsa.
Statistical analysis.
According to the distribution of the data, either a classical one-way analysis of variance or a Kruskal-Wallis test on ranks was performed. The Tukey-Kramer multiple test or the Kruskal-Wallis multiple test was used to assess the differences between the groups.
RESULTS
Amoxicillin administration to rat pups alters the gut microbiota.
The impact of amoxicillin administration on the gut microbiota during and after the amoxicillin administration was monitored by quantifying the lactobacilli, enterobacteria, and enterococci in the colonic contents by using classical methods of culture.
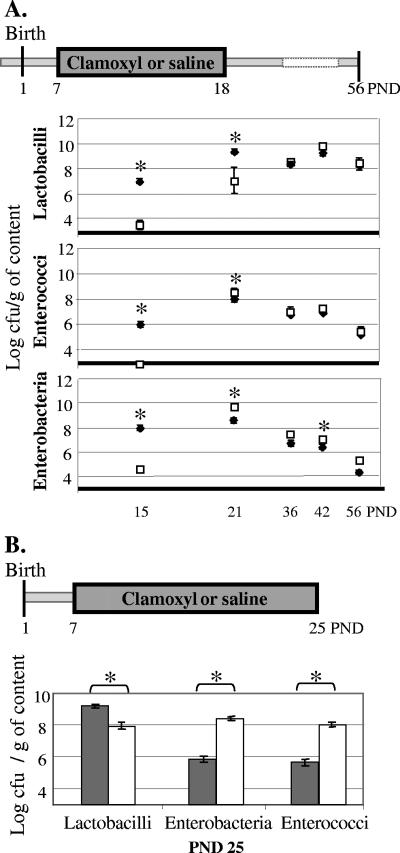
As described previously (35), when amoxicillin was administered to rats from PND7, drastic reductions in the amounts of the three bacterial genera were observed in the colon at PND15 (Fig. 1A). Three days after the end of the administration (PND21), the colonic levels of lactobacilli remained lower in the amoxicillin-treated group than in the saline-treated group (Fig. 1A; 7.0 ± 0.9 log CFU/g and 9.4 ± 0.2 log CFU/g, respectively), but similar levels were observed in the two groups thereafter. Populations of members of the family Enterobacteriaceae and the genus Enterococcus not only were recovered from amoxicillin-treated rats at PND21 but also reached even slightly higher levels than those in the saline-treated rats. In the case of the Enterobacteriaceae, the levels in the amoxicillin-treated group were still significantly elevated at PND42 (Fig. 1A).
FIG. 1.
Effect of amoxicillin (Clamoxyl) on the colonic microbiota during and after antibiotic treatment. (A) Concentrations of lactobacilli, enterobacteria, and enterococci in colonic contents of saline-treated rats (closed diamonds) and amoxicillin-treated (PND7 to PND18) rats (open squares) at PNDs 15, 21, 36, 42, and 56 (the results of one of five representative experiments are presented; n = 5 to 8 per group). (B) Concentrations of lactobacilli, enterobacteria, and enterococci at PND25 in the colonic contents of saline-treated rats (gray bars) and amoxicillin-treated rats (PND7 to PND25) (white bars) (n = 8 per group; the results of one of two representative experiments are represented). Data are expressed as log CFU/g of luminal content (mean ± standard error of the mean). *, significant differences between saline- and amoxicillin-treated groups (P < 0.05). The detection limit was 3 log CFU/g intestinal content.
When the antibiotic administration was pursued until PND25, Lactobacillus counts in the colonic contents were about 20 times lower in the amoxicillin-treated group than in the saline-treated group (P < 0.005) (Fig. 1B). Conversely, the counts of the Enterobacteriaceae and Enterococcus significantly increased with antibiotic administration (P < 0.02) (Fig. 1B).
In summary, these results show that the colonic counts of lactobacilli, enterobacteria, and enterococci were drastically reduced during antibiotic administration (namely, at PND15). However, their recovery was rapid; and no major differences in the colonic counts were observed 11 days after the cessation of antibiotic administration, other than a mild but long-lasting overgrowth of enterobacteria. By contrast, when the administration was prolonged for more than 2 weeks (namely, at PND25), we observed an important overgrowth of enterobacteria and enterococci.
Amoxicillin altered specific humoral response induction.
In order to test the potential impact of the antibiotic administration on OT induction (estimated by suppression of specific humoral immune responses), the rats were fed a high dose of OVA at PND15 (Fig. 2A), a time when the microbiota was strongly altered, as described above. Induction of specific humoral responses to OVA prior to challenge with OVA and KLH suppressed the humoral response to OVA, as shown by the significantly lower titers of the specific anti-OVA IgE, IgG, and Th2-dependent IgG1 in the tolerized group compared to those in the nontolerized group at PND36 (Fig. 2B). Interestingly, in the tolerized animals, significantly higher anti-OVA IgE, IgG, and IgG1 titers were observed in the plasma of amoxicillin-treated rats compared to those observed in the saline-treated rats (log5 titers, 4.0 ± 0.3 and 3.1 ± 0.3, respectively, for IgE; 5.4 ± 0.5 and 4.3 ± 0.3, respectively, for IgG; and 4.1 ± 0.53 and 2.4 ± 0.36, respectively, for IgG1) (Fig. 2B). Suppression of the humoral response was specific to OVA. Total IgE levels were not affected by the feeding of antigen (Fig. 2C), and the KLH-specific IgE and IgG titers (Fig. 2D) measured in rats not fed the antigen were similar to those obtained in rats fed the antigen, regardless of the antibiotic administration. Under our experimental conditions, cellular immunity, as assessed by proliferation experiments, did not seem to be altered (data not shown).
Amoxicillin did not affect the maintenance of specific humoral responses or the specific humoral responses induced later in life.
We tested the effect of antibiotic administration on the long-term persistence (PND56) of the specific humoral response in rats tolerized in early life. To this end, rats treated or not treated with antibiotics were fed OVA at PND15 and were subsequently challenged two times (at PND21 and PND41) in order to monitor the humoral immune response at PND56 (Fig. 3A). As expected, OVA-specific IgE titers were lower in the tolerized animals than in the nontolerized animals. However, no differences were observed between tolerized saline-treated rats and tolerized amoxicillin-treated rats (Fig. 3B). Similar results were obtained for OVA-specific IgG and IgG1 (data not shown). The impact of early antibiotic administration on the OT induced later in life was also examined. Oral OVA administration was performed at PND43 (Fig. 3C), i.e., 25 days after the end of antibiotic administration. OT was induced in both the saline-treated and the amoxicillin-treated groups, and the levels of OVA-specific IgE (Fig. 3D) and IgG and IgG1 (data not shown) were similar in both groups.
In the last two experiments, the humoral response was specific, as shown by similar levels of total IgE and KLH-specific IgE and IgG in the tolerized and the nontolerized groups, regardless of whether they received amoxicillin (data not shown).
Amoxicillin administration did not affect the humoral sensitization to OVA but increased the local level of RMCPII and the numbers of mast cells in small intestinal tissue.
The effect of amoxicillin on sensitization to OVA was monitored by quantifying the levels of plasma anti-OVA-specific IgE and IgG in both amoxicillin- and saline-treated animals 14 days after the subcutaneous injection of OVA at PND11 (Fig. 4A). Neither the IgE nor the IgG titer was significantly affected by the amoxicillin administration (Fig. 4B). The allergic reaction was monitored by quantifying the level of the mast cell mediator RMCPII in plasma after an intragastric challenge with OVA in previously sensitized animals. As expected, plasma RMCPII levels were highly increased in the challenged animals (Fig. 4C) but were not affected by the antibiotic administration (4.4 ± 0.7 and 5.5 ± 0.7 μg/ml in amoxicillin- and saline-treated groups, respectively).
The level of RMCPII in the intestinal tissues was also assessed as a marker of the allergic reaction at the local level. In contrast to the humoral response, no differences in tissue RMCPII levels between challenged and nonchallenged animals were observed (Fig. 4D). Strikingly, the antibiotic administration induced a significant increase in RMCPII levels in the small intestines of both the challenged and the nonchallenged animals. This antibiotic-related RMCPII increase was more important in the nonchallenged animals (+148%) than in the challenged animals (+34%).
The mast cells in small intestinal tissues were enumerated (Fig. 4E). Amoxicillin administration significantly (P < 0.05) increased the number of mast cells present in the tissue (1.65 ± 0.09 versus 1.41 ± 0.10 cells/mm2 in the amoxicillin-treated [Fig. 4Ec and d] and saline-treated [Fig. 4Ea and b] groups, respectively), whereas the challenge tended (P = 0.08) to decrease the number of stained mast cells in the tissue (1.63 ± 0.10 versus 1.42 ± 0.10 cells/mm2 in OVA-challenged [Fig. 4Eb and d] and saline-challenged [Fig. 4Ea and c] groups, respectively), as a consequence of degranulation and the release of mast cell mediators.
Despite the immune reaction induced by the OVA challenge in sensitized animals, neither clinical symptoms nor major histological changes were observed (Fig. 4F).
DISCUSSION
The goal of the present study was to determine the impact of an antibiotic intervention early in life on OT acquisition and maintenance and on the severity of an allergic response subsequent to the antibiotic treatment. The results showed that, as a consequence of the antibiotic administration, higher levels of specific anti-OVA IgE, IgG, and Th2-dependent IgG1 were induced in animals when they were tolerized with OVA during the antibiotic treatment in comparison to the levels induced in nontolerized animals (Fig. 2B). In a model of allergy, higher RMCPII levels (Fig. 4D) and higher numbers of mast cells (Fig. 4E) were quantified in the intestinal tissues of animals treated with antibiotics.
A commercial form of amoxicillin was chosen to alter the microbiota of suckling rats. Amoxicillin is a broad-spectrum antibiotic commonly used in pediatrics to treat diseases such as otitis media, bronchitis, and sinusitis (1). The dose of amoxicillin (100 mg/kg/day) given to the rat pups was in the range of that currently used for infant therapy (50 to 100 mg/kg/day). The antibiotic was administered during the suckling period (from PND7 to PND18 or PND25), when the intestinal microbiota is not yet fully established. As described earlier (35), the antibiotic administration induced strong alterations in the colonic levels of enterobacteria, enterococci, and lactobacilli, which constitute the major groups of bacteria colonizing the suckling rat intestine. After cessation of the antibiotic administration, the recovery of these populations was rapid (within a few days), although a long-lasting increase in the subdominant enterobacterial population was observed. Even though we cannot exclude the possibility that changes in additional groups of bacteria occurred, the changes described here are comparable to those reported in the fecal microbiota of young infants while they are receiving amoxicillin therapy (3). Our experimental model therefore seems to be more relevant to the clinical situation than the model that consists of germfree animals, which up to now have been widely used to study the impact of the microbiota on the immune response to luminal antigens.
A significant decrease in the efficacy of the specific humoral response in the amoxicillin-treated pups compared to the efficacy of the response in the saline-treated animals was observed. This was evidenced by a specific alteration of the anti-OVA IgE, IgG, and IgG1 responses subsequent to the challenge. On the contrary, no change in the cellular response was observed. The discrepancy between these two types of responses has already been reported (32).
The alteration of the specific humoral response was transient, because the antibiotic administration did not affect the maintenance of OT. These results contradict those presented in a recent paper, which described that depletion of the gut microbiota with oral antibiotics results not only in altered induction but also in impaired maintenance of OT to cow's milk proteins (31). These discrepancies could be attributed to major differences between the two studies. Pecquet et al. (31) used weaning mice, while we used suckling rats; both the strain and the age of the animals have been shown to affect the immune response. The antibiotics used in the two studies and their effects on the intestinal microbiota also differed and might also have affected the OT response.
The temporal association between the effects of amoxicillin on the intestinal microbiota and on the specific humoral response in our experiments suggests that a global decrease in the microbiota is more likely important for the alteration of oral tolerance than the modulation of a particular bacterial species. Those perturbations of the microbiota may have led to the observed alteration of the homeostasis of the gut immune system. Luminal bacteria influence innate and adaptive immunity through pattern recognition receptors, such as toll-like receptors (TLRs), which are selectively activated by specific microbe-associated molecular patterns (43). The generation and expansion of T regulatory cells have been shown to depend on bacterial antigens and DNA (28, 40). In line with these findings, several studies have highlighted the effects of bacterial components, through TLR2 and TLR4 signaling, on OT and the allergic response in animal models (2, 44, 46). Interestingly, in a recent study with humans (45), TLR-mediated inhibition of allergic inflammation by lipopolysaccharide was observed only in atopic children and not in adults. Taken together, these data suggest that the alteration of the specific humoral responses that we observed could be at least partially explained by a modified signaling through TLRs induced by the antibiotic-mediated alteration of the gut microbiota in the immature animal.
Contrary to specific humoral responses, amoxicillin administration did not significantly affect the sensitization to ovalbumin, i.e., the specific IgE and IgG levels in plasma, when the antigen was administered subcutaneously to naive animals together with a Th2-skewing adjuvant [Al(OH)3], which was necessary to achieve sensitization. Accordingly, the plasma levels of histamine (data not shown) and RMCPII were similar in amoxicillin- and saline-treated animals after intragastric OVA challenge. Those markers were quantified 2 h after the challenge, a time point relevant to allergic reaction assessment; but a potential effect of the antibiotic treatment on the RMCPII kinetics cannot be ruled out. In contrast, amoxicillin-treated animals showed increased RMCPII levels and higher number of mast cells in the intestinal tissue in comparison to those in the saline-treated groups, independently of the intragastric challenge. No significant morphological changes (neither major inflammatory infiltrates nor epithelial lesions) accompanied the increase in RMCPII levels, which is consistent with published data obtained with an anaphylactic animal model (37). The mild increase in the number of mast cells present in the intestinal mucosa, together with the higher levels of expression of mast cell protease genes previously observed in antibiotic-treated animals compared to those observed in control animals (16, 35), could explain this difference and suggests a local more than a systemic immune response. The mechanism responsible for the increased expression of RMCPII by the antibiotic administration is not clear. To our knowledge, no direct effect of any antibiotic on RMCPII levels has ever been reported. Interestingly, it has recently been shown that TLR3 activation by double-stranded RNA decreases the level of mast cell attachment to extracellular matrix proteins and reduces the level of IgE-mediated mast cell degranulation (20). Similarly, coincubation of human mast cells with Escherichia coli downregulated FcɛRI-mediated degranulation (21). Also, Ikeda et al. (16) showed that pretreatment of mice with immunostimulatory CpG oligonucleotides prevented the accumulation of peribronchial mast cells in a model of chronic allergic airway inflammation. These data may explain the increase in mast cell number and the level of mediator expression in antibiotic-treated animals, in which the microbial load—and, likewise, the level of TLR stimulation—was much reduced.
In the intestine, mast cell chymases have been suggested to participate in the local allergic response by increasing intestinal permeability (36) or by inducing mucin secretion (15). Intestinal mast cells have also been associated with inflammatory bowel diseases (for a review, see reference 14). A high level of mast cell tryptase was measured in colorectal samples from patients with Crohn's disease or ulcerative colitis compared to the levels in control patients (33). Interestingly, the rising incidence of Crohn's disease has been linked to antibiotic use early in life (5). Therefore, in our model, the increased numbers of mast cells and mast cell mediators, although not accompanied by any clinical manifestation, might reflect the early stages of an alteration of the gut immune system.
Globally, our data suggest that antibiotic administration early in life negatively affects the acquisition of tolerance to a luminal antigen when the antigen is first introduced during antibiotic administration. This impaired specific humoral response leads us to hypothesize that antibiotic treatment early in life might facilitate allergic sensitization to novel food antigens. Although these results should be validated in appropriate clinical trials, a practical recommendation resulting from them may be to avoid the introduction of new food ingredients in the infant diet during an antibiotic treatment (31) in order to reduce the risk of food allergy onset.
Acknowledgments
We are grateful to José Sanchez-Garcia, Christophe Maubert, and Massimo Marchesini for their skillful help with animal experiments. We warmly thank Gloria Reuteler for her technical assistance with the microbiota analyses; Corinne Vachier for the development of the program to perform the morphometric analyses; Julie Moulin for statistical analyses; and Christine Cherbut, Sophie Pecquet, Adrian Zuercher, and Rodolfe Fritsché for fruitful discussions and for critical reading of the manuscript.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Albu, R. E. 1998. Amoxicillin: a pharmacologic description. Clin. Excell. Nurse Pract. 2:260-262. [PubMed] [Google Scholar]
- 2.Bashir, M. E., S. Louie, H. N. Shi, and C. Nagler-Anderson. 2004. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 172:6978-6987. [DOI] [PubMed] [Google Scholar]
- 3.Bennet, R., M. Eriksson, and C. E. Nord. 2002. The fecal microflora of 1-3-month-old infants during treatment with eight oral antibiotics. Infection 30:158-160. [DOI] [PubMed] [Google Scholar]
- 4.Bjorksten, B., P. Naaber, E. Sepp, and M. Mikelsaar. 1999. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 29:342-346. [DOI] [PubMed] [Google Scholar]
- 5.Card, T., R. F. Logan, L. C. Rodrigues, and J. G. Wheeler. 2004. Antibiotic use and the development of Crohn's disease. Gut 53:246-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 7.Celedon, J. C., A. A. Litonjua, L. Ryan, S. T. Weiss, and D. R. Gold. 2002. Lack of association between antibiotic use in the first year of life and asthma, allergic rhinitis, or eczema at age 5 years. Am. J. Respir. Crit. Care Med. 166:72-75. [DOI] [PubMed] [Google Scholar]
- 8.Chehade, M., and L. Mayer. 2005. Oral tolerance and its relation to food hypersensitivities. J. Allergy Clin. Immunol. 115:3-12. [DOI] [PubMed] [Google Scholar]
- 9.Droste, J. H., M. H. Wieringa, J. J. Weyler, V. J. Nelen, P. A. Vermeire, and H. P. Van Bever. 2000. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 30:1547-1553. [DOI] [PubMed] [Google Scholar]
- 10.Duchmann, R., I. Kaiser, E. Hermann, W. Mayet, K. Ewe, and K. H. Meyer zum Buschenfelde. 1995. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 102:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritsché, R. 2003. Animal models in food allergy: assessment of allergenicity and preventive activity of infant formulas. Toxicol. Lett. 140-141:303-309. [DOI] [PubMed] [Google Scholar]
- 12.Furrie, E., M. Turner, and S. Strobel. 1995. The absence of gut flora has no effect on the induction of oral tolerance to ovalbumin. Adv. Exp. Med. Biol. 371B:1239-1241. [PubMed] [Google Scholar]
- 13.Guigoz, Y., F. Rochat, G. Perruisseau-Carrier, I. Rochat, and E. Schiffrin. 2002. Effect of oligosaccharide on the fecal flora and non-specific immune system in elderly people. Nutr. Res. 22:13-25. [Google Scholar]
- 14.He, S. H. 2004. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 10:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, S. H., and J. Zheng. 2004. Stimulation of mucin secretion from human bronchial epithelial cells by mast cell chymase. Acta Pharmacol. Sin. 25:827-832. [PubMed] [Google Scholar]
- 16.Ikeda, R. K., M. Miller, J. Nayar, L. Walker, J. Y. Cho, K. McElwain, S. McElwain, E. Raz, and D. H. Broide. 2003. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J. Immunol. 171:4860-4867. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, C. C., D. R. Ownby, S. H. Alford, S. L. Havstad, L. K. Williams, E. M. Zoratti, E. L. Peterson, and C. L. Joseph. 2005. Antibiotic exposure in early infancy and risk for childhood atopy. J. Allergy Clin. Immunol. 115:1218-1224. [DOI] [PubMed] [Google Scholar]
- 18.Kalliomaki, M., P. Kirjavainen, E. Eerola, P. Kero, S. Salminen, and E. Isolauri. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Kalliomaki, M., S. Salminen, T. Poussa, H. Arvilommi, and E. Isolauri. 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361:1869-1871. [DOI] [PubMed] [Google Scholar]
- 20.Kulka, A., and D. D. Metcalfe. 2006. TLR3 activation inhibits human mast cell attachment to fibronectin and vitronectin. Mol. Immunol. 43:1579-1586. [DOI] [PubMed] [Google Scholar]
- 21.Kulka, M., N. Fukuishi, M. Rottem, Y. A. Mekori, and D. D. Metcalfe. 2006. Mast cells, which interact with Escherichia coli, up-regulate genes associated with innate immunity and become less responsive to FcɛRI-mediated activation. J. Leukoc. Biol. 79:339-350. [DOI] [PubMed] [Google Scholar]
- 22.Leder, L. D. 1979. The chloroacetate esterase reaction. A useful means of histological diagnosis of hematological disorders from paraffin sections of skin. Am. J. Dermatopathol. 1:39-42. [PubMed] [Google Scholar]
- 23.Maeda, Y., S. Noda, K. Tanaka, S. Sawamura, Y. Aiba, H. Ishikawa, H. Hasegawa, N. Kawabe, M. Miyasaka, and Y. Koga. 2001. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer's patches under germfree conditions. Immunobiology 204:442-457. [DOI] [PubMed] [Google Scholar]
- 24.Matricardi, P. M. 2004. The role of early infections, hygiene and intestinal microflora. Pediatr. Pulmonol. Suppl. 26:211-212. [DOI] [PubMed] [Google Scholar]
- 25.Moreau, M. C., and G. Corthier. 1988. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect. Immun. 56:2766-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau, M. C., and V. Gaboriau-Routhiau. 1996. The absence of gut flora, the doses of antigen ingested and aging affect the long-term peripheral tolerance induced by ovalbumin feeding in mice. Res. Immunol. 147:49-59. [DOI] [PubMed] [Google Scholar]
- 27.Noverr, M. C., R. M. Noggle, G. B. Toews, and G. B. Huffnagle. 2004. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72:4996-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obermeier, F., U. G. Strauch, N. Dunger, N. Grunwald, H. C. Rath, H. Herfarth, J. Scholmerich, and W. Falk. 2005. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut 54:1428-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouwehand, A., E. Isolauri, and S. Salminen. 2002. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 41(Suppl. 1):I32-I37. [DOI] [PubMed] [Google Scholar]
- 30.Oyama, N., N. Sudo, H. Sogawa, and C. Kubo. 2001. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J. Allergy Clin. Immunol. 107:153-159. [DOI] [PubMed] [Google Scholar]
- 31.Pecquet, S., G. Prioult, J. Campbell, B. German, and M. Turini. 2004. Commonly used drugs impair oral tolerance in mice. Ann. N. Y. Acad. Sci. 1029:374-378. [DOI] [PubMed] [Google Scholar]
- 32.Prioult, G., I. Fliss, and S. Pecquet. 2003. Effect of probiotic bacteria on induction and maintenance of oral tolerance to beta-lactoglobulin in gnotobiotic mice. Clin. Diagn. Lab. Immunol. 10:787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raithel, M., S. Winterkamp, A. Pacurar, P. Ulrich, J. Hochberger, and E. G. Hahn. 2001. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand. J. Gastroenterol. 36:174-179. [DOI] [PubMed] [Google Scholar]
- 34.Saklayen, M. G., A. J. Pesce, V. E. Pollak, and J. G. Michael. 1984. Kinetics of oral tolerance: study of variables affecting tolerance induced by oral administration of antigen. Int. Arch. Allergy Appl. Immunol. 73:5-9. [DOI] [PubMed] [Google Scholar]
- 35.Schumann, A., S. Nutten, D. Donnicola, E. M. Comelli, R. Mansourian, C. Cherbut, I. Corthesy-Theulaz, and C. Garcia-Rodenas. 2005. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol. Genomics 23:235-245. [DOI] [PubMed] [Google Scholar]
- 36.Scudamore, C. L., M. A. Jepson, B. H. Hirst, and H. R. Miller. 1998. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur. J. Cell Biol. 75:321-330. [DOI] [PubMed] [Google Scholar]
- 37.Scudamore, C. L., E. M. Thornton, L. McMillan, G. F. Newlands, and H. R. Miller. 1995. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J. Exp. Med. 182:1871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soille, P. 2003. Morphological image analysis: principles and applications. Springer-Verlag, New York, NY.
- 39.Strachan, D. P. 2000. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55(Suppl. 1):S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauch, U. G., F. Obermeier, N. Grunwald, S. Gurster, N. Dunger, M. Schultz, D. P. Griese, M. Mahler, J. Scholmerich, and H. C. Rath. 2005. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut 54:1546-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strobel, S., and A. M. Mowat. 1998. Immune responses to dietary antigens: oral tolerance. Immunol. Today 19:173-181. [DOI] [PubMed] [Google Scholar]
- 42.Sudo, N., S. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 43.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji, R. F., K. Hoshino, Y. Noro, N. M. Tsuji, T. Kurokawa, T. Masuda, S. Akira, and B. Nowak. 2003. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin. Exp. Allergy 33:249-258. [DOI] [PubMed] [Google Scholar]
- 45.Tulic, M. K., P. O. Fiset, J. J. Manoukian, S. Frenkiel, F. Lavigne, D. H. Eidelman, and Q. Hamid. 2004. Role of toll-like receptor 4 in protection by bacterial lipopolysaccharide in the nasal mucosa of atopic children but not adults. Lancet 363:1689-1697. [DOI] [PubMed] [Google Scholar]
- 46.Velasco, G., M. Campo, O. J. Manrique, A. Bellou, H. He, R. S. Arestides, B. Schaub, D. L. Perkins, and P. W. Finn. 2005. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am. J. Respir. Cell Mol. Biol. 32:218-224. [DOI] [PubMed] [Google Scholar]
- 47.Wannemuehler, M. J., H. Kiyono, J. L. Babb, S. M. Michalek, and J. R. McGhee. 1982. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J. Immunol. 129:959-965. [PubMed] [Google Scholar]
- 48.Wickens, K., N. Pearce, J. Crane, and R. Beasley. 1999. Antibiotic use in early childhood and the development of asthma. Clin. Exp. Allergy 29:766-771. [DOI] [PubMed] [Google Scholar]
- 49.Wills-Karp, M., J. Santeliz, and C. L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69-75. [DOI] [PubMed] [Google Scholar]