Abstract
The recent outbreaks of West Nile virus (WNV) infection in the northeastern United States and other regions of the world have made it essential to develop efficient, sensitive, and rapid protocols for virus surveillance. Laboratory testing is the backbone of any surveillance program. Protocols to detect the presence of WNV have been refined since 1999 for sensitivity, speed, efficiency, and specificity. This paper presents the protocols currently used by the New York State Department of Health to handle vertebrate and mosquito specimens that have been submitted for WNV testing to the Arbovirus Laboratories of the Wadsworth Center.
West Nile virus (WNV) belongs to the family Flaviviridae. The virion contains single-stranded, positive-sense RNA approximately 11 kb in length. In the summer and fall of 1999, an outbreak of WNV infection in the northeastern United States was responsible for 62 human cases, including seven deaths. Extensive mortality in crows (Corvus spp.) and deaths of several exotic birds at a zoological park in the same geographic area were concurrently observed (13). This was the first evidence of WNV in North America (1, 11; T. Briese, X. Y. Jia, C. Huang, L. J. Grady, and W. I. Lipkin, Letter, Lancet 354:1261-1262, 1999 [Erratum, 354:1650]). The virus recurred in 2000 and expanded its range to include 12 states in the northeastern United States (3). In 2001, the virus spread dramatically, and by October it had extended its range along the East Coast of the United States from Vermont to Florida and west past the Mississippi River. Its presence was documented in a total of 25 states and the District of Columbia, as well as in Canada (4). The spread was even more dramatic in 2002, with the virus reaching 44 states across the United States and five provinces in Canada and infecting over 3,700 humans, with at least 200 fatalities (5). Due to the intensive surveillance that was instituted in 2000 in New York state, the spread of WNV among mosquitoes, birds, and other vertebrates was monitored effectively. Accumulated data on viral infection among various species of mosquitoes and among birds as well as other vertebrates (e.g., horses) in an expanding geographic area during 2000, 2001, and 2002 have provided valuable information for prevention, control, and prediction of viral activity.
The continuing incursion of WNV into the United States and the increasing numbers of samples submitted to the laboratory for testing necessitate efficient detection procedures for analysis of submitted specimens, which consist of mosquitoes, bird and mammal tissues, sera from human, mammal, and avian sources with suspected infections, and sera from sentinel animals that were placed in locations designed for monitoring of the presence of the virus. Assays were developed for both antibody and virus detection. WNV-specific antibody was detected by standard indirect enzyme-linked immunosorbent assay (6) and plaque reduction neutralization assays. Detection of virus was accomplished through molecular procedures that detected viral nucleic acid, cell culture procedures that detected live virus, and immunologic procedures that detected viral antigen. During the 2000, 2001, and 2002 surveillance seasons, our lab used a combination of these methods to evaluate tissues from more than 12,400 dead birds and 24,000 mosquito pools collected in New York state.
MATERIALS AND METHODS
Specimen collection.
Mosquitoes were collected in the field and sorted by species. Pools of 50 or fewer were placed into 2-ml safe-lock microfuge tubes (Eppendorf cat. no. 2236335-2), each containing one 4.5-mm-diameter zinc-plated steel BB (Daisy brand), and shipped to the Arbovirus Laboratories on dry ice (15). For evaluation of RNA purification with the use of a robotic workstation, Culex pipiens mosquitoes were inoculated with 10 PFU of WNV, incubated for 4 days at 27°C, and subsequently frozen at −80°C. Dead vertebrates were necropsied at the Wildlife Pathology Unit of the New York State Department of Environmental Conservation, and tissues (kidneys, brains, livers, hearts, and spleens) were shipped to the Arbovirus Laboratories in individual jars on dry ice.
RNA extraction.
Mosquito pools were homogenized in 0.5 to 1 ml of mosquito diluent (phosphate-buffered saline supplemented with 20% fetal bovine serum, 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 0.25 μg of amphotericin B/ml) by using a high-speed mechanical homogenizer (Mixer Mill MM300; Qiagen, Inc., Valencia, Calif.) for 30 s at 24 cycles/s. Each homogenized pool was centrifuged at room temperature for 4 min at 5,796 × g (Sigma centrifuge 4-15C and plate rotor 2 × 96; Qiagen), and the supernatant was removed and stored at −70°C for virus isolation. The remaining mosquito pellet was lysed in 0.8 to 1 ml of either guanidine isothiocyanate-containing RNA lysis buffer (RLT from RNeasy mini kit; Qiagen) or 1× lysis buffer (Applied Biosystems, Foster City, Calif.) and homogenized again as described above. RNA was extracted from the lysate by using RNeasy or the ABI Prism 6700 nucleic acid workstation (Applied Biosystems), with some modifications of the procedure described previously (12). For the RNeasy method, a 350-μl aliquot of the lysed mosquito homogenate was used for extraction of RNA, according to the manufacturer's directions for animal tissue, with elution in a final volume of 50 μl. For ABI robot extraction, 200 μl of lysed mosquito homogenate was vacuumed through tissue prefilter plates either in the ABI 6700 workstation or in the ABI Prism 6100 nucleic acid prep station to reduce the viscosity of the homogenized tissue lysate and to remove particles from the sample. Then RNA was purified by robot from a 50-μl aliquot of the prefiltered material, eluting in 100 μl of elution buffer. For comparison of RNeasy and ABI Prism RNA extraction methods, 50 μl of RLT-extracted RNA was purified with RNeasy and eluted in a 100-μl volume. For validation of the 6700 workstation, pools of 50 mosquitoes were spiked with a leg, head, thorax, or abdomen of a mosquito that had been infected by intrathoracic injection with 10 PFU of WNV and incubated at 32°C for 4 days.
Vertebrate specimens were prepared for RNeasy extraction by excision of approximately 50 mg (3 by 3 by 6 mm) of frozen kidney tissue, which was homogenized directly in 800 μl of RNeasy RLT buffer as described above for mosquitoes except that the time was increased to 4 min. RNA was extracted by the RNeasy method from 350 μl of homogenate in a final elution volume of 50 μl. For purification with the ABI Prism 6700 workstation, 25 mg (3 by 3 by 3 mm) was homogenized directly in 1 ml of 1× lysis buffer and RNA was purified from 100 μl of the homogenized lysate. If kidney tissue was not available, brain or heart tissue was excised. The remaining tissue was returned to −80°C.
Standard RT-PCR.
WNV RNA was detected by one-step reverse transcription-PCR (RT-PCR) (Qiagen) amplification of a 431-bp region (nucleotides [nt] 212 to 643) containing the junction between the capsid and premembrane genes. The primers used in this assay were forward primer 5′-TTGTGTTGGCTCTCTTGGCGTTCTT-3′ (nt 212 to 236) and reverse primer 5′-CAGCCGACAGCACTGGACATTCATA-3′ (nt 619 to 643) (8). The amplification reaction was carried out as described previously (12).
Real-time RT-PCR.
Three sets of primers and probes, each targeting a different region of WNV RNA, were used in the 5′ nuclease real-time RT-PCR assays. The sequences of the primer-probe sets were generously provided by Robert Lanciotti (8, 12). All probes contained a 5′ reporter, FAM (6-carboxyfluorescein), and a 3′ quencher, TAMRA (6-carbox-N,N,N′,N′-tetramethylrhodamine). Set 1, targeting the envelope gene, included forward primer 5′-TCAGCGATCTCTCCACCAAAG-3′ (nt 1160 to 1180), reverse primer 5′-GGGTCAGCACGTTTGTCATTG-3′ (nt 1229 to 1249), and probe 5′-TGCCCGACCATGGGAGAAGCTC-3′ (nt 1186 to 1197). Set 2, targeting the NS1 gene, included forward primer 5′-GGCAGTTCTGGGTGAAGTCAA-3′ (nt 3111 to 3131), reverse primer 5′-CTCCGATTGTGATTGCTTCGT-3′ (nt 3239 to 3259), and probe 5′-TGTACGTGGCCTGAGACGCATACCTTGT-3′ (nt 3136 to 3163). Set 3, targeting the 3′ untranslated region, included forward primer 5′-CAGACCACGCTACGGCG-3′ (nt 10668 to 10684), reverse primer 5′-CTAGGGCCGCGTGGG-3′ (nt 10770 to 10784), and probe 5′-TCTGCGGAGAGTGCAGTCTGCGAT-3′ (nt 10691 to 10714). The assay was performed on an ABI Prism 7700 sequence detector by using TaqMan one-step RT-PCR master mix (Applied Biosystems). The reaction mixture included a total volume of 50 μl, including 1 μM (each) primer pairs, 0.2 μM probe, and 5 to 10 μl of extracted RNA. Negative controls had water in place of extracted RNA. Tenfold dilutions of WNV standards (800 to 0.08 PFU) were used routinely in all assays. These standards were prepared from RNA extracted from WNV stock that had been amplified in Vero cells with titers determined on Vero cells. The standards were divided into aliquots, stored at −80°C, and used over several months. Thermal cycling consisted of 48°C for 30 min for RT, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed by first identifying the amplification cycle at which fluorescence increased above the threshold (CT), which was fixed at 0.1, and then determining the relative change in fluorescence (Rn) by performing a plate read function at the end of amplification. A sample was determined to be positive if the CT value was equal to or less than the threshold CT value and the Rn value was two or more times the average Rn value of eight negative wells (8). Results were expressed as CT values or relative numbers of PFU calculated by linear regression from the standard curve.
Multiplex RT-PCR.
A multiplex real-time RT-PCR assay was developed to incorporate two sets of primers and probes for WNV into one reaction mixture by using the ABI Prism 7700 sequence detector (TaqMan). The assay used primer-probe sets 1 (env) and 2 (NS1), with the probes of sets 1 and 2 labeled with the fluorescent reporter dyes FAM and VIC respectively. The assay was performed exactly as described for single real-time RT-PCR, except that two primer-probe sets were included in the reaction mix, and the concentrations of the primers, 0.5 μM each, and probes, 0.1 μM each, were half of those used in the single-assay TaqMan protocol. The WNV standards were tested in triplicate in both single and multiplex assays to determine sensitivity. Ten replicates of each dilution of the WNV standards were tested to determine reproducibility. RT-PCR conditions were set for 30 min at 48°C and 10 min at 95°C, followed by 40 cycles of 10 s at 95°C and 60 s at 55°C.
Cell culture.
African green monkey kidney cells (Vero; ATCC CCL-81) were grown in minimal essential medium (Gibco; Invitrogen Corp., Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, Utah), 2 mM l-glutamine, 0.3% sodium bicarbonate, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. A 150-μl aliquot of the clarified supernatant of mosquito suspensions and vertebrate tissues was inoculated onto subconfluent monolayers in T-25 flasks and observed daily for evidence of cytopathic effect (CPE). When CPE was observed, infected cells were spotted onto slides, fixed with acetone, and stained by indirect immunofluorescence assay (IFA) with an immunoglobulin M (IgM) monoclonal antibody (MAb), H5.46, specific for WNV envelope protein (9) and with goat anti-mouse IgM (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). If immunofluorescence was not observed, new flasks of Vero cells were inoculated with the supernatant harvested from the first passage, infected cells were spotted onto slides, and grouping antibodies against alphaviruses, flaviviruses, rhabdoviruses, and bunyaviruses (developed in-house) were used to determine etiology. Virus isolates of the family Bunyaviridae were identified to serogroup level by using polyclonal sera against the Bunyamwera and California groups and to species level by using RT-PCR amplification and nucleotide sequencing as described by Huang and others (7).
IFA of frozen sections.
Indirect IFAs were performed on avian kidney, brain, heart, and spleen. Frozen tissues were sectioned into a thickness of 6 um (Microm Crysotat; Richard-Allan Scientific, Kalamazoo, Mich.) and fixed in acetone at −20°C for periods ranging from 1 h to overnight. Tissue sections were stained with an IgM MAb (H5.46) specific for WNV envelope protein (9), washed five times in phosphate-buffered saline, stained with goat anti-mouse IgM fluorescein conjugate (Kirkegaard & Perry Laboratories), and washed twice in phosphate-buffered saline. Fluorescence was evaluated by using an Olympus BH-2 microscope equipped with a fluorescein isothiocyanate filter set. Negative controls included negative tissues and a rabies-specific IgM antibody. A similar protocol was used with IgG MAbs (WN3F1, WN5H10, and WN9H8) specific for WNV (developed by G. Ludwig; purchased from Bioreliance, Rockville, Md.) and goat anti-mouse IgG fluorescein conjugate (Kirkegaard & Perry Laboratories).
RESULTS
An algorithm for testing vertebrate and mosquito specimens at the Wadsworth Center has been followed for three consecutive surveillance seasons: 2000, 2001, and 2002. Mosquito pools and dead bird tissues were screened initially by real-time RT-PCR using the envelope region-specific WNV primer-probe set. Avian samples for which the initial test result was negative were declared to be negative, and no further testing was done. However, negative mosquito samples, other than Culex pipiens complex and Culex restuans, were inoculated onto cell culture (see below). All samples that were positive in the initial assay were tested further by a second real-time RT-PCR assay using either the NS1- or the 3′ untranslated region-specific WNV primer-probe set to confirm positivity. Specimens that were positive with two sets of primers were reported as confirmed positive samples. Those samples for which a positive result was not confirmed with a second primer set were tested by standard RT-PCR, IFA on frozen sections (avian tissues only), and/or cell culture. These additional confirmatory tests were used also to further evaluate specimens that represented new species or new locations that had not previously been reported to be associated with WNV. For mammals, all submitted tissues, including brains, kidneys, hearts, livers, and spleens, were screened by real-time RT-PCR. The same algorithm used for mosquito pools and birds was followed; i.e., two positive tests on at least one tissue were required for confirmed positives. If one TaqMan primer-probe set yielded positive results and the other yielded negative results, the standard RT-PCR and/or cell culture methods were used as confirmatory tests (the IFA was not used, due to problems with nonspecific staining on mammalian tissue). Several mammal cases were classified as “indeterminate”; i.e., one TaqMan primer-probe set consistently gave positive results with one or more tissues, but results of the other tests were negative. In 2000, 2001, and 2002, a total of more than 12,500 vertebrate specimens and 25,000 mosquito pools were tested by one or more TaqMan assays at the Wadsworth Center.
Cell culture assays for the detection of infectious virus by inoculation of specimens onto Vero cell monolayers and identification of etiology of virus-induced CPE by indirect IFA were used for selected positive mosquito and vertebrate species. For example, all new vertebrate species that were positive for WNV RNA and negative mosquito species other than the Culex pipiens complex and Culex restuans were tested. Occasionally, viruses other than WNV were detected in mosquito homogenates, including Flanders, Trivittatus, Cache Valley, and Highlands J viruses (2). From 2000 to 2002, a total of 1,300 vertebrate specimens and 6,326 mosquito pools were tested in cell culture at the Wadsworth Center Arbovirus Laboratories.
The indirect IFA, using a WNV-specific IgM MAb, detected viral antigen in avian kidney, brain, heart, and spleen. During the 2000 surveillance season, this was an important confirmatory assay for bird tissues. However, this assay resulted in nonspecific binding of the IgM MAb in mammalian tissues, precluding its use as a confirmatory assay for mammals. WNV-specific IgG MAbs (Bioreliance) yielded results similar to those yielded by IgM MAb. This test was used infrequently for surveillance in 2001 and 2002.
During the latter half of the 2001 surveillance season, RNA purification by RNeasy kits was replaced with purification by the ABI Prism 6700 robotic workstation, which significantly improved throughput time for processing of both bird and mosquito samples. Before the conversion was implemented, critical comparisons of the two purification procedures were performed by using naturally infected bird tissue and mosquitoes that were infected by inoculation with WNV. The accuracy of WNV purification by 6700 robot compared to that by the Qiagen RNeasy procedure was determined by analysis of parallel RNA extractions from bird tissue by real-time RT-PCR. RNA was extracted by RNeasy or by 6700 robot from five separate tissue samples that were excised from the kidneys of six different WNV-infected American crows. The RT-PCR results obtained by using the WNV env-specific primer-probe set are presented in Table 1. The ratios of robot to RNeasy CT values ranged from 0.8 to 1.02, demonstrating that recovery of WNV RNA by the ABI 6700 robot was equal to or greater than that by the RNeasy method. Standard deviations were close to or less than one, indicating that sampling from WNV-infected crow kidneys is relatively uniform. The higher standard deviation for bird F, which had a lower viral load, can be explained by the focal nature of infection, which would be more pronounced in a bird with low-level infection (Table 1).
TABLE 1.
Comparison of CT values derived from real-time RT-PCR analysis of RNA samples extracted from bird kidneys naturally infected with WNV
| Birda | Results obtained with:
|
Ratioc | |||
|---|---|---|---|---|---|
| ABI Prism 6700
|
RNeasy
|
||||
| CTb | SD | CT | SD | ||
| A | 14.97 | 0.8 | 16.56 | 0.7 | 0.90 |
| B | 17.85 | 0.6 | 18.45 | 0.6 | 0.97 |
| C | 14.59 | 0.6 | 16.93 | 0.5 | 0.86 |
| D | 14.71 | 0.7 | 16.78 | 0.6 | 0.88 |
| E | 17.57 | 0.4 | 21.95 | 1.3 | 0.80 |
| F | 30.13 | 1.5 | 29.54 | 5.5 | 1.02 |
| G | 40.00 | 0.0 | 40.00 | 0.0 | 1.00 |
American crows A through F were infected naturally with WNV; bird G was uninfected.
Each CT value presented here is the average from five separate tissue pieces processed from each bird kidney.
Ratio was determined by dividing the CT of the ABI Prism 6700 sample by the CT of the RNeasy sample.
Precision of the RNA extraction method using the ABI Prism 6700 workstation was measured by excision of tissue samples from WNV-infected birds, homogenization in lysis buffer, and isolation of RNA by robot from multiple aliquots of the homogenate. Within-assay variability (repeatability) was measured by performing real-time RT-PCR on RNA isolated on the same day by robot from four aliquots of tissue lysate taken from 13 different WNV-infected birds (Table 2). Coefficients of variation (CVs) for tissues tested from each of the birds ranged from 0.2 to 5.5%, indicating very good repeatability of the RNA extraction process. RT-PCR results with each of the two primer-probe sets, specific for WNV envelope or NS1, were similar. Reproducibility (interassay variability) was measured by isolating RNA with the 6700 workstation on three separate days from aliquots of 14 different tissue lysates. The results (Table 3) demonstrate excellent precision, with CVs ranging from 1.9 to 11.8%
TABLE 2.
Repeatability (within-assay variability) of WNV RNA purification by ABI Prism 6700 workstation
| Birda | CT values obtained with:
|
|||||
|---|---|---|---|---|---|---|
| WNV NSIb
|
WNV envc
|
|||||
| Meand | SD | CVe (%) | Mean | SD | CV (%) | |
| 227 | 16.7 | 0.51 | 3.0 | 15.8 | 0.39 | 2.5 |
| 228 | 16.2 | 0.35 | 2.2 | 15.4 | 0.22 | 1.5 |
| 229 | 15.9 | 0.19 | 1.2 | 15.3 | 0.11 | 0.7 |
| 230 | 15.8 | 0.22 | 1.4 | 15.2 | 0.13 | 0.8 |
| 231 | 16.9 | 0.31 | 1.8 | 16.0 | 0.32 | 2.0 |
| 232 | 17.1 | 0.17 | 1.0 | 16.1 | 0.03 | 0.2 |
| 233 | 16.5 | 0.90 | 5.5 | 15.7 | 0.62 | 3.9 |
| 234 | 15.8 | 0.25 | 1.6 | 15.6 | 0.16 | 1.0 |
| 235 | 16.4 | 0.11 | 0.7 | 15.4 | 0.06 | 0.4 |
| 236 | 16.1 | 0.05 | 0.3 | 15.3 | 0.03 | 0.2 |
| 302 | 16.4 | 0.20 | 1.2 | 15.4 | 0.23 | 1.5 |
| 303 | 16.9 | 0.42 | 2.5 | 15.4 | 0.21 | 1.4 |
| 304 | 16.6 | 0.53 | 3.2 | 15.1 | 0.13 | 0.8 |
WNV-infected American crows.
WNV NS1-specific primer-probe set.
WNV envelope-specific primer-probe set.
Average CT value, taken from results for four tissue lysate aliquots from each bird, each purified separately by robot.
CV, percentage of standard deviation relative to the mean.
TABLE 3.
Reproducibility (interassay variability) of WNV RNA purification by ABI Prism 6700 workstation
| Birda | Tissue homogenateb | Mean CTc | SD | CVd (%) |
|---|---|---|---|---|
| 2445 | P126 | 16.1 | 0.94 | 5.9 |
| P127 | 15.1 | 1.06 | 7.0 | |
| P128 | 16.6 | 0.77 | 4.6 | |
| P129 | 15.8 | 0.59 | 3.7 | |
| P130 | 15.4 | 1.10 | 7.1 | |
| 473 | P152 | 18.1 | 0.39 | 2.2 |
| P153 | 18.4 | 0.51 | 2.8 | |
| P154 | 17.8 | 0.38 | 2.1 | |
| P155 | 18.6 | 0.44 | 2.4 | |
| P156 | 18.3 | 1.51 | 8.2 | |
| 3200 | P217 | 15.3 | 0.29 | 1.9 |
| P218 | 15.7 | 0.30 | 1.9 | |
| P219 | 15.9 | 1.88 | 11.8 | |
| P220 | 15.6 | 1.27 | 8.1 |
WNV-infected American crows.
Each homogenate was prepared from the designated bird by excision of a piece of kidney tissue and homogenization in lysis buffer. On three different days, an aliquot was taken from each homogenate, and RNA was extracted and assayed by real-time RT-PCR.
Each CT value is the average from three separate aliquots processed and assayed on different days. CT values of 15 to 18 correspond to approximately 80 to 100 PFU of WNV.
CV, percentage of standard deviation relative to the mean.
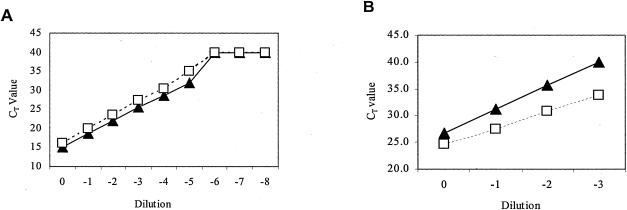
To determine the linearity and range of the two RNA purification methods, kidney tissue from a WNV-infected crow was homogenized in ABI lysis buffer or RNeasy RLT lysis buffer and then serially diluted with uninfected bird tissue homogenates prepared in the same manner. RNA was purified separately from three different aliquots of each dilution by using the ABI Prism 6700 workstation or RNeasy kits and then assayed by real-time RT-PCR by using the WNV NS1-specific primer-probe set. The results (Fig. 1A) demonstrate that CT values were linear across 6 logs of viral RNA concentration, with correlation coefficients of 0.9997 and 0.9989 for 6700 robot and RNeasy methods, respectively. Each dilution within this range demonstrated excellent precision for both the 6700 robot and RNeasy methods (CVs ranged from 0.3 to 4.2%). The accuracy of the ABI 6700 robot method of RNA purification within this range was very good, since the mean robot CT values were lower than the RNeasy values for each dilution (Fig. 1A). Linearity at higher levels has not been determined.
FIG. 1.
Comparison of the linearities and ranges of detection of WNV RNA extracted from birds and mosquitoes by ABI 6700 workstation and RNeasy methods. Kidney tissue from a WNV-positive crow (A) and a pool of 50 mosquitoes spiked with the head from an infected mosquito (B) were homogenized. Each sample was serially diluted with homogenates prepared from uninfected birds or mosquitoes. RNA was extracted from triplicate aliquots of each dilution by ABI 6700 robot (filled triangles) or RNeasy (empty squares) and assayed by real-time RT-PCR using WNV-specific primers and probe.
Isolation of RNA from mosquito pools by the ABI 6700 robot proved more difficult than that from bird tissue. For efficient RNA isolation, homogenization time was reduced to 30 s, and the lysed material was vacuumed through a prefilter before application to the RNA purification plate to prevent clogging of the columns. To test accuracy, linearity, and range of detection, pools of 50 mosquitoes were spiked with heads, thoraxes, abdomens, or legs of mosquitoes that had been infected by inoculation with WNV. RNA from these spiked pools was then extracted by 6700 robot or the RNeasy method and assayed by real-time RT-PCR (Table 4). Results, expressed as CT values and relative number of PFU per 10-μl sample tested in an assay, are presented in Table 4. WNV-specific RNA could be detected in pools of 50 uninfected mosquitoes spiked with as little as one leg from an infected mosquito. Although it is not possible to compare directly 6700 robot and RNeasy processing, since each sample was prepared from a different mosquito or mosquito part, the numbers of derived PFU generally agree to within 1 log. The linearity and range of detection for WNV RNA extraction from mosquitoes was compared in a manner similar to that used for birds (Fig. 1B). CT values were linear across 4 logs of viral RNA concentration, with correlation coefficients of 0.9999 and 0.9984 for the 6700 robot and RNeasy methods, respectively. Linearity at higher RNA concentrations has not been determined.
TABLE 4.
Comparison of results for WNV RNA purified from infected mosquitoes by using either the 6700 workstation or RNeasy
| WNV-positive mosquito parta (no.) | Results obtained with:
|
|||
|---|---|---|---|---|
| 6700 robot
|
RNeasy
|
|||
| CT | No. of PFUb | CT | No. of PFU | |
| Leg (1) | 31 | 1.4 | 32 | 1.0 |
| 39 | NDc | 33 | 0.3 | |
| 38 | ND | 36 | ND | |
| 40 | ND | 32 | 0.8 | |
| 32 | 1.2 | 40 | ND | |
| Legs (2) | 30 | 3.2 | 28 | 1.1 |
| 30 | 4.4 | 31 | 2.9 | |
| 30 | 3.3 | 32 | 0.9 | |
| Head | 28 | 7.6 | 27 | 16 |
| 26 | 1.8 | 27 | 43 | |
| 27 | 1.6 | 25 | 136 | |
| Abdomen | 27 | 20 | 25 | 79 |
| 24 | 313 | 25 | 101 | |
| Thorax | 26 | 35 | 22 | 593 |
| 25 | 144 | 23 | 387 | |
| 23 | 603 | 23 | 442 | |
Pools of 50 uninfected mosquitoes were spiked with the indicated part of an infected mosquito.
Relative number of PFU per sample determined by linear regression from WNV standards included in the assay.
Not detected; below limit of detection of the assay.
To reduce the time commitment and cost of testing surveillance samples, a multiplex real-time RT-PCR utilizing two primer-probe sets in a single reaction was developed. Analysis of sensitivity indicated that 0.8 PFU was the minimum limit of detection by the multiplex assay (Table 5). This assay is 10-fold less sensitive than the single primer-probe assay (12). The correlation coefficients from the standard curve analysis with FAM and VIC were 0.992 and 0.9989, respectively (data not shown). Reproducibility of the multiplex assay results was measured by testing 10 replicates of several dilutions of the WNV standards (Table 6). Reproducibility was excellent, with CV values ranging from 0.4 to 1.2%.
TABLE 5.
Sensitivity of the real-time RT-PCR multiplex assay
| WNV standard (PFU) | Results obtained with:
|
|||
|---|---|---|---|---|
| FAMa
|
VICb
|
|||
| CTc | SD | CT | SD | |
| 8,000 | 17.20 | 0.15 | 17.54 | 0.16 |
| 800 | 20.75 | 0.08 | 21.07 | 0.08 |
| 80 | 24.37 | 0.12 | 24.62 | 0.14 |
| 8 | 27.66 | 0.12 | 28.34 | 0.14 |
| 0.8 | 30.55 | 0.24 | 31.97 | 0.24 |
| 0.08 | 40 | 0 | 40 | 0 |
| 0.008 | 40 | 0 | 40 | 0 |
WNV envelope-specific primer-probe set, with probe labeled with FAM fluorescent dye.
WNV NS1-specific primer-probe set, with probe labeled with VIC fluorescent dye.
Mean of CT values of 10 replicate samples.
TABLE 6.
Reproducibility of results of the real-time RT-PCR multiplex assay
| WNV standard (PFU) | Results obtained with:
|
|||||
|---|---|---|---|---|---|---|
| FAMa
|
VICb
|
|||||
| CTc | SD | CVd | CT | SD | CV | |
| 8,000 | 17.26 | 0.11 | 0.63 | 17.31 | 0.07 | 0.40 |
| 800 | 20.80 | 0.10 | 0.48 | 21.08 | 0.15 | 0.71 |
| 80 | 24.23 | 0.10 | 0.41 | 24.52 | 0.13 | 0.53 |
| 8 | 27.58 | 0.34 | 1.23 | 28.05 | 0.20 | 0.71 |
WNV envelope-specific primer-probe set, with probe labeled with FAM fluorescent dye.
WNV NS1-specific primer-probe set, with probe labeled with VIC fluorescent dye.
Mean of CT values of 10 replicate samples
CV, percentage of standard deviation relative to the mean.
The cost and time requirements for surveillance of WNV in mosquito and vertebrate specimens at the Wadsworth Center were evaluated (Table 7). The expenses of processing and testing RNA samples were similar for RNeasy and 6700 workstation procedures, i.e., $7.25 and $6.60, respectively, for birds and $7 and $7.10, respectively, for mosquitoes. However, the hands-on time required for the ABI 6700 robotic workstation was approximately one-fourth that required for the RNeasy procedures, 1.5 and 6 h, respectively, per 96-well plate. Note that these times are for one TaqMan plate containing 80 test samples, 10 standards, and six negative controls. Most of the bird specimens are tested with two different primer-probe sets, raising the cost to $10.50 and $10.45 for RNeasy and 6700 workstation plates, respectively. Mosquito pools are tested with only one primer-probe set at the beginning and end of the season, when there are few positive specimens. Any specimens that test positive are then tested with another primer-probe set for confirmation.
TABLE 7.
Arbovirus Laboratory assay costs and time commitments
| Task | Sample type(s) | Assay cost per sample ($)a | Time (h) |
|---|---|---|---|
| Recording of specimen information and results and reporting of results | 2.5/50 samples | ||
| Tissue sorting, excision, and homogenization | Birds and mammals | 0.75 | 2/96-well plateb |
| Mosquitoes | 0.50 | 3/96-well plate | |
| Isolation of RNA | |||
| Using RNeasy method | Birds and mammals | 3.25 | 5/96-well plate |
| Mosquitoes | 3.25 | 5/96-well plate | |
| Using ABI 6700 robotc | Birds and mammals | 2.00 | 1/96-well plate |
| Mosquitoes | 2.75 | 1/96-well plate | |
| Real-time RT-PCR (TaqMan) | |||
| Using manual setup | 3.25 | 1/96-well plate | |
| Using ABI 6700 robotc | 3.85 | 0.5/96-well plate | |
| Virus isolation | |||
| Birds and mammals | 5.00 | 2.5/10 samples | |
| Mosquitoes | 1.50 | ||
| IFA (Excising of tissue, cutting of frozen sections, and application of immunocytochemistry) | 7.50 | 5/10 samples |
These costs are for specific supplies only and do not reflect costs for equipment, personnel, or general laboratory supplies and services, such as gloves, Kimwipes, underpads, disinfectant, and autoclaving.
Each 96-well plate contains 80 samples, 12 WNV standards, and four negative controls.
ABI 6700 run time, 85 min for RNA extraction, 45 min for TaqMan setup.
DISCUSSION
While growth in cell culture represents the “gold standard” for viral detection, the procedure requires time for the virus to grow and to subsequently be identified, most commonly by IFA staining of infected cells spotted onto slides. Cell culture, however, allows for detection of any virus that grows in the cells. Nucleic acid-based techniques, especially RT-PCR, have the advantages of speed, specificity, and sensitivity for detection of viral RNA. The procedures involve extraction of RNA from specimens, amplification by RT-PCR, and product detection. The recent development of real-time RT-PCR has combined the steps of amplification and detection and has thus dramatically increased throughput, as well as improved quantitation, specificity, and accuracy (10). The disadvantages of both standard and real-time RT-PCR are the potential for cross contamination, the occurrence of equivocal results needing further confirmation, and the limitation of detection to the specific viral sequence determined by the primers. Development of multiplex assays has increased the number of viruses detectable in a single assay. The multiplex real-time RT-PCR developed here, which combines two sets of primers in one reaction mixture, allows for significant savings in time and materials but loses sensitivity by a factor of 10; i.e., the limit of detectability is 0.8 PFU compared to 0.08 PFU by individual RT-PCR. Therefore, the laboratory must make a decision as to whether that loss is acceptable. The epidemiological significance and the contribution to the transmission cycle of those specimens with 0.08 PFU are not clear. Infectious virus usually is not detectable in these specimens, most likely because it is at too low a concentration to infect Vero. Such levels of detection represent approximately 500 and 50 copy numbers, corresponding to 0.8 and 0.08 PFU, respectively (12). A more accurate determination of copy numbers by using electron microscopy is in progress.
The indirect IFA was a very valuable confirmatory assay for avian samples. This procedure is very sensitive and rapid, but tissue section preparation may be too hazardous and time-consuming for the method to be useful for screening large numbers of tissues. The technician must work with extreme caution, each tissue must be handled carefully, and the results must be read individually. In contrast to its success with bird tissues, the indirect IFA was problematic with mammalian tissues due to nonspecific binding of the IgM MAb and, in some species, the second antibody.
The change from manual to automated processing with the ABI Prism 6700 workstation resulted in a considerable reduction in the hands-on time required for purification of RNA and preparation of real-time RT-PCR plates. The feasibility of this procedure using virus-spiked negative bird tissue was described in a previous publication (12). In the present study, naturally infected birds and mosquitoes infected by inoculation with WNV were used to test the procedure more extensively. Direct comparison of RNA purification by the ABI Prism 6700 workstation to that by the RNeasy kit (considered the gold standard for these assays) demonstrates that the robot method is very accurate, with a sensitivity equivalent to that of RNeasy. Excellent inter- and intra-assay precision was demonstrated by the ABI robot. The linearities and ranges of detection were comparable for extraction by the ABI robot and RNeasy; CT values were linear across 6 logs of viral RNA concentration for bird tissue and 4 logs for mosquito samples. Linearity might be extended over several more logs, since higher concentrations have not yet been tested. Isolation of RNA from mosquito pools by the ABI Prism 6700 workstation proved more difficult than that from bird tissue. The homogenization time was reduced to 30 s, since longer times produced particulate material that clogged the purification columns. Clogging was further reduced by vacuuming the homogenate through a prefilter before application to the RNA purification columns. Even with these precautions, the amount of material that could be purified on one column was reduced fivefold compared to the amount that could be purified with RNeasy. However, the sensitivity of the method with mosquitoes was again comparable to that of the RNeasy method.
Cost and time requirements of the assays performed as part of WNV surveillance at the Wadsworth Center were analyzed. The cost of testing one bird tissue or one mosquito pool with two TaqMan assays is approximately $10.50 with either RNeasy or the ABI workstation. The real difference between the two methods is the hands-on time needed for processing samples from a 96-well plate: 6 h for RNeasy versus 1.5 h for the ABI workstation. This equals a savings of 640 h for processing the 11,400 samples tested in 2002. This timesaving advantage should be weighed against the initial cost of the robot.
Since WNV is assigned to biosafety level 3 (BSL-3), all mosquito and vertebrate specimen testing is performed in a BSL-3 laboratory by following procedures recommended in Biosafety in Microbiological and Biomedical Laboratories, 4th ed. (14). There is no immunization against West Nile infection, and all laboratory personnel are monitored annually by serological testing. A laboratory-specific safety manual has been developed, which is available in the lab and is required reading for all new personnel, in addition to one-on-one training. According to the Centers for Disease Control and Prevention, routine diagnostic procedures for WNV infection may be carried out in a BSL-2 facility providing that the exhaust air from the laboratory room is discharged to the outdoors, the ventilation to the laboratory is balanced to provide directional airflow into the room, access to the laboratory is restricted when work is in progress, and all standard BSL-3 procedures are rigorously followed (14).
Acknowledgments
We thank Ward Stone and the Department of Environmental Conservation for necropsy of birds and mammals. We also are grateful to the New York State Department of Health Division of Epidemiology, particularly Millicent Eidson, director of the Zoonoses Program, and Dennis White, director of the Arthropod-Borne Disease Program, for facilitating submission of birds and mosquitoes. We are grateful to George Ludwig, USAMRIID, for providing us with the IgG MAbs. We also thank Charles Trimarchi, chief of the Laboratory for Zoonotic Disease and Epidemiology, Robert Rudd of the Rabies Lab for preparation and staining of frozen sections from birds and mammals, and Morris Safford, Jr., of the Rabies Lab for his significant technical contributions to the early optimization of IFA conditions and to first and second antibody selection. We thank the Wadsworth Center Molecular Genetics Core for providing the primers used in this paper.
We are appreciative of Centers for Disease Control and Prevention support for this work (grant no. 15-0022-06), especially the many helpful discussions with Robert Lanciotti. Additional support was provided by the New York State Department of Health.
REFERENCES
- 1.Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Wakem, R. A. French, A. E. Garmendia, and H. J. Van Kruiningen. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331-2333. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, K. A., J. G. Maffei, S. A. Jones, E. B. Kauffman, G. D. Ebel, A. P. Dupuis II, K. A. Ngo, D. C. Nicholas, D. M. Young, P.-Y. Shi, V. L. Kulasekera, M. Eidson, D. J. White, W. B. Stone, and L. D. Kramer. 2001. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 7:679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Update: West Nile virus activity—northeastern United States, January-August 7, 2000. Morb. Mortal. Wkly. Rep. 49:714-718. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. West Nile virus activity—United States, 2001. Morb. Mortal. Wkly. Rep. 51:497-501. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. West Nile virus activity—United States, November 7-13, 2002. Morb. Mortal. Wkly. Rep. 51:1026-1027. [Google Scholar]
- 6.Ebel, G. D., A. P. Dupuis, D. Nicholas, D. Young, J. Maffei, and L. D. Kramer. 2002. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg. Infect. Dis. 8:979-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, C., B. Slater, W. Campbell, J. Howard, and D. White. 2001. Detection of arboviral RNA directly from mosquito homogenates by reverse-transcription-polymerase chain reaction. J. Virol. Methods 94:121-128. [DOI] [PubMed] [Google Scholar]
- 8.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 10.Mackay, I. M., K. E. Arden, and A. Nitsche. 2002. Real-time PCR in virology. Nucleic Acids Res. 30:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melnick, J. L. 1979. Taxonomy of viruses, 1979. Prog. Med. Virol. 25:160-166. [PubMed] [Google Scholar]
- 12.Shi, P.-Y., E. B. Kauffman, P. Ren, A. Felton, J. H. Tai, A. P. Dupuis II, S. A. Jones, K. A. Ngo, D. C. Nicholas, J. G. Maffei, G. D. Ebel, K. A. Bernard, and L. D. Kramer. 2001. High throughput detection of West Nile virus RNA. J. Clin. Microbiol. 39:1264-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele, K. E., M. J. Linn, R. J. Schoepp, N. Komar, T. W. Geisbert, R. M. Manduca, P. P. Calle, B. L. Raphael, T. L. Clippinger, T. Larsen, J. Smith, R. S. Lanciotti, N. A. Panella, and T. S. McNamara. 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 37:208-224. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. U.S. Government Printing Office, Washington, D.C.
- 15.White, D. J., L. D. Kramer, P. B. Backenson, G. Lukacik, G. Johnson, J. Oliver, J. J. Howard, R. G. Means, M. Eidson, I. Gotham, V. Kulasekera, and S. Campbell. 2001. Mosquito surveillance and polymerase chain reaction detection of West Nile virus, New York state. Emerg. Infect. Dis. 7:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]