Abstract
Paracoccidioidomycosis (PCM) is a granulomatous disease caused by the dimorphic fungus Paracoccidioides brasiliensis. The immunoglobulin classes and isotypes of antibodies directed to acidic glycosphingolipids (GSLs) and glucosylceramide of P. brasiliensis were determined by enzyme-linked immunosorbent assay of sera from 31 PCM patients. The reactivities of 38 serum samples were analyzed by considering the stage of treatment: before antifungal treatment (n = 10), during 1 to 4 months of treatment (T1-4; n = 9), during 5 to 12 months of treatment (T5-12; n = 9), and posttreatment (PT; n = 10). Sera from healthy subjects (n = 12) were used as controls. Only the GSL Pb-1 antigen, which presents the carbohydrate structure Galfβ1-6(Manα1-3)Manβ1, was reactive with the PCM patient sera. The PCM patient sera did not react with Pb-2, which lacks the Galf residue and which is considered the biosynthetic precursor of Pb-1, indicating that the Galf residue is essential for antibody reactivity. The Pb-1 glycolipid from nontreated patients elicited a primary immune response with immunoglobulin M (IgM) production and subsequent switching to IgG1 production. The IgG1 titer increased after the start of antifungal treatment (T1-4 group), and general decreases in the anti-Pb-1 antibody titers were observed after 5 months of treatment (T5-12 and PT groups). The Pb-1 antigen, an acidic GSL with terminal Galf residue, has potential application as an elicitor of the host immune response in patients with PCM.
Paracoccidioidomycosis (PCM) is a systemic granulomatous fungal disease caused by the thermally dimorphic fungus Paracoccidioides brasiliensis, which grows in the yeast form at 36°C and in the mycelium form at 23°C.
P. brasiliensis infection is endemic in most countries of South and Central America, particularly Brazil, Argentina, Colombia, and Venezuela (3). It is estimated that as many as 10 million people in areas where PCM is endemic, mostly farm workers, may be infected by inhalation of P. brasiliensis microconidia from the soil, which is suspected to be the natural habitat of this fungus (3, 16). Clinical manifestations range from mild pulmonary lesions to severe disseminated infection involving many organs, most frequently the lungs, oropharyngeal mucosae, skin, lymph nodes, adrenal glands, and central nervous system.
Many antigenic compounds expressed in the yeast forms of P. brasiliensis are recognized by antibodies raised in human patients (5-7, 14, 18, 23). The specificity of the serologic tests depends on the antigen used. P. brasiliensis crude antigens have components that are common with those of other fungi, which can explain the cross-reactivity with the sera of patients with other fungal diseases, such as histoplasmosis or Jorge Lobo's disease (4). Glycoprotein 43 (gp43), the major exoantigen secreted by P. brasiliensis, is a reliable antigen for immunodiagnostic assays (6, 15); however, the glycoepitopes of gp43 may be common to different fungi and may explain the cross-reactivity with heterologous sera, which is frequently abolished by the deglycosylation of gp43 (26). Some patients may be serologically negative for gp43 at the time of the diagnosis, whereas others exhibit persistent levels of specific antibodies following recovery from PCM (4). Therefore, a definitive diagnosis of PCM requires visualization or isolation of the fungus from infected tissue. Clinicians use specific antibody detection mainly to evaluate the patient's immune response to antifungal therapy, since decreasing levels of specific anti-P. brasiliensis gp43 antibodies have been documented in patients with good clinical responses to antifungal therapy (4).
Besides gp43, other proteins, glycoproteins, and glycosphingolipids (GSLs) present in P. brasiliensis are antigenic (i.e., provoke an immune response) in humans (6, 12, 14, 18, 23). GSLs are present in all eukaryotic cells and vary widely in their glycan and ceramide structures, depending on the species, tissue, and stage of differentiation/development. We showed previously that glucosylceramide (GlcCer) is the only neutral GSL in the yeast and the mycelium forms of P. brasiliensis but that two glycosylinositolphosphorylceramide (GIPC) antigens (acidic GSLs), termed Pb-1 and Pb-2, are present (11, 23, 24). The sera of patients with PCM reacted strongly with Pb-1, which comprises 90 to 95% of the acidic GSLs of the yeast forms, whereas the sera of patients with histoplasmosis and Jorge Lobo's disease showed no cross-reactivity with Pb-1 (23). The structure of Pb-1 was elucidated as Galfβ1-6(Manα1-3)Manβ1-2Ins-P-Cer (11). Pb-2 comprises the remaining 5 to 10% of the acidic GSLs of yeast forms. It differs from Pb-1 in the absence of a terminal Galf residue and probably represents the biosynthetic precursor of Pb-1.
In order to identify new antigens potentially useful as markers of fungal infection, we evaluated the clearance of antibodies directed to P. brasiliensis GSLs in serum samples of PCM patients during antifungal treatment. Serum samples were also tested to evaluate the immunoglobulin (Ig) classes and IgG subclasses of host antibodies directed to the fungal GSLs.
MATERIALS AND METHODS
Serum samples.
Serum samples were collected from 31 patients with chronic PCM before and/or after the start of antifungal treatment. A total of 38 serum samples were analyzed, with seven serum samples obtained from the same patients at different stages of treatment. The diagnosis of PCM for the patients enrolled in the study was confirmed by the observation of characteristic P. brasiliensis fungal elements on histopathologic examination, direct KOH examination, and/or culture from clinical specimens. Serum samples were aliquoted and frozen at −70°C for no longer than 2 years before they were tested.
The clearance of antibodies directed to P. brasiliensis GSLs in serum samples of PCM patients was evaluated by testing samples collected (i) before treatment (BT; n = 10), (ii) during the first 4 months of treatment (T1-4; n = 9), (iii) from months 5 to 12 of treatment (T5-12; n = 9), and (iv) after 3 years of successful therapy with antifungal drugs (posttreatment [PT]; n = 10).
Table 1 summarizes the clinical characteristics of the PCM patients enrolled in the study. Forty-three percent of the study patients were from São Paulo State, 20% were from Paraná, 26% were from Minas Gerais, and 11% were from other states of Brazil (Paraíba, Bahia, Mato Grosso do Sul, and Rio Grande do Sul). All PCM patients were successfully treated with sulfamethoxazole-trimethoprim at doses ranging from 800/160 to 1,200/240 mg twice a day for at least 2 years (19). In addition, 6 serum samples from patients with invasive candidiasis, 14 serum samples from patients with pulmonary aspergillosis, and 9 serum samples from patients with the chronic form of Chagas' disease were also analyzed. Twelve serum samples from healthy volunteers (blood donors) were included as controls.
TABLE 1.
Sera of patients with PCM
| Patient group | Age (yr) | Clinical form of PCM (no. of patients) | No. of patients with the following immunodiffusion test result at the indicated titersa:
|
|||
|---|---|---|---|---|---|---|
| Negative | Positive
|
|||||
| 1:1-1:2 | 1:4-1:8 | 1:16 | ||||
| BT | 39-53 | Multifocal (9) | 1 | 4 | 3 | 1 |
| 64 | Unifocal (1) | 1 | ||||
| T1-4 | 38-62 | Multifocal (8) | 1 | 5 | 2 | |
| 50 | Unifocal (1) | 1 | ||||
| T5-12 | 39-61 | Multifocal (8) | 3 | 5 | ||
| 47 | Unifocal (1) | 1 | ||||
| PT | 43-71 | Multifocal (9) | 6 | 3 | ||
| 61 | Unifocal (1) | 1 | ||||
Immunodiffusion was performed with the Paracoccidioides brasiliensis somatic antigen as described previously (5).
All patients were informed and signed a consent form for this investigation. The study protocol was approved by the Institutional Ethics Committee of Universidade Federal de São Paulo.
Culture of P. brasiliensis.
P. brasiliensis B-339 (ATCC 200273) was provided by Zoilo P. Camargo, Cellular Biology Discipline, Universidade Federal de São Paulo. The yeast and the mycelium forms were grown at 36°C and 23°C, respectively, in PGY (peptone, 5 g/liter; glucose, 15 g/liter; yeast extract, 5 g/liter) supplemented with thiamine (0.1 g/liter) and asparagine (1.4 g/liter) by using 2.5-liter Fernbach flasks in a shaker at 100 rpm. After 1 week, the yeast and the mycelium forms were inactivated with 0.1% thimerosal, and fungi were collected 48 h later by filtration on Whatman no. 1 filter paper (20).
Extraction and purification of GSLs.
GSLs were extracted by homogenizing the yeast or the mycelium forms (∼30 g) in an Omni-mixer (Sorvall Inc., Wilmington, DE) three times with 200 ml isopropanol-hexane-water (55:20:25; the upper phase was discarded) and twice with 200 ml chloroform-methanol (2:1) (all solvent ratios are by volume) (21). The five extracts were pooled, dried on a rotary evaporator, dialyzed against water, and lyophilized.
GSL preparations were separated by DEAE-Sephadex chromatography into (i) neutral GSLs eluted with chloroform-methanol-water (30:60:8) and (ii) acidic GSLs eluted with 0.2 M sodium acetate in methanol (12). Acidic GSLs were dried in a rotary evaporator, resuspended in water, dialyzed against water for 72 h, and dried. The Pb-1 and Pb-2 GSLs were purified by high-performance liquid chromatography on an Iatrobeads 8010 (Iatron Laboratories, Inc., Tokyo, Japan) column with a gradient of isopropanol-hexane-water from 55:43:2 to 55:20:15 over 175 min with a flow rate of 1.0 ml/min. Fractions of 1 ml were collected. The identity and purity of each fraction were assessed by analytical high-performance thin-layer chromatography (HPTLC). Fractions 62 to 64 (corresponding to Pb-2) and fractions 67 to 74 (corresponding to Pb-1) were used for all subsequent experiments. The monohexosylceramide present in the neutral fraction was further purified from other contaminants by chromatography on Silica Gel 60 by using a stepwise gradient of chloroform-methanol (98:2, 94:6, 92:8, 90:10, 70:30, 50:50). Glucosylceramide was the only GSL present in the fraction eluted with chloroform-methanol (90:10). The purities of GlcCer, Pb-1, and Pb-2 were determined by HPTLC on Silica Gel 60 plates (Merck, Darmstadt, Germany) with the solvent chloroform-methanol-CaCl2 (0.02%; 60:40:9) and by staining with 0.5% orcinol (Sigma Chemical Co., St. Louis, MO) in 3.0 M H2SO4 (the plate was sprayed and briefly heated to 120°C, and the glycolipids were visualized as purple bands), 0.01% primulin (Aldrich Chemical Co., Milwaukee, WI) in 80% aqueous acetone (the GSL bands were visualized under UV light after the plate was sprayed), and the molybdenum blue spray reagent (Sigma Chemical Co.) for the phospholipids.
ELISA for anti-GSL antibodies.
Purified GSLs (25 μl of a 4-μg/ml solution in ethanol) were added to 96-well plates (Microtest III flexible assay plates; Falcon, Oxnard, CA) and dried at 37°C, and the wells were blocked with 0.01 M phosphate-buffered saline (PBS; pH 7.2) containing 5% bovine serum albumin (BSA) and 10% skim milk for 3 h at 25°C. The plates were incubated sequentially with (i) sera from patients or control subjects overnight at 4°C; (ii) anti-human IgG (heavy and light chains) biotinylated antibody (Dako, Denmark) diluted 1:1,000 in BSA-PBS or biotinylated specific anti-human IgG (γ chain), IgG1, IgG2, IgG3, IgG4, IgM (μ chain), IgA, and IgE (Southern Biotechnology, Inc., Birmingham, AL) for 2 h at 25°C; and (iii) horseradish peroxidase-streptavidin (Dako) (1:2,000) for 1 h at 25°C. Antibody levels were expressed as the mean absorbance values (at 492 nm) for duplicate assays minus the absorbance value for the blank (absorbance values for each serum dilution in plates without adsorbed GSL).
The cutoff for each GSL was determined by using 12 serum samples from healthy donors. The enzyme-linked immunosorbent assay (ELISA) was performed on 96-well plates adsorbed with 100 ng of each GSL per well. Cutoff values were calculated as the mean ± 3 standard deviations (2).
Antibody titers were determined as the last serum dilution with an absorbance value higher than the cutoff value.
IgG avidity to Pb-1 antigen.
The avidities of the anti-Pb-1 antibodies were estimated by the method of Jenum et al. (9) from their capacities to remain bound to the antigen in the presence of 6.0 M urea. Each serum sample was analyzed in a fourfold dilution range (starting at 1:25) in two rows (rows A and B) overnight at 4°C. After incubation, row A was washed three times with PBS containing 6.0 M urea at 25°C to remove the low-avidity antibodies from their binding sites. Row B (control) was washed with PBS only at 25°C. During each washing step, the microtiter plate was shaken vigorously for 5 min. Subsequent steps of the reaction were the same as those of the standard ELISA. The secondary antibody used was anti-human γ chain biotinylated antibody. For each serum sample, avidity was calculated as (titer in row A/titer in row B) × 100 and the titer was calculated a 1/(dilution x × 10n), where dilution x is the highest dilution giving an absorbance value higher than the cutoff (0.22) and n is equal to log 4 [(ODx − 0.220)/(ODx − ODy)], where 4 is the dilution factor, ODx is the absorbance (optical density) at dilution x, 0.220 is the cutoff OD, and ODy is the absorbance at the next dilution after dilution x.
Statistical analysis.
The values obtained for different PCM treatment groups were compared by the Mann-Whitney test by using GraphPad Prism software, version 3.0. Differences were considered significant if the P value was <0.05.
RESULTS
Reactivities of sera from PCM patients with GSL antigens of P. brasiliensis.
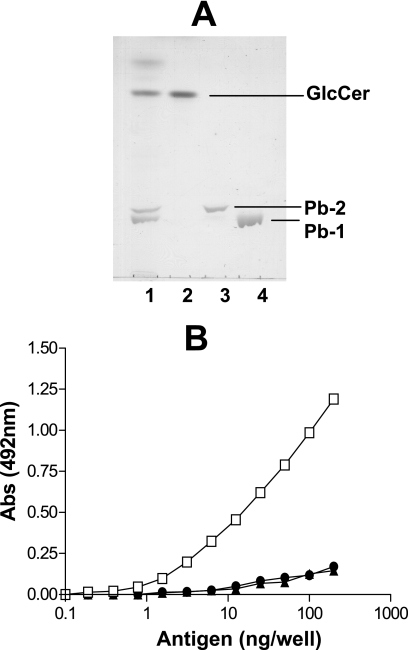
The glucosylceramide, Pb-1, and Pb-2 antigens were purified from the yeast forms of P. brasiliensis as described in Materials and Methods. The chromatographic profiles of the purified GSLs used for ELISA are shown in Fig. 1A. Sera pooled from five PCM patients before treatment were initially tested with serially diluted antigens, using a starting concentration of 200 ng/well. Only Pb-1 was recognized by patient sera. Fifty percent of the maximal reactivity was observed with approximately 25 ng Pb-1 per well (Fig. 1B).
FIG. 1.
Purified GSLs from P. brasiliensis and their immunogenicities. (A) HPTLC pattern of purified GSLs from P. brasiliensis obtained by using the solvent chloroform-methanol-CaCl2 (0.02%) and staining with orcinol-H2SO4. Lane 1, total P. brasiliensis GSLs; lane 2, purified GlcCer; lane 3, purified Pb-2; lane 4, purified Pb-1. (B) Reactivities of sera from PCM patients (n = 5; diluted 100 times) with Pb-1 (squares), Pb-2 (triangles), and GlcCer (circles) purified from P. brasiliensis by ELISA before treatment. Various concentrations of GSLs were adsorbed on 96-well plates, and the plates were blocked and incubated overnight at 4°C with pooled patient sera (n = 5). The amount of Ig bound to each GSL was determined by using biotinylated anti-IgG (heavy and light chains), as described in Materials and Methods. Abs, absorbance.
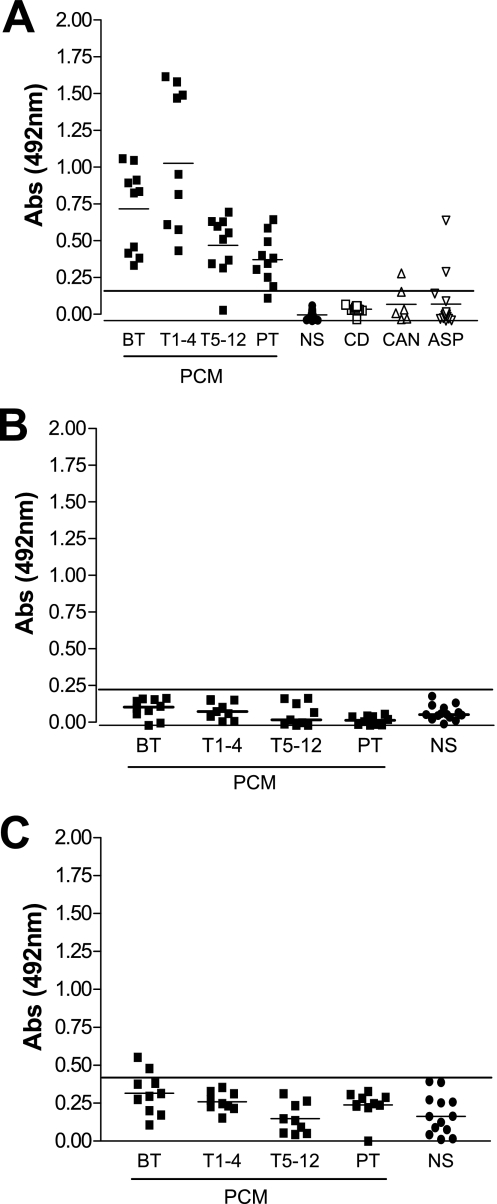
In order to evaluate the expression and clearance of anti-P. brasiliensis GSL antibodies during PCM treatment, the sera were analyzed by ELISA with the three GSLs, as described above, at 100 ng/well. Antibody reactivity against Pb-1 (Fig. 2A) but not against Pb-2 (Fig. 2B) or glucosylceramide (Fig. 2C) was observed. The level of expression of the anti-Pb-1 antibody was significantly higher in the BT group or the T1-4 group than in the T5-12 or the PT group. Since the sera of the PCM patients were reactive with Pb-1 [Galfβ1-6(Manα1-3)Manβ1-2Ins-Cer] but not with Pb-2 (Manα1-3Manβ1-2Ins-Cer), the Galf residue appears to be a key molecule for antibody recognition. Similarly, the sera of patients with Chagas' disease contained antibodies directed to Trypanosoma cruzi GIPCs with a terminal Galf residue β1-3 linked to mannose (8, 27). However, the sera from patients with Chagas' disease showed no reactivity with the Pb-1 antigen (Fig. 2A). Also, as shown in Fig. 2A, only 1 of 6 serum samples from patients with invasive candidiasis (n = 6) and 2 of 14 serum samples from patients with pulmonary aspergillosis presented cross-reactivity with Pb-1 by ELISA.
FIG. 2.
Reactivities of sera of patients with PCM and Chagas' disease with various GSLs. Samples (∼100 ng) of Pb-1 (A), Pb-2 (B), and GlcCer (C) were adsorbed on 96-well plates and incubated with sera from PCM patients (diluted 100 times) taken at various stages of treatment (BT, n = 10; T1-4, n = 9; T5-12, n = 9; PT, n = 10) or with sera from patients with Chagas' disease (CD; n = 9), patients with invasive candidiasis (CAN; n = 6), or patients with pulmonary aspergillosis (ASP; n = 14). The amount of Ig bound to each GSL was determined by using biotinylated anti-IgG (heavy and light chains), as described in Materials and Methods. Serum reactivity was analyzed by ELISA. Short horizontal lines, mean absorbance for each group; long horizontal lines, cutoff determined for sera from healthy subjects (NS; n = 12). Abs, absorbance.
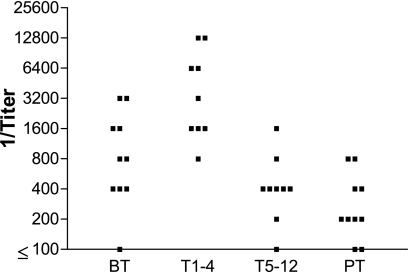
Figure 3 presents the anti-Pb-1 antibody titers, determined by ELISA, in four groups of patients at different stages of antifungal treatment. Although the samples are not matched, it is possible to note that the highest anti-Pb-1 titers were observed during the first 4 months of treatment (T1-4 group) and that the titers were significantly higher (P < 0.05) than the titers observed in the BT, T5-12, and PT groups. It is worth mentioning that all patients responded positively to the treatment. The mean value of the anti-Pb-1 antibody titer in patients during the first 4 months of treatment (T1-4 group) was almost four times higher than the mean value for the BT group. On the other hand, after 5 months of treatment, as the clinical symptoms declined, the anti-Pb-1 antibody titers also declined to almost 1/10.
FIG. 3.
Pb-1 antibody titers in PCM patient serum. Purified Pb-1 antigen (100 ng/well) was adsorbed on a 96-well plate and incubated with serially diluted sera from the PCM patient groups, as defined in the legend to Fig. 2. The amount of bound antibody was determined by ELISA with biotinylated mouse anti-human IgG (heavy and light chains). The titer was determined as the last serum dilution with an absorbance value higher than 0.1.
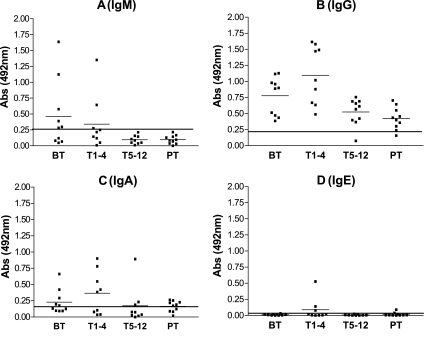
In order to determine the titers of the anti-Pb-1 antibodies and isotype expression during PCM treatment, the sera of the four patient groups were assayed by using specific anti-human IgG, IgM, IgA, and IgE antibodies. Anti-Pb-1 IgM antibodies were detected at levels above the cutoff value in 60% of the BT group and 20% of the T1-4 group (Fig. 4A). IgM is clearly the initial response to the Pb-1 antigen. The IgM titer decreased during the course of treatment, indicating IgM-IgG switching; no anti-Pb-1 IgM was detected in the T5-12 or PT group. On the other hand, IgG was the main class directed to the Pb-1 antigen in the four PCM groups; i.e., these antibodies were detected at levels above the cutoff vlaue in 100% of the BT and T1-4 groups and 90% of the T5-12 and PT groups (Fig. 4B). The level of expression of anti-Pb-1 IgA was found to be above the cutoff value in ∼50% of the BT and T1-4 groups (Fig. 4C). No statistically significant expression of anti-Pb-1 IgE was detected in the four PCM groups, although three patients in the T1-4 group, as well as two patients of the posttreatment group, presented absorbance values above the cutoff (Fig. 4D).
FIG. 4.
Expression of Ig directed to Pb-1 antigen in PCM patient sera. Purified Pb-1 (100 ng/well) was adsorbed on a 96-well plate and incubated with sera from the PCM patient groups, as defined in the legend to Fig. 2. The amount of bound antibody was determined by ELISA with biotinylated mouse anti-human Ig, as described in Materials and Methods. (A) IgM (μ chain); (B) IgG (γ chain); (C) IgA; (D) IgE. The short and the long horizontal lines are as defined in the legend to Fig. 2. Abs, absorbance.
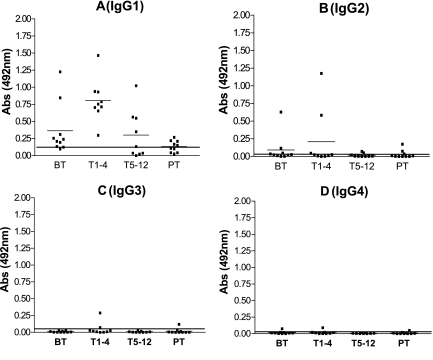
After the determination that IgG is the main class of anti-Pb-1 antibody, we investigated the reactive subclasses. Approximately 80% of the BT group and 100% of the T1-4 group expressed anti-Pb-1 IgG1 at levels above the cutoff value (Fig. 5A). The median absorbance at 492 nm was significantly higher in the T1-4 group than in the BT group (0.806 and 0.327, respectively; P = 0.0133). Lower levels of anti-Pb-1 IgG1, but levels above the cutoff, were detected in 50% of the T5-12 group, and weak reactivity with IgG1 was detected in 50% of the PT group. No statistically significant expression of IgG2, IgG3, or IgG4 directed to the Pb-1 antigen was detected in any of the groups of PCM patients (Fig. 5B to D). It is worth mentioning that one serum sample from the BT group and two serum samples from the T1-4 group showed titers of anti-Pb-1 IgG2 antigen above the cutoff, which probably compensated for the low levels of anti-Pb-1 IgG1 and IgM detected in these sera (Fig. 5B).
FIG. 5.
Expression of IgG subclass directed to Pb-1 antigen in PCM patient sera. Purified Pb-1 (100 ng/well) was adsorbed on a 96-well plate and incubated with sera from the PCM patient groups, as defined in the legend to Fig. 2. The amount of bound antibody was determined by ELISA with biotinylated mouse anti-human Ig, as described in Materials and Methods. (A) IgG1; (B) IgG2; (C) IgG3; (D) IgG4. The short and the long horizontal lines are as defined in the legend to Fig. 2. Abs, absorbance.
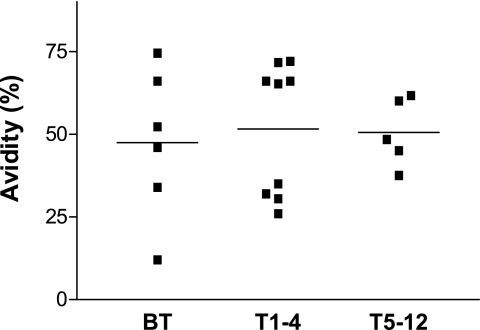
Sera from PCM patients were subjected to ELISA to test the capacity of anti-Pb-1 IgG antibodies to remain bound to the Pb-1 antigen (“avidity”) after the 96-well plates were washed with PBS containing 6.0 M urea, as described in Materials and Methods. The avidities of the antibodies to Pb-1 expressed in the sera of patients with the chronic form of PCM varied from 12 to 74% (mean, 50%) in the BT, T1-4, and T5-12 groups (Fig. 6). However, it should be noted that no statistically significant differences in the avidities of the anti-Pb-1 antibodies were observed among the four groups.
FIG. 6.
Avidity of anti-Pb-1 IgG antibody present in PCM patient sera. Sera from patients with chronic PCM were serially diluted and incubated in 96-well plates preadsorbed with 100 ng Pb-1. The avidities of the anti-Pb-1 antibodies, defined as the capacity to remain bound to the Pb-1 antigen in the presence of 6.0 M urea in PBS, were determined by ELISA. Antibody binding was determined with biotinylated anti-human IgG (γ chain) as the secondary antibody, as described in Materials and Methods. Bars, mean absorbance value for each group.
DISCUSSION
Among the GSLs expressed in P. brasiliensis, only Pb-1, a GIPC with a terminal Galf residue, is immunogenic for humans. Since Pb-2, the putative biosynthetic precursor of Pb-1, does not react with the sera of PCM patients and does not have a terminal Galf residue, the present data indicate that Galf is the essential residue that elicits the production of PCM serum antibodies. The Galf residue has also been shown to be immunogenic in other infectious diseases, such as Chagas' disease (8, 27), in which patients produce antibodies directed to the terminal Galf of the GIPCs of T. cruzi. However, the sera of patients with Chagas' disease do not cross-react with Pb-1, indicating that antibodies directed to the residues of beta-Galf expressed in patients with Chagas' disease and PCM present distinct specificities. Of all the heterologous serum samples tested (6 from patients with invasive candidiasis and 14 from patients with pulmonary aspergillosis), only 1 from a patient with candidiasis and 2 from patients with aspergillosis had positive reactivities. Toledo et al. (23) reported that sera from patients with histoplasmosis or Jorge Lobo's disease do not cross-react with the Pb-1 antigen. Therefore, Pb-1 appears to be a useful antigen for the immunodiagnosis of PCM. The ELISA with Pb-1 gave a sensitivity of 100% and a specificity of 96.1%. On the other hand, a lower specificity (about 90%) was observed when the P. brasiliensis somatic antigen was used in the immunodifusion test. For the ELISA with the Pb-1 antigen, the positive and the negative predictive values were 90% and 100%, respectively. Considering that the distributions of the antibody titers at the baseline and at the end of therapy were statistically different, there is also potential for the use of the ELISA not only for diagnosis but also as a surrogate marker of a good clinical response.
No differences in reactivities to GlcCer purified from P. brasiliensis between the sera from healthy donors and the sera from the PCM patients were detectable by ELISA. However, it should be noted that the cutoff for GlcCer was slightly higher than those for Pb-1 and Pb-2, suggesting that sera from healthy subjects may contain trace amounts of anti-GlcCer antibodies or antibodies that cross-react with fungal GlcCer. Our results are inconsistent with those of Rodrigues et al. (17), who detected antibodies directed to GlcCer of Cryptococcus neoformans, which is structurally identical to the GlcCer purified from P. brasiliensis, in the sera from patients with PCM or cryptococcal infection (24, 25). Even using a high concentration of GlcCer, i.e., 10 μg/well, we were not able to detect anti-GlcCer antibodies in the sera of patients with PCM by ELISA (data not shown). It was observed that the sera of patients with PCM present antibodies directed to Pb-1 but do not present antibodies directed to GlcCer.
P. brasiliensis GSL antigens, specifically, those that contain the Galf residue, elicit a primary IgM response and subsequently an IgG1 response. As patients begin antifungal treatment and develop a better immune response against P. brasiliensis, their titers of anti-Pb-1 IgG1 increase. After 5 months of treatment, as the clinical symptoms decline, the anti-Pb-1 antibody titers also decline. Similar patterns of immune response to gp43, the major exoantigen of P. brasiliensis, and to crude P. brasiliensis extract were observed in the sera of patients with chronic PCM (1, 10). Also, 3 of 10 patients in the T1-4 group showed values of IgE above the cutoff (Fig. 4D). Interestingly, Biselli et al. (2) described that IgE antibodies directed to gp43 are produced at high levels by most patients with the acute form of PCM, whereas anti-gp43 IgE antibody titers above the cutoff were detected in only 27% of patients with the chronic form of PCM.
The present study shows that antibodies directed to P. brasiliensis GSLs in patients with chronic PCM are of the IgG1 isotype and display a 50% avidity to the Pb-1 antigen. IgG1 may have an important role in fungal clearance, since Pb-1 is highly expressed on the yeast surface (22). P. brasiliensis coated with IgG1 could bind specifically to Fcγ receptors on the surfaces of phagocytes (monocytes and neutrophils), which remove pathogens and their degradation products from extracellular spaces. Furthermore, natural killer (NK) T cells have the ability to destroy antibody-coated target cells through the antibody-dependent cell-mediated cytotoxicity (ADCC) triggered by the interaction between IgG1 on the yeast surface and the FcγRIII (CD16) expressed on NK cells (13). This receptor recognizes IgG1 and IgG3 antibodies and is involved in the release of perforin and granzymes from cytoplasmic granules. ADCC is well documented as a mechanism for antigen-specific attack by an effector cell; however, such an attack by an effector cell that itself has a role in defense against fungal infection has not been fully established.
These results provide an important advance in our knowledge of the immune responses in patients with PCM, particularly those against GSL antigens. GIPCs with terminal galactofuranose residues have clear potential application as elicitors of the host immune response.
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Pesquisa (CNPq).
We thank Stephen Anderson for editing of the manuscript.
Footnotes
Published ahead of print on 29 November 2006.
REFERENCES
- 1.Baida, H., P. J. C. Biselli, M. Juvenale, G. J. S. Del Negro, M. J. S. Mendes-Giannini, A. J. S. Duarte, and G. Benard. 1999. Differential antibody isotype expression to the major Paracoccidioides brasiliensis antigen in juvenile and adult form paracoccidioidomycosis. Microbes Infect. 1:273-278. [DOI] [PubMed] [Google Scholar]
- 2.Biselli, P. J. C., M. Juvenale, M. J. S. Mendes-Giannini, A. J. S. Durte, and G. Benard. 2001. IgE antibody response to the main component of Paracoccidioides brasiliensis in patient with paracoccidioidomycosis. Med. Mycol. 39:475-478. [DOI] [PubMed] [Google Scholar]
- 3.Brummer, E., E. Castaneda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Camargo, Z. P., and M. F. Franco. 2000. Current knowledge on pathogenesis and immunodiagnosis of paracoccidioidomycosis. Rev. Iberoam. Micol. 17:41-48. [PubMed] [Google Scholar]
- 5.De Camargo, Z. P., C. Unterkircher, S. P. Campoy, and L. R. Travassos. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion test. J. Clin. Microbiol. 26:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Camargo, Z. P., C. Unterkicher, and L. R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 27:407-412. [PubMed] [Google Scholar]
- 7.Ferreira-da-Cruz, M. F., A. C. Francesconi-do-Vale, M. C. D. Espinera, B. Wanke, and B. Galvão-Castro. 1990. Study of antibodies in paracoccidioidomycosis: follow-up of patients during and after treatment. J. Med. Vet. Mycol. 28:151-157. [DOI] [PubMed] [Google Scholar]
- 8.Golgher, D. B., W. Colli, T. Souto-Padron, and B. Zingales. 1993. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 60:249-264. [DOI] [PubMed] [Google Scholar]
- 9.Jenum, P. A., B. Stray-Pedersen, and A.-G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juvenale, M., G. M. B. Del Negro, A. J. S. Duarte, and G. Benard. 2001. Antibody isotypes to a Paracoccidioides brasiliesnis somatic antigen in sub-acute and chronic form paracoccidioidomycosis. J. Med. Microbiol. 50:127-134. [DOI] [PubMed] [Google Scholar]
- 11.Levery, S. B., M. S. Toledo, A. H. Straus, and H. K. Takahashi. 1998. Structure elucidation of sphingolipids from the mycopathogen Paracoccidioides brasiliensis: an immunodominant β-galactofuranose residue is carried by a new glycosylinositol phosphorylceramide antigen. Biochemistry 37:8764-8775. [DOI] [PubMed] [Google Scholar]
- 12.Levery, S. B., M. S. Toledo, E. Suzuki, M. E. K. Salyan, S. Hakomori, A. H. Straus, and H. K. Takahashi. 1996. Structural characterization of a new galactofuranose-containing glycolipid antigen of Paracoccidioides brasiliensis. Biochem. Biophys. Res. Commun. 222:639-645. [DOI] [PubMed] [Google Scholar]
- 13.Nabavi, N., and J. W. Murphy. 1986. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neofarmans. Infect. Immun. 51:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz, B. L., A. M. Garcia, A. Restrepo, and J. G. McEwen. 1996. Immunological characterization of a recombinant 27-kilodalton antigenic protein from Paracoccidioides brasiliensis. Clin. Diagn. Lab. Immunol. 3:239-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puccia, R., and L. R. Travassos. 1991. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidiomycosis, histoplasmosis, or Jorge-Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restrepo, A., J. G. McEwen, and E. Castañeda. 2001. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med. Mycol. 39:233-241. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rosental, W. de Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San-Blas, G., G. Niño-Vega, and T. Iturriaga. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40:225-242. [DOI] [PubMed] [Google Scholar]
- 19.Shiganai-Yasuda, M. A., F. de Q. Telles Filho, R. P. Mendes, A. L. Colombo, and M. L. Moretti. 2006. Guidelines in paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 39:297-310. [DOI] [PubMed] [Google Scholar]
- 20.Straus, A. H., E. Freymuller, L. R. Travassos, and H. K. Takahashi. 1996. Immunochemical and subcellular localization of the 43 kDa glycoprotein antigen of Paracoccidioides brasiliensis with monoclonal antibodies. J. Med. Vet. Mycol. 34:181-186. [DOI] [PubMed] [Google Scholar]
- 21.Straus, A. H., S. B. Levery, M. G. Jasiulionis, M. E. K. Salyan, S. J. Steele, L. R. Travassos, S. Hakomori, and H. K. Takahashi. 1993. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J. Biol. Chem. 268:13723-13730. [PubMed] [Google Scholar]
- 22.Suzuki, E., M. S. Toledo, H. K. Takahashi, and A. H. Straus. 1997. A monoclonal antibody directed to terminal residue of β-galactofuranose of a glycolipid antigen isolated from Paracoccidioides brasiliensis: cross-reactivity with Leishmania major and Trypanosoma cruzi. Glycobiology 7:463-468. [DOI] [PubMed] [Google Scholar]
- 23.Toledo, M. S., E. Suzuki, A. H. Straus, and H. K. Takahashi. 1995. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J. Med. Vet. Mycol. 33:247-251. [DOI] [PubMed] [Google Scholar]
- 24.Toledo, M. S., S. B. Levery, A. H. Straus, E. Suzuki, M. Momamy, J. Glushka, J. M. Moulton, and H. K. Takahashi. 1999. Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Δ3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 38:7294-7306. [DOI] [PubMed] [Google Scholar]
- 25.Toledo, M. S., E. Suzuki, S. B. Levery, A. H. Straus, and H. K. Takahashi. 2001. Characterization of monoclonal antibody MEST-2 specific to glucosylceramide of fungi and plants. Glycobiology 11:105-112. [DOI] [PubMed] [Google Scholar]
- 26.Travassos, L. R. 1994. Immunochemistry of Paracoccidioides brasiliensis, p. 67-86. In M. F. Franco, C. S. Lacaz, A. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Inc., Boca Raton, FL.
- 27.Zingales, B., D. B. Golgher, P. G. Marmorato, R. P. Souto, and A. Gruber. 1993. Molecular approaches to diagnosis of Chagas disease: use of defined antigens and a target ribosomal RNA sequence. Biol. Res. 26:89-100. [PubMed] [Google Scholar]