Abstract
Thottapalayam virus (TPMV), a member of the genus Hantavirus in the family Bunyaviridae, was isolated from an insectivore, Suncus murinus (musk shrew), captured in southern India in 1964. While the isolation of TPMV predates the discovery of the prototype Hantaan virus, little is known about its genetics and biology. To date, preliminary evidence suggests that TPMV differs significantly, both antigenically and genetically, from all known rodent-borne hantaviruses. However, since detailed epizootiological studies have not been conducted, it is unclear if TPMV is naturally harbored by an insectivore host or if TPMV represents a “spillover” from its natural rodent reservoir host. Moreover, to what extent TPMV causes infection and/or disease in humans is not known. To address these issues, we first studied the antigenic profile of TPMV using monoclonal antibodies against Hantaan and Seoul viruses and polyclonal immune sera against Puumala virus and TPMV. Armed with this newfound information, we developed an enzyme-linked immunosorbent assay system for the diagnosis of TPMV infections in shrews and humans, using a recombinant TPMV N antigen manipulated to have an E5/G6 epitope to be captured by monoclonal antibody clone E5/G6. Using this assay, we found anti-TPMV antibodies in sera from a patient with high fever of unknown etiology in Thailand and from two shrews captured in Indonesia. Seropositivity was verified by the indirect immunofluorescence antibody test, Western blotting analysis, and focus reduction neutralization test. Collectively, our data indicate that TPMV is harbored by Suncus murinus as its host in nature and is capable of infecting humans.
Like other viruses in the family Bunyaviridae, members of the genus Hantavirus are enveloped viruses with a tripartite, negative-stranded RNA genome, consisting of large (L), medium (M), and small (S) segments. The L segment encodes an RNA-dependent RNA polymerase; the M segment encodes a glycoprotein precursor, which is cleaved into surface glycoproteins, Gn and Gc; and the S segment encodes a nucleocapsid protein (N) (15). Some hantaviruses cause zoonotic diseases in humans, known as hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome (HPS) (14). Presently, 22 species are classified within the genus Hantavirus based on antigenic and genetic differences (9). In the Old World, four antigenically related and genetically distinct hantaviruses are known to cause hemorrhagic fever with renal syndrome: Hantaan virus (HTNV), Seoul virus (SEOV), Puumala virus (PUUV), and Dobrava virus (DOBV). Several sigmodontine rodent-borne hantaviruses in the New World, including Sin Nombre virus (SNV) and Andes virus, cause HPS. For both diseases, virus transmission to humans occurs via aerosolization of infectious rodent excreta (6).
Each hantavirus appears to have coevolved with a specific rodent species, in which it maintains an enzootic cycle. As the only known presumed exception, Thottapalayam virus (TPMV) was isolated from an insectivore, Suncus murinus (musk shrew) captured in southern India in 1964 (3). Either very low or no antigenic cross-reactivity has been observed between TPMV and other hantaviruses (4, 5). And as evidenced by nucleotide and amino acid sequence analyses of the full-length S segment, TPMV is the most genetically divergent of all other hantaviruses (6, 17). Analyses of the recently acquired full-length M and L segments of TPMV are congruent (J.-W. Song and R. Yanagihara, unpublished observations). However, since detailed epizootiological and epidemiological surveys of TPMV infection have not been conducted, the fundamental biology of TPMV, including its true natural host and pathogenicity to humans, is unclear.
Previously, we have developed enzyme immunoassays using baculovirus-expressed recombinant N (rN) antigens of various hantaviruses (including HTNV, SEOV, PUUV, and DOBV) for the serological diagnosis of hantavirus infections (1, 7, 8, 18). With this method, the monoclonal antibody (MAb) clone E5/G6 is utilized as an effective capture antibody, since it binds to a linear epitope of the N protein among all hantaviruses (11, 18). Thus, after determining the antigenic profile of TPMV, we developed a robust serological assay to diagnose TPMV infections in animals and humans, using the TPMV rN antigen manipulated to contain specific amino acid substitutions to allow binding with MAb E5/G6. Using this assay, we detected anti-TPMV antibodies in a human with febrile illness and in two musk shrews. These results indicate that TPMV is carried by musk shrews in nature and is capable of causing infections in humans.
MATERIALS AND METHODS
Viruses and cells.
The prototype VRC-66412 strain of TPMV, originally isolated in suckling mice (3) and subsequently adapted to growth in the E6 clone of Vero cells (CRL 1586; American Type Culture Collection), was used. HTNV strain 76-118, SEOV strain SR-11, and PUUV strain CG1820 were used as representative rodent-borne hantaviruses. Viruses were propagated in Vero E6 cells maintained in Eagle's minimal essential medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% nonessential amino acids (Gibco). High Five cells (Invitrogen, Carlsbad, CA) were maintained in Grace's insect growth medium (Gibco) supplemented with 10% fetal bovine serum. Recombinant baculoviruses of HTNV, PUUV, and SNV were prepared as described previously (1).
MAbs and immune sera.
Monoclonal antibodies (MAbs) and immune rabbit sera for N of HTNV and SEOV and MAbs to Gn and Gc of HTNV, as described previously, were used (2, 18). Immune rabbit serum for PUUV N was kindly provided by Hiroaki Kariwa of the Graduate School of Veterinary Medicine, Hokkaido University. Immune rabbit serum to TPMV N was prepared by intradermal injections of an 11-week-old Std:JW/CSK rabbit (specific-pathogen-free rabbit; SLC, Shizuoka, Japan) with 350 μg of TPMV rN expressed in Escherichia coli and 500 μg of Freund's complete adjuvant. A booster immunization of the same antigen with Freund's incomplete adjuvant was administered at 24 days, and blood was collected at 58 days. Immune mouse sera to TPMV were obtained 4 weeks following intraperitoneal inoculation of BALB/c mice (CLEA Japan, Osaka, Japan) with 2.0 × 103 focus-forming units of native TPMV (indirect immunofluorescence antibody [IFA] titer against TPMV was 1:12,800). Finally, sera were obtained from shrews (CLEA Japan) inoculated subcutaneously with 5.2 × 104 focus-forming units of native TPMV at 40 days postinoculation.
Human patient and wild shrew sera.
Of the 478 human sera available for testing, 284 were collected between 2003 and 2004 from patients in Surin Province who had leptospirosis-like symptoms but who were serologically negative for both Leptospira and dengue virus. The other 194 sera were collected from patients with febrile illnesses of unknown etiology as part of the Emerging and Re-emerging Infectious Diseases collaborative project, conducted by the Thai National Institute of Health and the Japan International Cooperation Agency in Nongkhai Province in 2005. In addition, sera were collected from 14 wild shrews (Suncus murinus) captured in Thousand Islands, Indonesia, in July and October 2005.
Preparation of recombinant TPMV N antigen.
Culture supernatant of TPMV-infected Vero E6 cells was ultracentrifuged (265,000 × g, 4 h, 4°C), and RNA was isolated from the viral pellet and dissolved with Isogen (Invitrogen) following the manufacturer's instructions. Reverse transcription-PCR was performed using the KOD-plus system (Toyobo, Tokyo, Japan) to amplify the entire TPMV N-coding S segment with primers 5′-TTCAG AATTC GATGA CTCAA GGGAA AATGA CTCCC GAAGA-3′ and 5′-TATCC TCGAG TTACA GTTTA ATAGG CTCCT GACTT GAAAT C-3′ (the EcoRI and XhoI sites are shown in italics). After amplification, the DNA fractions were subcloned into the pET-43b(+) vector using restriction enzymes that recognized the restriction sites added by PCR and transformed into E. coli strain Origami (Invitrogen). A single colony was inoculated into Circle growth medium (BIO101 systems, Carlsbad, CA) containing tetracycline, kanamycin, and ampicillin for small-scale culture incubation at 37°C overnight. The culture fluid was then centrifuged, the collected cells were inoculated into 100 ml of fresh medium, and isopropyl-β-d-thiogalactopyranoside induction was performed according to the procedure for pET system expression. The cultured cells were collected by centrifugation, resuspended in 5 ml of 0.5 M NaCl binding buffer (0.5 M NaCl, 20 mM imidazole, 20 mM potassium phosphate), and sonicated four times for 15 s each on ice. Thereafter, the fusion protein was purified using a His trap column (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions.
IFA test.
An IFA test was performed using previously described procedures (18). Acetone-fixed smears of Vero E6 cells infected with hantavirus or High Five cells infected with recombinant baculovirus were used as antigens. Alexa Fluor 488 goat anti-mouse immunoglobulin G (IgG) (heavy plus light chains) antibody (1:2,000; Molecular Probes, Eugene, OR) was used as a secondary antibody to MAbs. For rabbit and human sera, fluorescein isothiocyanate-conjugated protein A (1:2,000; Sigma, St. Louis, MO) was used. IFA titers were expressed as the reciprocal of the highest serum dilution that produced characteristic intracytoplasmic fluorescence.
Peptide synthesis and antigenic analysis.
Peptides were synthesized and analyzed by previously published methods (11). Briefly, using an Autospot ASP222 peptide synthesizer (ABiMED, Langenfeld, Germany), a variety of 10-mer peptides were spotted on a membrane. The spotting membrane was blocked in Block Ace (Yukijirushi Co., Tokyo, Japan) for 30 min at room temperature, stained with an E5/G6 hybridoma culture supernatant for 60 min, and detected using horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (1:500; Zymed, South San Francisco, CA) and 3-amino-9-ethylcarbazole (Sigma).
Construction of recombinant baculovirus expressing TPMV N with an E5/G6 epitope.
The subcloned DNA fragment was excised from pET-43(+), described above, by digestion with the same enzyme and inserted in the donor plasmid pFAST-BAC1 (Gibco). Based on the results of the E5/G6 epitope analysis of TPMV N, amino acid-altering nucleotide mutations required for E5/G6 binding were added, using the GeneTailor site-directed mutagenesis system (Invitrogen). TPMV wild-type rN (rN/wt) and TPMV rN with the E5/G6 epitope (rN/E5G6) were expressed using the Bac-to-Bac baculovirus expression system according to the manufacturer's instructions (Gibco). These baculoviruses were inoculated into High Five cells to acquire the rN antigen, using previously described methods (1).
Western blotting analysis.
Western blotting was performed using previously published methods (19). The infected High Five and Vero E6 cells were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (ATTO, Tokyo, Japan). Immune rabbit serum to hantavirus N was used to detect antigen on the membrane. Binding antibodies were detected using HRP-conjugated protein A (Prozyme, San Leandro, CA), and 4-chloro-1-naphthol (Sigma) was used as the peroxidase substrate.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) using whole HTNV, PUUV, SNV, and TPMV rN were performed according to previously described methods (1, 7, 8, 18). MAb E5/G6 (2 μg/ml) was used as a capture antibody to coat 96-well plates for 60 min at 37°C. Nonspecific binding was blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS). After a 60-min incubation, the plates were washed three times with PBS containing 0.05% Tween 20. Each antigen was added and incubated for 60 min at 37°C, followed by three washings. For detection of rabbit IgG, HRP-conjugated goat anti-rabbit IgG antibody (1:5,000; Jackson, Bar Harbor, ME) was used as the secondary antibody, and o-phenylenediamine (Sigma) was added as the peroxidase substrate. Absorbance at 450 nm was measured using a SpectraMax 340 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). For detection of human IgG, alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific) antibody (1:2,000; Sigma) was used as the secondary antibody and p-nitrophenyl phosphate (Sigma) was added as the substrate before measuring the absorbance at 405 nm. For detection of shrew IgG, HRP-conjugated protein A (1:5,000; Prozyme) was used as the secondary antibody and o-phenylenediamine was added as the peroxidase substrate.
FRNT.
Endpoint titers of neutralizing antibodies were determined by the focus reduction neutralization test (FRNT), as described elsewhere (1). Foci of virus-infected cells were detected by staining the cells with Alexa Fluor 488-labeled MAb 5B7, which recognizes the Gc of hantaviruses (10). FRNT titers were expressed as the reciprocal of the highest serum dilution leading to a greater than 80% reduction in the number of infected cell foci.
RESULTS
Antigenic profiling of TPMV using MAbs and polyclonal antibodies.
To characterize the TPMV antigenic profile, we performed the IFA test using a panel of MAbs against HTNV N, Gn, and Gc and SEOV N (Table 1). None of the MAbs against HTNV N and Gn cross-reacted with TPMV, while 8 of 16 MAbs against HTNV Gc did. By contrast, all other hantaviruses exhibited various degrees of cross-reactivity to MAbs against N and Gn, except for Prospect Hill virus, which did not react with MAbs against HTNV Gn. TPMV seemed to share partly common epitopes in the Gc region but not in the N or Gn regions, although all other rodent-borne hantaviruses had some common epitopes in each region.
TABLE 1.
Reactivities of MAbs to TPMV and rodent-borne hantaviruses
| Specificity | Epitope | MAb | Reactivity of MAb toa,b:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPMV | HTNV | SEOV | THAIV | DOBV | SaaV | PUUV | PHV | SNV | |||
| N (HTNV) | FDO3 | ± | + | + | + | + | + | + | + | NT | |
| KAO6 | − | + | + | + | + | + | − | − | NT | ||
| ECO2 | − | + | + | + | + | + | − | − | − | ||
| ECO1 | − | + | + | + | + | + | + | − | − | ||
| GBO4 | − | + | + | + | + | + | + | + | NT | ||
| E5G6 | − | + | + | + | + | + | + | + | + | ||
| C16D11 | − | + | + | + | + | + | + | + | − | ||
| F23A1 | − | + | + | + | + | + | − | + | + | ||
| C24B4 | − | + | − | − | ± | ± | − | − | − | ||
| BDO1 | − | + | − | − | − | − | − | − | − | ||
| G5 | − | + | − | − | − | − | − | − | + | ||
| N (SEOV) | DCO3 | − | − | + | − | − | − | − | − | − | |
| Gn/Gc (HTNV) | Gn-a | 8B6 | − | + | ± | + | ± | + | ± | − | NT |
| 6D4 | − | + | + | + | + | + | − | − | NT | ||
| 10F11 | − | + | + | + | + | + | + | − | NT | ||
| Gn-b | 2D5 | − | + | − | − | − | − | − | − | NT | |
| 3D5 | − | + | − | − | − | − | − | − | NT | ||
| 16D2 | − | + | − | − | − | − | − | − | NT | ||
| Gc-a | HCO2 | − | + | + | + | − | − | − | − | NT | |
| 16E6 | ± | + | + | + | + | + | ± | − | NT | ||
| Gc-b | EBO6 | − | + | + | − | ± | ± | − | − | NT | |
| Gc-c | 11E10 | − | + | ± | − | + | − | + | + | NT | |
| Gc-e | 17G6 | + | + | ± | + | + | + | + | ± | NT | |
| 3D7 | + | + | + | + | + | + | + | ± | NT | ||
| 5B7 | + | + | + | + | + | + | + | ± | NT | ||
| Gc-e | 20D3 | + | + | ± | + | + | + | − | − | NT | |
| Gc-f1 | 8E10 | + | + | + | + | + | + | + | ± | NT | |
| 1C6 | + | + | + | + | + | + | + | ± | NT | ||
| 1G8 | − | + | + | + | + | + | + | ± | NT | ||
| 23G10-2 | + | + | + | + | + | + | + | ± | NT | ||
| 3B6 | ± | + | + | + | + | + | + | ± | NT | ||
| Gc-f2 | 23G10-1 | + | + | + | + | + | + | − | − | NT | |
| 7G6 | − | + | + | + | + | + | − | − | NT | ||
| 18F5 | − | + | ± | + | + | + | − | − | NT | ||
TPMV, Thottapalayam virus; HTNV, Hantaan virus; SEOV, Seoul virus; THAIV, Thailand virus; DOBV, Dobrava-Belgrade virus; SaaV, Saaremaa strain of DOBV; PUUV, Puumala virus; PHV, Prospect Hill virus; SNV, Sin Nombre virus; NT, not tested.
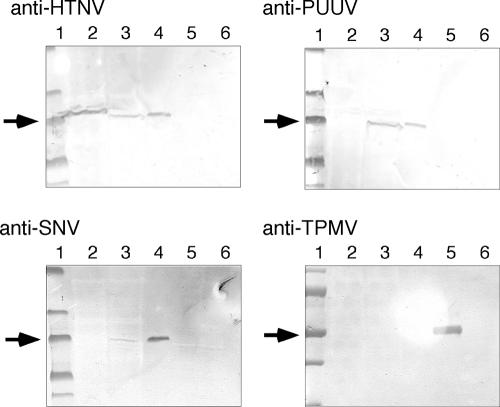
We next immunized a rabbit with TPMV rN expressed in E. coli and obtained a polyclonal immune serum with an IFA titer of 1:6,400, which strongly reacted also to reduced-TPMV antigens by Western blotting analysis (Fig. 1). Using this immune serum, however, TPMV did not cross-react with other hantaviruses (Fig. 1), suggesting that TPMV was the most antigenically divergent of all hantaviruses isolated to date.
FIG. 1.
Western blotting analysis of hantavirus antigens using polyclonal rabbit immune sera. We tested the cross-reactivity of each hantavirus, including TPMV, using sera from rabbits immunized with rN antigens expressed in E. coli. For HTNV, PUUV, and TPMV antigens, viruses were inoculated on Vero E6 cells, harvested, dissolved, and used. For SNV antigen, High Five cells expressing SNV rN by use of recombinant baculovirus were used (7). Lanes 1, molecular weight marker; lanes 2, HTNV; lanes 3, PUUV; lanes 4, SNV; lanes 5, TPMV; lanes 6, uninfected Vero E6 cells. Arrows indicate the band at 50 kDa.
Epitope analysis and construction of TPMV rN possessing the E5/G6 epitope.
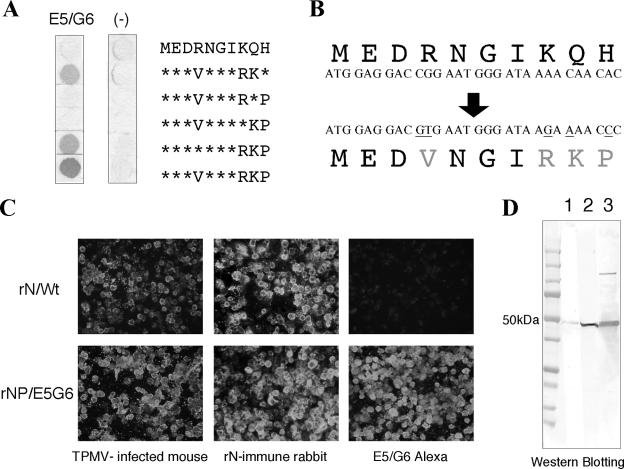
We have developed an ELISA system for diagnosing hantavirus infections with excellent specificity and sensitivity (1, 7, 8, 18). In this assay, baculovirus-expressed recombinant hantavirus antigen is captured on 96-well plates coated with MAb E5/G6 (18). Although there are several amino acid variations, the N protein of all 21 rodent-borne hantavirus species reacted with MAb E5/G6 (11). Therefore, MAb E5/G6 is an effective tool for capturing hantavirus N, with the only exception being TPMV (Table 1). Accordingly, to use this assay for TPMV, we manipulated the E5/G6 epitope region of TPMV rN to allow binding with MAb E5/G6. The MAb E5/G6 made by immunizing mice with HTNV rN reacted effectively with the sequence YEDVNGIRKP at 165 to 174 amino acids (11, 18). However, TPMV has the sequence MEDRNGIKQH at 175 to 184 amino acids for the corresponding E5/G6 epitope region and did not react with MAb E5/G6 (Table 1). Using a peptide synthesizer, we synthesized this 10-mer peptide and confirmed the effect of some amino acid mutations on MAb E5/G6. As a result, the peptide MEDVNGIRKP with four changes (R to V, K to R, Q to K, and H to P) reacted with MAb E5/G6 (Fig. 2A). Based on the E5/G6 epitope analysis, we inserted five nucleotide mutations in the TPMV S segment to produce the four amino acid changes (Fig. 2B) and prepared recombinant baculoviruses expressing TPMV rN with the E5/G6 epitope.
FIG. 2.
A. E5/G6 epitope analysis of TPMV N. Using a variety of synthesized 10-mer peptides, we confirmed E5/G6 reactivity against TPMV sequence. Further, we determined which amino acid changes in this region were essential for E5/G6 binding. The peptide changes at positions 178 (R→V), 182 (K→R), 183 (Q→K), and 184 (H→P) were sufficient for MAb E5/G6 binding. B. Insertion of several amino acid mutations changing E5/G6 binding. C. Confirmation of the antigenicitiy of each baculovirus-infected High Five cell antigens expressed by recombinant baculoviruses. The rN antigen having the original sequence (rN/wt) reacted with immune serum but not with MAb E5/G6. On the other hand, the rN with the E5/G6 epitope (rN/E5G6) reacted with immune serum, as well as MAb E5/G6. D. Western blotting analysis using sera from rabbits immunized with E. coli-expressed rN antigens. Both rN antigens (rN/wt and rN/E5G6) were detected by a band of about 50 kDa, which corresponded to authentic TPMV N. Lane 1, rN/wt; lane 2, rN/E5G6; lane 3, TPMV-infected Vero E6 cells.
High Five cells inoculated with the recombinant baculoviruses were harvested, and the antigenicities of TPMV rN/wt and TPMV rN/E5G6 were confirmed by the IFA test (Fig. 2C). Both rNs reacted with TPMV-infected mouse sera and rN-immune rabbit sera. But only rN/E5G6 reacted with MAb E5/G6, as expected. In addition, we confirmed the antigenicity of rN/wt and rN/E5G6 by Western blotting analysis using rN-immune rabbit sera (Fig. 2D). These data show that both TPMV rNs have the same band of approximately 50 kDa, which is the size of TPMV N, as well as TPMV-infected Vero E6 cell antigen. So, we succeeded in producing TPMV rN with the E5/G6 epitope, which has the antigenicity of TPMV as well as reactivity with MAb E5/G6.
Developing the E5/G6 capture ELISA system for TPMV.
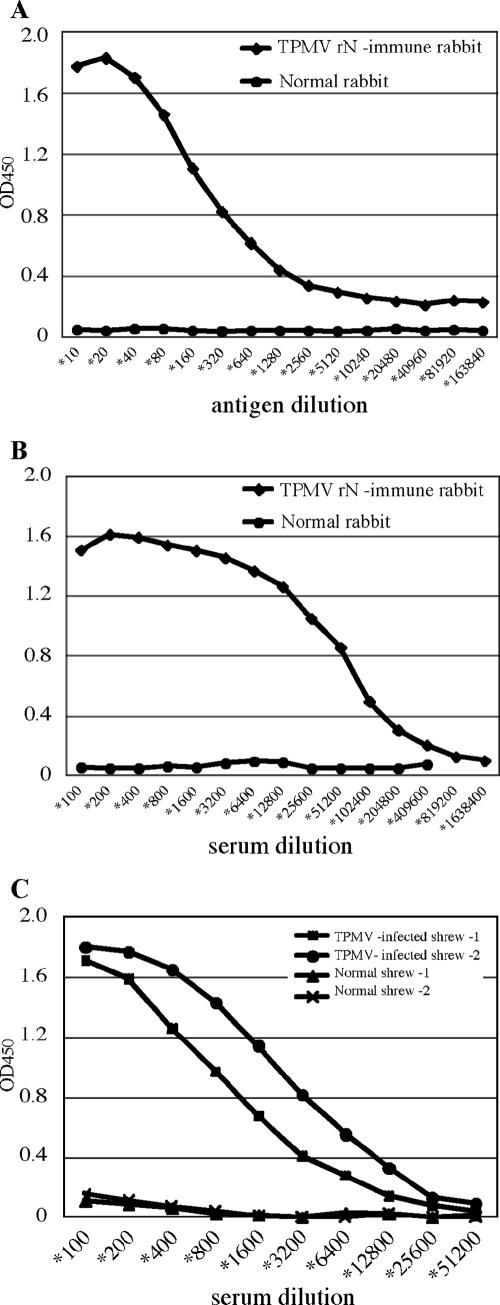
Using the TPMV rN/E5G6 antigen, we developed an IgG antibody-detecting capture ELISA system, according to methods described previously (1, 7, 8, 18). Figure 3A shows the results of an ELISA with twofold dilutions of TPMV rN/E5G6 to a constant dilution of rN-immune rabbit sera (1:200 dilution), and Fig. 3B shows the results of an ELISA with a constant amount of TPMV rN/E5G6 antigen (1:40 dilution) to serial twofold dilutions of rN-immune rabbit sera. The ELISA assay system using the TPMV rN/E5G6 antigen detected anti-TPMV antibodies with high sensitivity. Results with sera from shrews experimentally infected with TPMV also supported the sensitivity of this assay system (Fig. 3C).
FIG. 3.
A. To determine a suitable dilution of the antigen for the ELISA, we tested the reactivities of serial twofold dilutions of rN/E5G6 to a constant amount (1:200 dilution) of antibodies from TPMV rN-immune rabbit. The 1:20 to 1:40 dilution seemed to be appropriate. B. The results of ELISA using a constant amount of rN/E5G6 antigen (1:40) to twofold dilutions of the immune rabbit serum. TPMV antibodies could be detected at serum dilutions at or exceeding 1:200,000. C. Detection of antibodies against TPMV in sera from shrews experimentally infected with TPMV.
In addition, we compared the antigenic cross-reactivities of TPMV and other hantaviruses using this ELISA system (Table 2). In the reactions with each homologous combination, the optical density value was remarkably high. Although the heterologous combinations showed a variety of reactivities, according to the antigenic similarity between viruses, TPMV antigen did not cross-react with other antihantavirus antibodies. This result indicated that rN/E5G6 is a useful tool for the specific detection of anti-TPMV antibodies.
TABLE 2.
Cross-reactivities in capture ELISA among TPMV and representative disease-causing hantaviruses
| Source of antigen | Cross-reactivity of immune rabbit serum to:
|
|||
|---|---|---|---|---|
| HTNV | PUUV | SNV | TPMV | |
| HTNV | 0.781 | 0.453 | 0.037 | 0.015 |
| PUUV | 0.671 | 1.487 | 0.669 | 0.000 |
| SNV | 0.614 | 1.362 | 1.672 | 0.036 |
| TPMV | 0.011 | 0.007 | 0.002 | 1.578 |
Serological survey of TPMV infection among febrile patients in Thailand.
Employing the newly developed capture ELISA system, we tested 478 serum samples from patients with fever in Thailand who were serologically negative for leptospirosis and dengue fever. Each serum was tested with whole HTNV, PUUV, SNV, and TPMV rN for serotyping (Fig. 4A). Serum samples no. B-3 and no. B-4, which were from the same patient during different phases of illness, were positive for anti-TPMV IgG antibodies. Sera from seven other cases were weakly positive to HTNV (data not shown). Sera no. B-3 and no. B-4 also reacted with TPMV-infected Vero E6 cells by the IFA test and Western blotting analysis (Fig. 5A and B) and by FRNT using native TPMV (Table 3). However, virus-specific IgM was not detected (data not shown). Therefore, this patient may have been infected with TPMV previously, although it is not clear if he had shown symptoms.
FIG. 4.
Serological surveys for TPMV infection in Southeast Asia. We examined 478 sera from patients with fever in Thailand and found two sera (B-3 and B-4 from the same patient at different phases of illness) with anti-TPMV IgG antibodies. Results of the ELISA are shown in panel A. Sera B-3 and B-4 reacted with TPMV antigen. HTNV, PUUV, and HPS patient immune sera served as positive controls. In addition, we tested sera from 14 wild shrews (Suncus murinus) captured in Indonesia in 2005 (B). Serum no. 69 was positive for anti-TPMV IgG antibodies, while sera no. 2, 49, and 79 were weakly positive.
FIG. 5.
A. Results of IFA test using TPMV-infected Vero E6 cell antigens. As a positive shrew serum control, serum from a shrew experimentally infected with TPMV was used. Sera no. 49 and 69 were positive against TPMV antigen. But sera no. 2 and 79 were negative by the IFA test (data not shown). B. Western blotting analysis of TPMV-positive sera with TPMV antigen. The human positive sera B-3 and B-4 in ELISAs also reacted with TPMV-infected Vero E6 antigen by Western blotting analysis. On the other hand, for shrews, only no. 49 and 69 showed a band at 50 kDa with TPMV antigen, and sera no. 2 and 79 did not. Lanes 1 (B-3) and 2 (B-4) are human positive samples in ELISA; lane 3 (no. 2), lane 4 (no. 49), lane 5 (no. 69), and lane 6 (no. 79) are shrew positive samples in ELISA; lane 7 (no. 2 serum from a shrew experimentally infected with TPMV) is a positive control. Lane 6 (normal shrew serum) is a negative control.
TABLE 3.
FRNT with native TPMV in human and shrew seraa
| Serum no. | FRNT titer |
|---|---|
| Human | |
| B-3 | 40 |
| B-4 | 80 |
| (−) | <40 |
| Shrew | |
| 49 | 80 |
| 69 | <40 |
| (+) | 320 |
| (−) | <40 |
Human (−) is a serum from a healthy individual as a negative control. Shrew (+) is a serum from a shrew experimentally infected with TPMV as a positive control, and (−) is no. 2 serum from a normal uninfected shrew.
This anti-TPMV-antibody-positive patient was a 58-year-old Laotian male who fell ill in Laos and came to a hospital in Nongkhai Province, along the border of Thailand and Laos, in April 2005. He presented with high fever, chills, headache, cough, sore throat, vomiting, diarrhea, abdominal pain, and exhaustion. The patient recovered fully after being hospitalized for several weeks. However, these symptoms were not necessarily related to TPMV infection directly, because he lacked IgM against TPMV. Unfortunately, no information is available about his occupation or his exposure to shrews or wildlife.
Serological survey of TPMV infection in wild shrews captured in Indonesia.
Of sera collected from 14 shrews captured in Indonesia in 2005, one (no. 69) was positive for anti-TPMV IgG antibodies by ELISA. Sera from three other shrews (no. 2, 49, and 79) were weakly positive by ELISA (Fig. 4B). By contrast, in the IFA test using TPMV-infected Vero E6 cells as antigen, sera no. 49 and 69 were positive (Fig. 5A), whereas sera no. 2 and 79 were negative. Sera no. 49 and 69 were also positive by Western blotting analysis using TPMV-infected Vero E6 cell antigens (Fig. 5B). Only no. 49 was positive by FRNT (Table 3).
DISCUSSION
Long unclassified, TPMV is now known to be a member of the genus Hantavirus. Surprisingly little is known about TPMV, however, despite the fact that its isolation predates that of Hantaan virus. For example, until very recently sequences of the full-length S-, M- and L-genomic segments of TPMV were not known. Also, although TPMV was isolated from tissues of a musk shrew, the identity of its natural reservoir host has remained shrouded in some uncertainty, with some believing that TPMV must represent spillover from a rodent host. The dearth of information about TPMV can largely be attributed to the lack of systematic, well-designed studies focusing on its epizootiology and pathogenicity. One of the barriers to conducting such studies has been the unavailability of highly sensitive and specific serological assays.
To address this limitation, we first compared the antigenic profile of TPMV with those of representative hantaviruses, which segregate into three groups according to the subfamilies of their rodent reservoir hosts: that is, Murinae-, Arvicolinae- and Sigmodontinae-associated hantaviruses (13, 16). Viruses in each group have antigenic properties similar to each other's (5, 7). As determined by the IFA test using MAb and polyclonal immune sera, TPMV had the most divergent antigenic profile among hantaviruses, which conforms to data from an earlier report using the plaque reduction neutralization test (5). Moreover, immune serum, prepared by inoculating BALB/c mice with TPMV, had a high IFA titer against TPMV of 1:12,800. However, in Western blotting analysis, the mouse immune serum did not detect TPMV antigen in TPMV-infected Vero E6 cell lysates or in TPMV rN antigen prepared with E. coli, whereas other hantavirus N proteins were detected by mouse serum immunized with the respective hantavirus (data not shown). These data suggest that TPMV induces either no or very low levels of linear epitope-recognizing antibodies in mice. The antigenic difference of TPMV N from that of other hantaviruses indicated a requirement for a new ELISA system for the serological diagnosis of TPMV infection.
We have developed an E5/G6 capture ELISA system which has excellent specificity and sensitivity profiles for the diagnosis of hantavirus infection (1, 7, 8, 18). In this ELISA system, each rN antigen is captured in wells coated with MAb E5/G6. Since TPMV seemed to have no affinity to MAb E5/G6, we inserted several amino acid mutations into the region corresponding to the E5/G6 epitope of TPMV N. Because antibodies against the E5/G6 epitope are not induced in hantavirus-infected patient sera and E5/G6 does not compete with other antibodies induced by hantavirus infections (18), we expected that inserting amino acid-altering point mutations within this region would not change its antigenicity. Finally, we succeeded in developing an E5/G6 capture ELISA which can identify TPMV rN-immune rabbit sera and sera of shrews experimentally infected with TPMV with high specificity.
We previously proposed that three kinds of whole-length rN antigens of HTNV, PUUV, and SNV were required for the serological diagnosis of rodent-borne hantavirus infections (7). Now, by adding TPMV rN/E5G6, it is possible to diagnose both rodent- and insectivore-borne hantavirus infections. Using these four rN antigens, we examined 478 serum samples from patients with high fever in Thailand who were serologically negative for leptospirosis and dengue fever and found two anti-TPMV IgG antibody-positive sera from a single individual. Anti-TPMV IgG antibodies in these sera were confirmed by IFA, Western blotting, and FRNT. Because the patient came to the hospital after his condition had worsened, the relationship between his illness and TPMV infection could not be accurately determined. Thus, while this case suggests the infectivity of TPMV for humans, its pathogenicity for humans remains uncertain.
In testing sera from 14 wild shrews captured in Indonesia in 2005, one sample (no. 69) reacted strongly against TPMV, and three other samples (no. 2, 49, and 79) reacted weakly by ELISA. In the IFA test, using TPMV-infected Vero E6 cells as the antigen, two of these sera (no. 49 and 69) were positive, and this was confirmed by Western blotting analysis. However, only serum no. 49 neutralized TPMV by FRNT, suggesting the possible existence of TPMV variants or other antigenically distinct insectivore-borne hantaviruses in nature. To fully demonstrate that shrews are the natural reservoir of TPMV, it is necessary to survey additional species and detect the viral genome using RT-PCR assays in the future.
This is the first report of TPMV infection serologically confirmed with both humans and shrews. Our data indicate that TPMV can infect humans and be maintained in musk shrews as its natural host. The availability of newly developed serological assays for TPMV will facilitate future studies aimed at further elucidating the epizootiology and molecular phylogeny of insectivore-borne hantaviruses. Moreover, such studies will provide important insights about the role of TPMV and TPMV-like hantaviruses in the pathogenesis of febrile illnesses.
Acknowledgments
M.O. is a research fellow of the Japan Society for the Promotion of Science (JSPS) and was supported by JSPS Research Fellowships for Young Scientists. This work was also supported in part by a grant from the 21st Century COE Program, “Program of Excellence for Zoonosis Control,” and Grants-in-Aid for Scientific Research and the Development of Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Araki, K., K. Yoshimatsu, M. Ogino, H. Ebihara, A. Lundkvist, H. Kariwa, I. Takashima, and J. Arikawa. 2001. Truncated hantavirus nucleocapsid proteins for serotyping Hantaan, Seoul, and Dobrava hantavirus infections. J. Clin. Microbiol. 39:2397-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikawa, J., A. L. Schmaljohn, J. M. Dalrymple, and C. S. Schmaljohn. 1989. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J. Gen. Virol. 70:615-624. [DOI] [PubMed] [Google Scholar]
- 3.Carey, D. E., R. Reuben, K. N. Panicker, R. E. Shope, and R. M. Myers. 1971. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 59:1758-1760. [PubMed] [Google Scholar]
- 4.Chu, Y. K., G. Jennings, A. Schmaljohn, F. Elgh, B. Hjelle, H. W. Lee, S. Jenison, T. Ksiazek, C. J. Peters, P. Rollin, and C. Schmaljohn. 1995. Cross-neutralization of hantaviruses with immune sera from experimentally infected animals and from hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome patients. J. Infect. Dis. 172:1581-1584. [DOI] [PubMed] [Google Scholar]
- 5.Chu, Y. K., C. Rossi, J. W. Leduc, H. W. Lee, C. S. Schmaljohn, and J. M. Dalrymple. 1994. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology 198:196-204. [DOI] [PubMed] [Google Scholar]
- 6.Meyer, B. J., and C. S. Schmaljohn. 2000. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 8:61-67. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto, T. 2004. Antigenic characterization of Sin Nombre virus and establishment of serodiagnosis of hantavirus pulmonary syndrome. Jpn. J. Vet. Res. 52:54-55. [Google Scholar]
- 8.Morii, M., K. Yoshimatsu, J. Arikawa, G. Zhou, H. Kariwa, and I. Takashima. 1998. Antigenic characterization of Hantaan and Seoul virus nucleocapsid proteins expressed by recombinant baculovirus: application of a truncated protein, lacking an antigenic region common to the two viruses, as a serotyping antigen. J. Clin. Microbiol. 36:2514-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichol, S. T., B. J. Beaty, R. M. Elliott, R. Goldbach, A. Plyusnin, C. S. Schmaljohn, and R. B. Tesh. 2005. Bunyaviridae, p. 695-716. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: classification and nomenclature of viruses. Eighth report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
- 10.Ogino, M., K. Yoshimatsu, H. Ebihara, K. Araki, B. H. Lee, M. Okumura, and J. Arikawa. 2004. Cell fusion activities of Hantaan virus envelope glycoproteins. J. Virol. 78:10776-10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okumura, M., K. Yoshimatsu, K. Araki, B. H. Lee, A. Asano, T. Agui, and J. Arikawa. 2004. Epitope analysis of monoclonal antibody E5/G6, which binds to a linear epitope in the nucleocapsid protein of hantaviruses. Arch. Virol. 149:2427-2434. [DOI] [PubMed] [Google Scholar]
- 12.Pattamadilok, S., B. H. Lee, S. Kumperasart, K. Yoshimatsu, M. Okumura, I. Nakamura, K. Araki, Y. Khoprasert, P. Dangsupa, P. Panlar, B. Jandrig, D. H. Kruger, B. Klempa, T. Jakel, J. Schmidt, R. Ulrich, H. Kariwa, and J. Arikawa. 2006. Geographical distribution of hantaviruses in Thailand and potential human health significance of Thailand virus. Am. J. Trop. Med. Hyg. 75:994-1002. [PubMed] [Google Scholar]
- 13.Plyusnin, A. 2002. Genetics of hantaviruses: implications to taxonomy. Arch. Virol. 147:665-682. [DOI] [PubMed] [Google Scholar]
- 14.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmaljohn, C. S. 1996. Molecular biology of hantaviruses, p. 63-90. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 16.Wang, H., K. Yoshimatsu, H. Ebihara, M. Ogino, K. Araki, H. Kariwa, Z. Wang, Z. Luo, D. Li, C. Hang, and J. Arikawa. 2000. Genetic diversity of hantaviruses isolated in china and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology 278:332-345. [DOI] [PubMed] [Google Scholar]
- 17.Xiao, S. Y., J. W. Leduc, Y. K. Chu, and C. S. Schmaljohn. 1994. Phylogenetic analyses of virus isolates in the genus Hantavirus, family Bunyaviridae. Virology 198:205-217. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimatsu, K., J. Arikawa, M. Tamura, R. Yoshida, A. Lundkvist, B. Niklasson, H. Kariwa, and I. Azuma. 1996. Characterization of the nucleocapsid protein of Hantaan virus strain 76-118 using monoclonal antibodies. J. Gen. Virol. 77:695-704. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimatsu, K., J. Arikawa, R. Yoshida, H. Li, Y. C. Yoo, H. Kariwa, N. Hashimoto, M. Kakinuma, T. Nobunaga, and I. Azuma. 1995. Production of recombinant hantavirus nucleocapsid protein expressed in silkworm larvae and its use as a diagnostic antigen in detecting antibodies in serum from infected rats. Lab. Anim. Sci. 45:641-646. [PubMed] [Google Scholar]