Abstract
Members of the genus Exophiala are often difficult to identify to the species level because of their variable morphological appearances. This paper describes the methods used to identify Exophiala mesophila and provides salient differential features for distinguishing other mesophilic members of the genus.
Dematiaceous fungi are increasingly reported as agents of disease in immunocompetent as well as immunocompromised persons (9, 34). The genus Exophiala contains a number of black yeasts that are recognized pathogens of vertebrates. At least eight species have been isolated from human patients with phaeohyphomycosis, causing infections that range from superficial skin lesions to disseminated disease (6). These fungi pose a significant challenge to physicians and laboratory personnel alike, as both appropriate therapeutic options and accurate species identification are problematic (5, 6, 7). Exophiala species are often isolated from oligotrophic water sources, such as sinks, drainpipes, swimming pools, and bathing facilities (21, 26). Although they have also been cultured from drinking water samples (16), the presence of black fungi in water systems has frequently been overlooked because of their slow growth under routine conditions of water quality assessment. In a recent investigation in Germany (12), Exophiala was found to be one of three genera that were disseminated efficiently by groundwater-derived public drinking water. During the course of a recent study to test the efficacy of a chemical waterline cleaner to reduce the bacterial load (2, 14, 24, 37) in dental unit waterlines, the authors isolated a black yeast that was subsequently identified as Exophiala mesophila (33). The public health significance of this finding is unknown, but an awareness of its existence is essential for dental professionals and laboratory personnel alike, in view of the ever-increasing numbers of immunocompromised persons who present at outpatient dental clinics. The purpose of this paper is to report on the laboratory identification of mesophilic Exophiala species and to review the morphological and physiologic features of mesophilic Exophiala species that may assist in their identification.
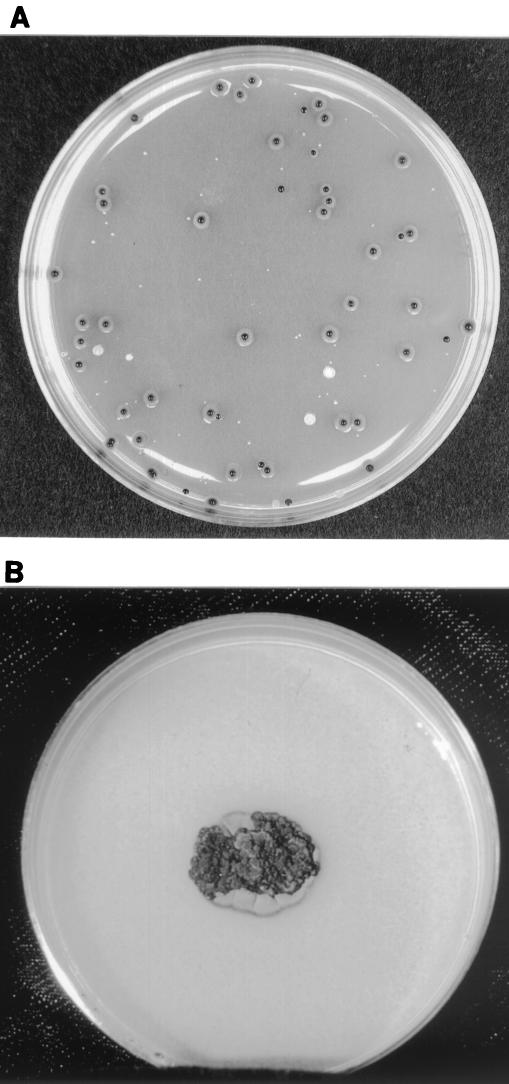
Details of the methods used for the dental unit waterline treatment study are described elsewhere (33). Briefly, 10-fold serial dilutions of water samples from treatment and control dental units were plated on low-nutrient R2A agar plates (Becton Dickinson Microbiology Systems, Sparks, Md.). This is the medium recommended for use to grow common water organisms (27). In week 9 of the study, the predominant isolates growing on R2A agar plates inoculated with water from one of the treated dental units appeared as small, dark, shiny colonies. Similar isolates were subsequently cultured from the source tap water and from other treated units. The typical appearance of these colonies on R2A agar after 7 days at 25°C can be seen in Fig. 1A.
FIG. 1.
(A) Growth of E. mesophila on R2A agar after 7 days at 25°C; (B) growth of E. mesophila on PFA after 14 days at 30°C.
Isolates recovered from the waterline samples (University of Texas Health Science Center at San Antonio accession number R-3282) were sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio for organism identification.
The macroscopic and microscopic morphologies of the isolates recovered were evaluated on potato flakes agar (PFA), prepared in-house (35). Colonies on PFA were black and yeast-like initially. After 14 days of incubation at 30°C, they developed filamentous areas that were gray-black and velvety and that measured approximately 18 mm in diameter, as can be seen in Fig. 1B. Growth on PFA occurred at 25 and 30°C but not at 35 or 40°C. The isolates assimilated nitrate (medium prepared in-house [32]) and grew on medium containing 0.4% cycloheximide (Remel, Lenexa, Kans.). Previous studies have suggested some utility for the API 20C yeast identification system for the identification of dematiaceous molds (8). By using a modified API 20C AUX system (BioMérieux, Marcy l'Etoile, France) for yeast identification, the isolates assimilated the following substrates: glucose, glycerol, 2-keto-d-gluconate, l-arabinose, xylose, adonitol (ribitol), N-acetyl-d-glucosamine, cellobiose, maltose, sucrose, trehalose, and melezitose. Substrates not assimilated included galactose, inositol, lactose, and raffinose. Questionable readings were obtained for wells containing xylitol, sorbitol, and methyl-d-glucoside. Modifications to the standard protocol for yeast identification included calibration of the inoculum to an approximately 3.0 McFarland standard and reading of the growth in the wells after 7 days of incubation at 30°C.
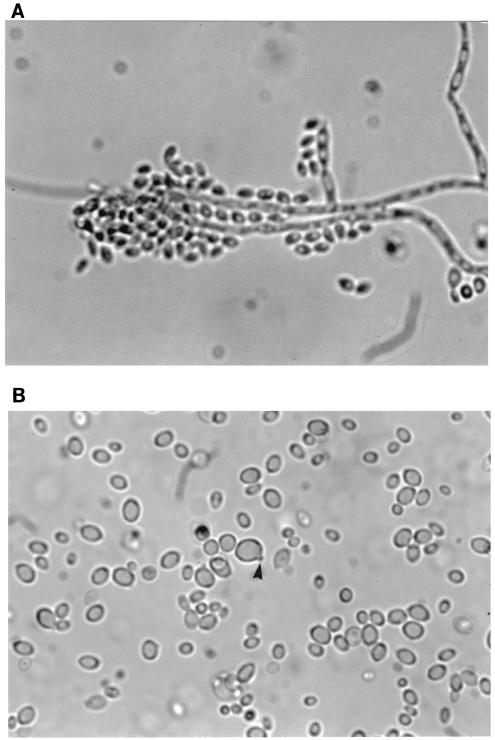
Microscopically, annellides were of medium length (9 to 12 μm) and tubular; conidia (2.5 by 4.8 μm) were formed at the apices of the annellides (Fig. 2A) as well as from intercalary loci on the vegetative hyphae. The black yeast synanamorph (Fig. 2B) was a striking feature of these isolates, with yeast cells displaying prominent annellated protrusions (arrow).
FIG. 2.
(A) Annellides and annelloconidia of E. mesophila; (B) yeast phase synanamorph with prominent annellated protrusions (arrow). Magnifications, ×920.
As the isolates could not be definitively identified to the species level on the basis of their macroscopic and microscopic morphologies, they were further evaluated by molecular methods. For molecular analysis, DNA was extracted from a loopful of the yeast synanamorph by proteinase K digestion, followed by chloroform-phenol extraction and isopropanol precipitation. A segment of the nuclear rRNA gene that included the entire internal transcribed spacer (ITS) 1 (ITS1) region and 5.8S subunit as well as portions of the 18S subunit and the ITS2 region was amplified with primers EXO1 (5′-CTCAGAGCCGGAAACTTGGTC-3′) and EXO2 (5′-CCGCCGTCATTGTCTTTGG-3′), which were previously designed by one of the authors (A.M.G.) for amplification of Exophiala spp. The resultant products were sequenced from both the 5′ and the 3′ ends with a dye-labeled terminator kit (Big Dye Terminator Cycle Sequencing Ready Reaction kit; Applied Biosystems, Foster City, Calif.) and an automated sequencer (ABI Prism 377 DNA sequencer; Applied Biosystems).
Sequencing of the amplicon produced with primers EXO1 and EXO2 yielded a 593-bp segment that included the entire ITS1 spacer region as well as the 5.8S subunit. E. mesophila was identified by comparison of the ITS1 sequence to approximately 1,200 comparable sequences of dematiaceous fungi maintained for research purposes at the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands. The sequence of our strain showed 99.1% identity with that of the type strain, CBS 402.95, from a shower joint, and 99.1% identity to that of a secondary strain of the same species from a swimming pool, CBS 836.95, both isolated in Germany. Our strain was a sister species to Exophiala castellanii, with sequence similarities of 95.9 to 94.6%. Five strains of E. castellanii held in the CBS collection came from drinking water, while the origin of the former type strain, CBS 158.58, was uncertain.
Species of Exophiala may be grouped according to their optimal and/or maximum temperatures for growth. Those considered psychrophilic, such as Exophiala psychrophila (30), have a maximum growth temperature of <25°C. The thermophilic group (species with a maximum growth temperature of 35°C or greater) includes the most common clinically significant human pathogens in the genus, such as Exophiala jeanselmei (18), Exophiala dermatitidis (Wangiella dermatitidis) (4, 15), Exophiala spinifera (40), E. castellanii (10), Exophiala bergeri (1), Exophiala moniliae (23), and Sarcinomyces phaeomuriformis (Exophiala phaeomuriformis [20]; when it is yeast-like, it is morphologically indistinguishable from W. dermatitidis and is physiologically distinguishable from W. dermatitidis only by a slightly lower maximum temperature of growth [20]). One of these species, E. jeanselmei, has recently been described as a nosocomial pathogen (28), and E. dermatitidis was implicated in a case of catheter-associated fungemia (25) and in the contamination of injectable steroid preparations (3). Mesophilic Exophiala species (those with maximum growth temperatures of 25 to 35°C) include Exophiala pisciphila (22), Exophiala alcalophila (11), Exophiala angulospora (16), Exophiala dopicola (17), Exophiala salmonis (29), and E. mesophila (19). Of these, E. pisciphila is the only mesophilic species that has been described as a human pathogen (38). It is unknown at present whether E. mesophila and other mesophilic species that lack the ability to grow at 35°C in vitro can proliferate at these temperatures in vivo. Seeliger (36) has shown that other dematiaceous genera (namely, Alternaria and Ulocladium) that fail to grow at 35°C in vitro do have the ability to invade tissue in compromised hosts. In addition, their melanized cell walls may interfere with disinfecting agents (31), thus eventually allowing bacterial growth.
Features that help to differentiate E. mesophila from other mesophilic species are displayed in Table 1. E. mesophila differs from E. alcalophila and E. salmonis by having smaller conidia (<5 μm), E. mesophila differs from E. dopicola by being dimorphic (having both a yeast synanamorph phase and a filamentous phase), and E. mesophila differs from E. pisciphila by having single-celled conidia. The key physiologic feature for this species is an optimal growth temperature between 28 and 30°C, with a maximum growth temperature of 32°C. E. mesophila is also capable of growth at pH 9.5 and fails to grow in 10% sodium chloride (19). On the basis of the assimilation patterns obtained by the modified method with the API 20C system, the following API codes could be obtained: 6716371, 6736371, 6714371, 6734371, 6712371, 6732371, 6710371, and 6730371.
TABLE 1.
Salient differential features for mesophilic Exophiala species
| Species | Temp (°C) | Conidia (μm) | Physiologic characteristics | Dimorphic | Comments (colony PDAa) |
|---|---|---|---|---|---|
| E. mesophila | Optimum, 28-30; maximum, 32 | <5 long; mean, 2.6 × 4.8 | Nitrate positiveb; growth at pH 9.5 and on 0.1% cycloheximide; no growth in 10% NaCl; melezitose positive | Yes; dimorphism distinguishes it from E. dopicola | Initially yeast-like, dark, olivaceous; brownish diffusable pigment on SDAc |
| E. angulospora | 37 =d | Angular to obovoid; 2.5-4 × 2-3 | Nitrate positive | Yes; budding cells present | From drinking water in Japan |
| E. alcalophila | 37 = | Globose, elliptical to reniform | Nitrate positive; growth at pH 10.4 | Yes | From Japanese soils |
| E. dopicola | 35 +e | Single celled, “bacillary”; 1.5-2.5 × 6-11 | Nitrate positive | No; yeast cells absent | From pine needles |
| E. pisciphila | Optimum, 23-29; maximum, 32 | >5 long; mean, 6-8 × 2.5-4 | Nitrate positive, lactose positive | No; yeast cells absent; occasionally detached conidia form one to a few secondary conidia | Expanding woolly colonies without a yeast phase; conidia broadly ellipsoidal, nearly all without septa; differs from E. salmonis by faster growth and smaller conidia (single celled) |
| E. salmonis | Optimum, 20-30; maximum, 35 | Large, 0-3 septa; >5 long with distinct unpigmented hilum; 5.6-6 × 2-2.4; two-celled (5-13.8 × 2.2-3.5) | Nitrate positive; lactose =f | No; nearly without a yeast phase | Expanding woolly colonies; conidia usually one septate |
PDA, potato dextrose agar.
Assimilated.
SDA, Sabouraud dextrose agar.
=, no growth.
+, growth.
=, not assimilated.
Despite these differential features, definitive identification of Exophiala species often requires molecular analyses. Although the primers used here have been successful for amplification and sequencing of the ITS1 regions of at least two different Exophiala species, they have failed to amplify DNA extracted from several other Exophiala species. A considerable amount of recent molecular data, mostly focusing on 18S and ITS rRNA gene sequences (13, 39, 40), will likely provide a resource for the future identification of opportunistic Exophiala species that may not have previously been recognized as vertebrate pathogens.
Nucleotide sequence accession number.
The sequence of 593 bp that included the entire ITS1 spacer region and the 5.8S subunit has been deposited in GenBank under accession number AF542377.
REFERENCES
- 1.Berger, L., and M. Langeron. 1949. Sur un type nouveau de chromomycose observé au Canada (Torula bergeri n. sp.). Ann. Parasitol. Hum. Comp. 24:574-599. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1993. Recommended infection control practices for dentistry. Morb. Mortal. Wkly. Rep. 41:1-12. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy—United States, July-November 2002. Morb. Mortal. Wkly. Rep. 51:1109-1112. [PubMed] [Google Scholar]
- 4.Chang, C. L., D.-S. Kim, D. J. Park, H. J. Kim, C. H. Lee, and J. H. Shin. 2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. J. Clin. Microbiol. 38:1965-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Hoog, G. S. 1977. Rhinocladiella and allied genera. Stud. Mycol. 15:1-140. [Google Scholar]
- 6.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, and Universitat Rovira i Virgili, Reus, Spain.
- 7.Dixon, D. M., and A. Polak-Wyss. 1991. The medically important dematiaceous fungi and their identification. Mycoses 34:1-18. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., M. R. McGinnis, D. H. Pincus, P. R. Goldson, and T. M. Kerkering. 1989. Evaluation of the API 20C yeast identification system for the differentiation of some dematiaceous fungi. J. Clin. Microbiol. 27:2565-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fothergill, A. W. 1996. Identification of dematiaceous fungi and their role in human disease. Clin. Infect. Dis. 22(Suppl. 2):S179-S184. [DOI] [PubMed] [Google Scholar]
- 10.Gold, W., L. H. Velland, I. E. Salit, I. Campbell, R. Summerbell, M. Rinaldi, and A. E. Simor. 1994. Successful treatment of systemic and local infections due to Exophiala species. Clin. Infect. Dis. 19:339-341. [DOI] [PubMed] [Google Scholar]
- 11.Goto, S., R. Aono, J. Sugiyama, and K. Horikoshi. 1981. Exophiala alcalophila, a new black, yeast-like hyphomycete with an accompanying Phaeococcomyces alcalophilus morph, and its physiological characteristics. Trans. Mycol. Soc. Jpn. 22:429-439. [Google Scholar]
- 12.Göttlich, E., W. van der Lubbe, B. Lange, S. Fiedler, I. Melchert, M. Reifenrath, H.-C. Flemming, and G. S. de Hoog. 2002. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health 205:260-279. [DOI] [PubMed] [Google Scholar]
- 13.Haase, G., L. Sonntag, B. Melzer-Crick, and G. S. de Hoog. 1999. Phylogenetic inference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Stud. Mycol. 43:80-97. [Google Scholar]
- 14.Hesselgren S.-G., and U. Nedlich. 1979. Microbial growth in dental units. Quintessence Int. 1343:103-107. [Google Scholar]
- 15.Hiruma, M., A. Kawada, H. Ohata, Y. Ohnishi, H. Takahashi, M. Yamazaki, A. Ishibashi, K. Hatsuse, M. Kakihara, and M. Yoshida. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Iwatsu, T., S. Udagawa, and T. Takase. 1991. A new species of Exophiala recovered from drinking water. Mycotaxon 61:321-328. [Google Scholar]
- 17.Katz, B., and M. R. McGinnis. 1980. A new species of Exophiala recovered from loblolly pine litter. Mycotaxon 11:182-184. [Google Scholar]
- 18.Kim, H. U., S. H. Kang, and T. Matsumoto. 1998. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei in a patient with advanced tuberculosis. Br. J. Dermatol. 138:351-353. [DOI] [PubMed] [Google Scholar]
- 19.Listemann, H., and H. Freiesleben. 1995. Exophiala mesophila spec. nov. Mycoses 39:1-3. [DOI] [PubMed] [Google Scholar]
- 20.Matos, T., G. Haase, A. H. G. Gerrits van den Ende, and G. S. de Hoog. 2003. Molecular diversity of oligotrophic and neurotropic members of the black yeast genus Exophiala, with accent on E. dermatitidis. Antonie Leeuwenhoek 83:293-303. [DOI] [PubMed] [Google Scholar]
- 21.Matos, T., G. S. de Hoog, A. G. de Boer, I. De Crom, and G. Haase. 2002. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 45:373-377. [DOI] [PubMed] [Google Scholar]
- 22.McGinnis, M. R., and L. Ajello. 1974. A new species of Exophiala isolated from channel catfish. Mycologia 66:518-520. [PubMed] [Google Scholar]
- 23.McGinnis, M. R., D. F. Sorell, R. L. Miller, and G. W. Kaminski. 1981. Subcutaneous phaeohyphomycosis caused by Exophiala moniliae. Mycopathol. Mycol. Appl. 73:69-72. [DOI] [PubMed] [Google Scholar]
- 24.Mills, S. E. 2000. The dental unit waterline controversy. J. Am. Dent. Assoc. 131:1427-1441. [DOI] [PubMed] [Google Scholar]
- 25.Nachman, S., O. Alpan, R. Malowitz, and E. D. Spitzer. 1996. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 34:1011-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura, K., M. Miyaji, H. Taguchi, and R. Tanaka. 1987. Fungi in bathwater and sludge of bathroom drainpipes. 1. Frequent isolation of Exophiala species. Mycopathology 97:17-23. [DOI] [PubMed] [Google Scholar]
- 27.Noce, L., D. Giovanni, and E. Putnins. 2000. An evaluation of sampling and laboratory procedures for determination of heterotrophic plate counts in dental unit waterlines. J. Can. Dent. Assoc. 66:262-269. [PubMed] [Google Scholar]
- 28.Nucci, M., T. Akiti, G. Barreiros, F. Silveira, S. G. Revankar, D. Sutton, and T. Patterson. 2001. Nosocomial fungemia due to Exophiala jeanselmei var. jeanselmei and a Rhinocladiella species. J. Clin. Microbiol. 39:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otis, E. J., and R. E. Wolke. 1985. Infection of Exophiala salmonis in Atlantic salmon (Salmo salar L.). J. Wildl. Dis. 21:61-64. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen, O. A., and F. Langvad. 1989. Exophiala psychrophila sp. nov., a pathogenic species of the black yeasts isolated from farmed Atlantic salmon. Mycol. Res. 92:153-156. [Google Scholar]
- 31.Phillips, G., H. McEwan, I. McKay, G. Crowe, and J. McBeath. 1988. Black pigmented fungi in the water pipe-work supplying endoscope washer disinfectors. J. Hosp. Infect. 40:250-251. [DOI] [PubMed] [Google Scholar]
- 32.Pincus, D. H., I. F. Salkin, N. J. Hurd, I. L. Levy, and M. A. Kemna. 1988. Modification of potassium nitrate assimilation test for identification of clinically important yeasts. J. Clin. Microbiol. 26:366-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porteous, N. B., S. W. Redding E. H. Thompson, A. M. Grooters, G. S. de Hoog, and D. A. Sutton. 2003. Isolation of an unusual fungus in treated dental unit waterlines. J. Am. Dent. Assoc. 134:853-858. [DOI] [PubMed]
- 34.Revankar, S. G., J. E. Patterson, D. A. Sutton, R. Pullen, and M. G. Rinaldi. 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 34:467-476. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi, M. G. 1982. Use of potato flakes agar in clinical mycology. J. Clin. Microbiol. 15:1159-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeliger, H. P. 1983. Infections of humans by opportunistic molds—their identification and nomenclature of their diseases. Mykosen 26:587-598. [PubMed] [Google Scholar]
- 37.Shearer, B. G. 1996. Biofilm and the dental office. J. Am. Dent. Assoc. 127:181-189. [DOI] [PubMed] [Google Scholar]
- 38.Sughayer, M., P. C. DeGirolami, J. Khettry, D. Korzeniowski, A. Grumney, L. Pasarell, and M. R. McGinnis. 1991. Human infection caused by Exophiala pisciphila: case report and review. Rev. Infect. Dis. 13:379-382. [DOI] [PubMed] [Google Scholar]
- 39.Untereiner, W. A. 2000. Capronia and its anamorphs: exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Stud. Mycol. 45:141-149. [Google Scholar]
- 40.Vitale, R. G., and G. S. de Hoog. 2002. Molecular diversity, new species and antifungal susceptibilities in the Exophiala spinifera clade. Med. Mycol. 40:545-556. [DOI] [PubMed] [Google Scholar]