Abstract
Stx2d is a recently described Shiga toxin whose cytotoxicity is activated 10- to 1,000-fold by the elastase present in mouse or human intestinal mucus. We examined Shiga toxigenic Escherichia coli (STEC) strains isolated from food and livestock sources for the presence of activatable stx2d. The stx2 operons of STEC were first analyzed by PCR-restriction fragment length polymorphism (RFLP) analysis and categorized as stx2, stx2c vha, stx2c vhb, or stx2d EH250. Subsequently, the stx2c vha and stx2c vhb operons were screened for the absence of a PstI site in the stx2A subunit gene, a restriction site polymorphism which is a predictive indicator for the stx2d (activatable) genotype. Twelve STEC isolates carrying putative stx2d operons were identified, and nucleotide sequencing was used to confirm the identification of these operons as stx2d. The complete nucleotide sequences of seven representative stx2d operons were determined. Shiga toxin expression in stx2d isolates was confirmed by immunoblotting. stx2d isolates were induced for the production of bacteriophages carrying stx. Two isolates were able to produce bacteriophages φ1662a and φ1720a carrying the stx2d operons. RFLP analysis of bacteriophage genomic DNA revealed that φ1662a and φ1720a were highly related to each other; however, the DNA sequences of these two stx2d operons were distinct. The STEC strains carrying these operons were isolated from retail ground beef. Surveillance for STEC strains expressing activatable Stx2d Shiga toxin among clinical cases may indicate the significance of this toxin subtype to human health.
Shiga toxigenic Escherichia coli (STEC) isolates are important food-borne pathogens that exist as commensal bacteria of ruminant animals. Shiga toxins (Stxs) comprise an A subunit that carries the toxic function and a B-subunit pentamer that binds the toxin to the eukaryotic cell receptor (for a review, see reference 22). Studies have indicated that Stx type 2 (Stx2), encoded by the stx2 operon, has an epidemiological relationship with the severe human disease conditions hemolytic-uremic syndrome and hemorrhagic colitis. In addition, toxins Stx2 and Stx2c, encoded by different genotypic subtypes of stx2, have also been associated with a greater severity of human disease. In contrast, the more recently described stx2 genotype, stx2d EH250 (26), is apparently of lesser clinical significance.
The nomenclature designating the Stx2d EH250 subtype is complicated by the prior use of Stx2d to designate activatable Stx2 (16, 18). Both Stx2d and Stx2d EH250 possess Ser291 and Glu297 residues in the Stx2A subunit toxin-coding region; however, only Stx2d is activatable. While STEC isolates carrying stx2d EH250 are commonly found, particularly from sheep (8), very few reports describe activatable stx2d genotype in STEC.
Activatable stx2d was originally detected in STEC strain B2F1. Two activatable operons, stx2d1 and stx2d2, have been identified in the B2F1 (17). Prior to their designation as stx2d1 and stx2d2, these operons were designated stx2c vha and stx2c vhb, respectively, due to the restriction fragment length polymorphisms (RFLPs) present in the stx2B genes. However, it is now clear that stx2d1 and stx2d2 are not synonymous with the stx2 subtypes stx2c vha and stx2c vhb, respectively; rather, stx2d1 and stx2d2 are subsets of stx2c vha and stx2c vhb, respectively, which are further defined by additional mutations in the stx2A subunit gene encoding the activatable toxin phenotype.
STEC strain B2F1 is extremely virulent in an orally infected streptomycin-treated mouse model, and the Stx2d toxins produced by this strain have increased cytotoxicities for Vero cells after incubation with mouse or human intestinal mucus (17). Stx2dA subunits possess two amino acid substitutions relative to the sequence of classical Stx2, Ser291 and Glu297. These residues are present in the A2 peptide and are believed to be associated with the property of activation. Ser291 and Glu297 contribute to a recognition motif that allows intestinal mucus or elastase purified from intestinal mucus to cleave the A2 peptide between Thr295 and Gly296, decreasing the length of the A2 peptide by two residues (16, 19). The modified A2 peptide is then able to interact with the Stx2B subunit pentamer, producing the activatable phenotype (19). Interestingly, the stx2d operons described by Piérard et al. (26) (stx2d EH250) also possess Ser291 and Glu297; however, these residues are adjacent to several other amino acid substitutions, which apparently mitigate the property of activation by intestinal mucus. Hence, Stx2d EH250 toxins are not activatable (A. R. Melton-Celsa and A. D. O'Brien, personal communication).
The aims of this study were (i) to examine the distribution of stx2 subtypes in livestock ruminant animals, (ii) determine if activatable stx2d operons were present, (iii) characterize stx2d isolates and operons, and (iv) determine if stx2d operons were borne on bacteriophages. We have demonstrated the presence of the activatable stx2d genotype in food and livestock sources and have shown by nucleotide sequencing that this stx2 subtype may be clearly distinguished from the stx2d EH250 subtype. We have further demonstrated that some stx2d operons are carried by inducible bacteriophages that may be propagated in E. coli K-12.
MATERIALS AND METHODS
Isolation of STEC.
STEC strains were isolated from food and livestock sources following enrichment broth culture, stx-specific PCR, and hydrophobic grid membrane filtration (20). The virulence determinants of each isolate were determined by a multiplex PCR for stx1, stx2, eae, and ehxA (24). Isolates carrying stx2d were kindly serotyped by Roger Johnson, Health Canada, Guelph, Ontario, Canada.
stx2 characterization and subtyping.
STEC isolates that carried stx2 were further characterized by PCR-RFLP analysis to determine the presence of stx2 subtypes. Primers GK5 and GK6 (30) were used to amplify the stx2B subunit gene. Amplicons were digested with FokI and HaeIII to discriminate stx2 from stx2c subtype genes. Use of restriction enzymes NciI and RsaI for PCR-RFLP analysis enabled classification of stx2c genes as stx2c vha, stx2c vhb, or stx2d EH250 (26). Isolates encoding stx2c vha and stx2c vhb were further examined to identify genes of this type which did not possess a PstI site in the 3′ region of the stx2A subunit gene. stx2c vha and stx2c vhb operons without PstI sites were sequenced in the 3′ region of the stx2A subunit gene. Putative stx2d genes showed identity to the sequence of activatable stx2d1 carried by O91:H21 strain B2F1 (11, 16).
DNA sequencing.
Complete stx2d operons were amplified with the primers 5′-GATGGCGGTCCATTATC-3′ (25) and 5′-ACTGAATTGTGACACAGATTA-3′ (A. R. Melton-Celsa, personal communication). Both strands of each stx2d operon amplicon were completely sequenced. Sequencing was performed with a Big Dye Cycle Sequencing kit following the instructions of the manufacturer and an ABI 377 fluorescent sequencer (Applied Biosystems, Foster City, Calif.) at the Australian Genome Research Facility, University of Queensland, Brisbane, Australia. Nucleotide sequence alignments were performed by the using BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) (1). The identities of any two sequences were compared by the using the BLAST 2 SEQUENCES program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html) (34). The predicted amino acid alignments presented in Fig. 1 and 2 were prepared by using the CLUSTALW program (version 1.8) at the Baylor College of Medicine Search Launcher (http://www.dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html) and the BOXSHADE program (version 3.21; http://www.ch.embnet.org/software/BOX_form.html).
FIG. 1.
Predicted amino acid sequences of the stx2d A-subunit genes from isolates identified in this study aligned with the published sequences of Stx2d1 from STEC isolate B2F1 (11, 17) and Stx2 (GenBank accession no. AB035143). Dots indicate residues identical to those in Stx2d1. Residues Ser291 and Glu297, which distinguish the Stx2d1A-subunit carboxy terminus from that of Stx2A, are boldface and underlined.
FIG. 2.
Predicted amino acid sequences of the stx2d B-subunit genes from isolates identified in this study aligned with the published sequences of Stx2d1 from STEC isolate B2F1 (11, 17) and Stx2 (GenBank accession no. AB035143). Dots indicate residues identical to those of Stx2d1. Residues Asn16 and Asp24, which distinguish the Stx2d1B subunit from that of Stx2B, are boldface and underlined.
Immunoblot detection of Stx expression from isolates with activatable stx2d.
Expression of stx2d operons was assayed by immunoblotting (2). Briefly, STEC isolates were patch inoculated onto nitrocellulose membranes placed on an agar plate surface. The production of Stx was determined by using rabbit anti-Stx antibodies reactive with all major Stx types and variants. Stx antisera were kindly provided by Roger Johnson, Health Canada.
Isolation and characterization of stx2d bacteriophages.
stx2d isolates were treated with mitomycin C (1 μg/ml) to induce the bacteriophage lytic cycle and production of phage. Lysed culture supernatants were filtered through a 0.45-μm-pore-size filter to ensure a cell-free lysate. The bacteriophage titers in the cell-free lysates were determined by serial dilution and propagation on E. coli Q358 (supE hsdR φ80r recA+) (13). Single phage plaques were picked from appropriately diluted indicator plates, resuspended in 100 μl of sterile H2O, and confirmed to carry stx2 by PCR of the plaque supernatant. Several purified single plaques from each isolate were confirmed to carry stx2d by PCR with primers GK5 and GK6, followed by RFLP analysis of the PCR amplicons.
Nucleotide sequence accession numbers.
The sequences of the complete stx2d operons from strains EC152a, EC173b, EC604a, EC782, EC1586, EC1720a, and EC1871 have been submitted to GenBank and given the accession numbers AF500187, AF500190, AF500192, AF500193, AF500188, AF500189, and AF500191, respectively.
RESULTS
stx2 genotypes among STEC isolates from livestock sources.
STEC isolates (n = 311) possessing stx2 were isolated from a variety of food and livestock sources. Each isolate was then characterized to designate a specific stx2 subtype (Table 1). The dominant stx2 subtype found in STEC strains of ovine origin was stx2d EH250, first described by Piérard et al. (26). A total of 79 of 103 (77%) isolates from lamb meat had the stx2d EH250 genotype, while 30 of 38 (79%) isolates from sheep carcasses and feces were stx2d EH250. In comparison, classical stx2 was dominant among STEC strains isolated from beef cattle (26 of 51; 51%) and dairy cattle (33 of 56; 59%) sources. STEC strains isolated from ground beef samples showed greater heterogeneity in stx2 genotypes, in which stx2 (29%), stx2d EH250 (21%), stx2c vha (11%), and stx2c vhb (11%) were characterized.
TABLE 1.
Genetic subtypes of stx2 present in STEC isolates of different animal or meat origins
| stx2 subtype | % STEC isolates from different sources possessing alternative stx2 genetic subtypesa
|
||||
|---|---|---|---|---|---|
| Lamb meat (n = 103) | Sheep (carcasses and feces) (n = 38) | Ground beef (n = 63) | Beef cattle (carcasses and feces) (n = 51) | Dairy cattle sources (n = 56) | |
| stx2 | 2 | 3 | 29 | 51 | 59 |
| stx2 and stx2c vha | 0 | 0 | 0 | 14 | 4 |
| stx2 and stx2c vhb | 0 | 0 | 3 | 2 | 11 |
| stx2 and stx2d EH250 | 3 | 0 | 0 | 0 | 0 |
| stx2c vha | 1 | 8 | 11 | 22 | 5 |
| stx2c vhb | 2 | 0 | 11 | 4 | 7 |
| stx2d EH250 | 77 | 79 | 21 | 6 | 2 |
| stx2c vha and stx2c vhb | 1 | 0 | 0 | 2 | 2 |
| stx2d EH250 and stx2c vha | 1 | 0 | 0 | 0 | 0 |
| stx2d EH250 and stx2c vhb | 1 | 0 | 0 | 0 | 0 |
| Negativeb | 8 | 0 | 16 | 0 | 11 |
| Otherc | 5 | 11 | 10 | 0 | 0 |
Percentages are rounded to the nearest integer value.
Primers GK5 and GK6 did not amplify the correct amplicon from the stx2B gene.
Primers GK5 and GK6 amplified amplicons of the correct size from the stx2B gene; however, the amplicons did not digest in a manner consistent with the anticipated RFLP scheme.
Presence of stx2d in STEC.
Isolates possessing stx2 were further characterized for the possession of putative stx2d operons. By PCR-RFLP analysis and preliminary sequencing of the DNA, 12 STEC isolates were found to possess stx2 operons, with the predicted amino acid sequences of the stx2A subunit showing carboxy-terminal modifications specific for stx2d. Seven putative stx2d operons from isolates that represented the diversity of isolates by original source of isolation and the stx2c vha, stx2c vhb, or non-stx2c vha and stx2c vhb classification were completely sequenced. The DNA sequences of the stx2d operons from isolates EC152a and EC604a were identical. The DNA sequences of the other five isolates were each unique and conformed with the PCR-RFLP analysis characterization for each stx2d operon. Isolates identified as carrying stx2d are listed in Table 2.
TABLE 2.
Characterization of STEC isolates with the stx2d genotype
| Isolate no. | Serotype | Isolate source | Virulence factor genotypea
|
stx2B subtypeb | Phage recovered | ||
|---|---|---|---|---|---|---|---|
| stx | eae | ehxA | |||||
| 152a | O?:H29 | Beef cattle feces | 2 | −d | − | 2 | Non-stx |
| 173b | O174:H21 | Beef cattle feces | 2 | − | − | vhb | Non-stx |
| 604a | O2:H29 | Dairy farm | 2 | − | − | 2 | Non-stx |
| 782 | O?:NMc | Beef cattle feces | 2 | − | − | vha | Non stx |
| 1564b | O1:H20 | Ground beef | 2 | − | + | vhb | |
| 1585 | O174:H21 | Dairy farm | 2 | − | − | vhb | Non-stx |
| 1586 | O174:H8 | Ground beef | 1 and 2 | − | + | 2 | stx1 |
| 1662a | O174:H21 | Ground beef | 2 | − | − | vha | stx2d |
| 1720a | O174:H21 | Ground beef | 2 | − | − | vha | stx2d |
| 1871a | O?:H11 | Dairy farm | 2 | − | + | vhb | Non-stx |
| 1995a | O174:H8 | Ground beef | 1 and 2 | − | + | vhb | stx1 |
| 2062a | O8:H19 | Lamb meat | 2 | − | − | vhb | |
STEC isolates were screened for the virulence factors stx (Stx genes stx1 and stx2), eae (E. coli attaching-and-effacing gene), and ehxA (enterohemolysin gene) by multiplex PCR (see Materials and Methods).
The genetic subtype of the toxin B subunit gene for each stx2d operon was classified by RFLP analysis and nucleotide sequencing (see Materials and Methods).
NM, nonmotile.
−, negative.
Comparison of the predicted amino acid sequences of the Stx2A subunit with the Stx2d sequence from O91:H21 strain B2F1 (11, 17) indicated that all the stx2d operons encoded Stx2A with Phe291Ser and Lys297Glu amino acid substitutions, characteristic of activatable Stx2d (17) (Fig. 1). Comparison of the predicted amino acid sequences of the B subunits also showed Asp16Asn and Asp24Ala substitutions characteristic of Stx2dB subunits. The one exception was EC1586, in which Asp24 was conserved in the Stx2dB subunit (Fig. 2). The stx2dB subunit genes of isolates EC152a, EC604a, and EC1586 did not conform to the stx2c vha or stx2c vhb RFLP designation that has been typical of other stx2d operons.
The nucleotide sequence of the stx2d operon from EC782 was identical to that of stx2d1 (11, 16). The identical nucleotide sequences of the stx2d operons from EC152a and EC604a differed by one nucleotide from the sequence of stx2-NV206 (3). Pairwise comparison and alignments of the stx2A and stx2B genes from EC173b, EC1586, EC1720a, and EC1871a with homologs from the activatable operons stx2d1, stx2d2, and stx2-NV206 (3, 11, 16) showed 98 to 100% identities and indicated that the new stx2d operons comprised mosaic sequences.
stx2d bacteriophage characterization.
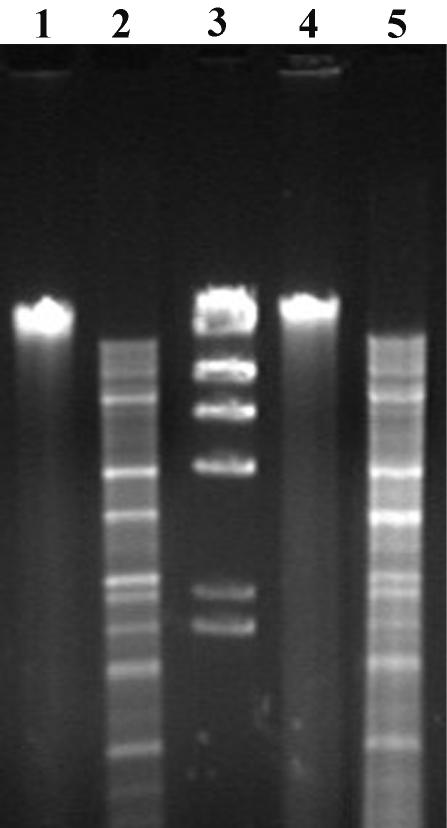
For each of the 12 stx2d-containing isolates identified, the bacteriophage lytic cycle was induced with mitomycin C. EC1564b and EC2062 cultures did not lyse in response to mitomycin C induction, and their culture supernatants did not show plaques on indicator plates. Phage plaques were identified from the induced culture lysates of all stx2d-carrying isolates except EC1564b and EC2062a. The phages from EC152a, EC173b, EC604a, EC782, EC1585, and EC1871a did not carry stx operons, while those from EC1586 and EC1995 were characterized as carrying stx1. The stx2 phages φ1662 and φ1720a were induced from EC1662 and EC1720a, respectively. PCR-RFLP analysis specific for the stx2B subunit genes of φ1662 and φ1720a showed that the stx2 operons of these phages corresponded to the stx2d operons sequenced from their respective STEC host isolates. RFLP analysis of φ1662 and φ1720a genomic DNA with the AvaI restriction enzyme indicated that these two phages were related; however, digestion of the DNA samples was complicated by contaminating nuclease activated during enzyme incubation (Fig. 3).
FIG. 3.
RFLP analysis of stx2d bacteriophages. Bacteriophage genomic DNA was isolated, digested with AvaI, and electrophoresed. Lanes: 1, undigested φ1720a; 2, φ1720a; 4, undigested φ1662a; and 5, φ1662a. Lane 3, DNA size markers of 23.1, 9.4, 6.6, 4.4, 2.3, and 2.0 kb.
Stx expression from stx2d strains.
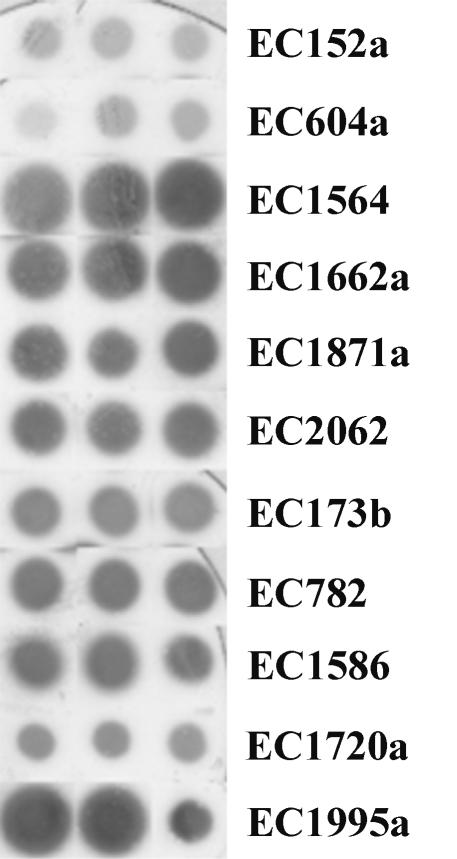
Eleven stx2d-containing isolates were examined for expression of Stx by immunoblotting (Fig. 4). Isolates EC1586 and EC1995a carried stx1 genes, in addition to stx2d, such that Stx expression from these isolates is the cumulative expression of both stx1 and stx2d. All other isolates examined carried only stx2d. Stx expression determined by the diameter and intensity of the zone of staining was variable between isolates. Of particular note were the different levels of Stx2d expression by EC1662a and EC1720a, isolates that were both serotype O174:H21, whose stx2B subunit genes had vha RFLP patterns, and that produced phages stx2d possessing similar genome RFLP profiles.
FIG. 4.
Immunoblot analysis of Stx expression from stx2d-containing isolates. Equivalent inocula of stx2d-containing isolates were grown on nitrocellulose membranes placed on tryptic soy agar plates and grown overnight. The Stx expressed by bacterial colonies was captured on a capture membrane precoated with rabbit anti-stx antibodies. The capture membrane was probed with alkaline phosphatase-labeled rabbit anti-mouse immunoglobulin G. Triplicate inocula were made for each isolate, as indicated.
DISCUSSION
In Australia, STEC strains that do not possess the locus of enterocyte effacement are the major causes of hemolytic-uremic syndrome and bloody diarrhea (9). We have begun to characterize the stx2 genotypes of STEC strains isolated from food-producing animal sources to determine if these stx2 operons are characterized by specific properties. STEC strains possess an array of stx2 genotypic variants (26, 32, 36); however, the roles of particular genotypic variants in human pathogenesis are not clear. We determined that STEC strains isolated from sheep or sheep meat sources most frequently carry stx2d EH250, while STEC strains isolated from cattle sources most frequently carry stx2 or stx2c variant stx2c vha or stx2c vhb. While cattle have been known to be a significant reservoir of STEC isolates, recent studies have also shown that stx2d EH250 is routinely associated with ovine STEC isolates (8) (K. S. Gobius et al., unpublished data). The predominance of stx2d EH250 among ovine STEC isolates compared with its prevalence among bovine STEC isolates suggests that particular stx2 subtypes may be associated with specific ruminant species.
Using screening by PCR-RFLP analysis, we were able to identify activatable stx2d variants in STEC isolates that were predominantly from bovine sources. Partial sequencing of the stx2 operons from 12 putative stx2d isolates further confirmed that the operons were consistent with the presence of stx2d. Further nucleotide sequencing of seven complete stx2 operons confirmed that they encoded the activatable stx2d genotype.
Each STEC isolate carrying activatable stx2d was recovered from a separate sampling location or on independent sampling dates, ensuring that the isolates were not clonal representatives of the same strain. The nucleotide sequence polymorphisms of the stx2d operons present in all isolates (except EC152a and EC604a, for which the stx2d operons were identical; data not shown) support the independent origins of these isolates. Similarly, the diversity of serotypes among the strains carrying stx2d also supports the conclusion that the isolates are not related. While four of the isolates were serotype O174:H21, the stx2d operons carried by each of the isolates were differentiated by RFLP analysis and/or nucleotide sequencing.
All the serotypes that carried stx2d in this study have previously been isolated from livestock sources in other countries, including Argentina, Brazil, Canada, France, Germany, New Zealand, and the United Kingdom (2, 4, 6, 7, 23, 28, 29, 31, 39); however, none of these serotypes have previously been associated with stx2d. Isolates of serotypes O1:H20, O2:H29, O174:H8, and O174:H21, which have been identified to carry stx2d, have previously been isolated from healthy or symptomatic humans in a variety of countries (14, 15, 27, 33).
Bertin et al. (3) recently described a novel stx2 subtype, stx2-NV206, present in STEC isolates of serotype O6:H10 isolated from a healthy cow. stx2-NV206 operons also possess Ser291 and Glu297; however, the stx2B gene of this subtype does not have restriction sites characteristic of stx2c vha or stx2c vhb (which have previously been associated with activatable stx2d operons). In our study, the identical stx2d operons carried by isolates EC152a and EC604a differed by a single nucleotide from the sequence of stx2-NV206. Similarly, the sequence of stx2d from EC782 was identical to that of stx2d1 from B2F1, originally isolated in North America. The occurrence of identical or nearly identical activatable stx2d operons in bovine STEC isolates from different global locations is intriguing. Their presence in the geographically separated locations of Australia, France, and North America suggests both conservation of these stx2 variants and the possibility of their global dissemination.
In contrast to the conserved stx2d variant operons present in isolates EC152a, EC604a, and EC782, we also identified stx2d operons whose sequences did not show complete identity to those of previously described stx2d operons. The stx2d operons from EC173b, EC1586, EC1720a, and EC1871a showed nucleotide sequence polymorphisms in mosaic blocks of sequence. The mosaic structures of stx bacteriophage genomes, gained through bacteriophage genome shuffling, have been understood for some time (10, 37). However, recombination in stx operons is more difficult to detect by sequence analysis if recombination occurs between identical sequences. Our data provide evidence that alternative activatable stx2d operons have arisen through recombination of independent stx2 sequences. Such stx2 operon recombination may account for the increasing number of stx2 variants that continue to be detected and described. Recombination in stx2 operons, coupled with bacteriophage lambda recombination and mosaicism, may create a rich genetic pool of stx phages capable of affecting horizontal gene transfer within the broad population of members of the family Enterobacteriaceae.
stx genes are bacteriophage borne or are associated with defective prophages (21). The stx2d1 operon from strain B2F1 has been shown to be carried by an inducible bacteriophage that formed small turbid plaques on E. coli K-12 strain DH5α (35). We were able to induce and propagate stx2d bacteriophages from only 2 of 12 stx2d-positive STEC isolates. Notwithstanding the limited number of propagating stx2d phages detected in our study, by infection of an E. coli K-12 indicator strain, we have confirmed that stx2d operons are potentially transferred by bacteriophage induction and infection of new enterobacterial hosts. In contrast to the work of Teel et al. (35), we did not detect stx phages by induction from strain EC782, which was shown to possess an stx2d operon identical to stx2d1. Since the stx2d bacteriophages were propagated on an E. coli K-12 strain, it is possible that other stx2d phages may have been induced from additional STEC isolates but that such a phage(s) was unable to propagate on the specific indicator strain used or readily formed lysogens (unable to be detected by plaque formation). James et al. (12) found that the stx phage φ24B::Kan was capable of infecting only a minority of wild E. coli strains tested. However, of the strains able to be infected, the majority were susceptible to lysogeny by this phage. Alternative explanations for the detection of a small number of stx2d phages following induction in our study may be (i) that the stx2d operons in these isolates are carried on prophage remnants no longer capable of lytic induction or (ii) stx2d phages were induced from these STEC isolates and rapidly formed Q358 lysogens so that detection by plaque formation was not possible.
The two stx2d phages (φ1662a and φ1720a) successfully induced from isolates EC1662a and EC1720a were related when they compared by using phage genome-specific RFLP analysis. The apparent nuclease contamination of phage DNA may be similar to that observed with plasmid DNA isolated from various STEC strains (5). Given that both phages were induced from isolates of the same O174:H21 serotype and that the stx2d operons showed RFLP patterns characteristic of the stx2c vha genotype, it was surprising that the level of Stx2d expression by EC1720a appeared to be less than that by EC1662a. Recently, Wagner et al. (38) have shown that stx2 transcription is regulated by the late promoter pR′ of lambda phages, such that expression of Stx2 is coordinated with induction of the phage lytic cycle. Therefore, we conclude that transcription from pR′ in φ1662a may be greater than that from pR′ in φ1720a, leading to a higher level of Stx2d expression by EC1662a.
In conclusion, we have identified the activatable stx2d genotype in STEC strains isolated from food-producing livestock sources. The STEC isolates carrying stx2d consisted of multiple serotypes, and the nucleotide sequences of the stx2d operons varied. In at least two isolates stx2d was carried by inducible stx phages that could infect and propagate on an E. coli K-12 strain, demonstrating the potential for the horizontal transfer of stx2d. It has been suggested previously that STEC strains involved in human infection, which carry stx2d, may be more virulent due to mucus activation of the toxin. The increased virulence of the Stx2d toxin may compensate for the absence of other virulence attributes such as expression of the attaching-and-effacing gene (eae) commonly found in other enterohemorrhagic E. coli strains (17). Therefore, surveillance for STEC strains expressing the activatable Stx Stx2d in human illness may indicate the significance of this toxin subtype to human health.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atalla, H. N., R. Johnson, S. McEwen, R. W. Usborne, and C. L. Gyles. 2000. Use of a Shiga toxin (Stx)-enzyme-linked immunosorbent assay and immunoblot for detection and isolation of Stx-producing Escherichia coli from naturally contaminated beef. J. Food Prot. 63:1167-1172. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, Y., K. Boukhors, N. Pradel, V. Livrelli, and C. Martin. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J. Clin. Microbiol. 39:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutin, L., D. Geier, S. Zimmermann, S. Aleksic, H. A. Gillespie, and T. S. Whittam. 1997. Epidemiological relatedness and clonal types of natural populations of Escherichia coli strains producing Shiga toxins in separate populations of cattle and sheep. Appl. Environ. Microbiol. 63:2175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boerlin, P., S. Chen, J. K. Colbourne, R. Johnson, S. De Grandis, and C. Gyles. 1998. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect. Immun. 66:2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, H. J., B. D. Mollison, K. A. Bettelheim, K. Matejka, K. A. Paterson, and V. K. Ward. 2001. Occurrence and virulence factors of non-O157 Shiga toxin-producing Escherichia coli in retail meat in Dunedin, New Zealand. Lett. Appl. Microbiol. 32:118-122. [DOI] [PubMed] [Google Scholar]
- 7.Cerqueira, A. M., B. E. Guth, R. M. Joaquim, and J. R. Andrade. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121. [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic, S. P., M. A. Hornitzky, G. Bailey, P. Gill, B. Vanselow, K. Walker, and K. A. Bettelheim. 2001. Virulence properties and serotypes of Shiga toxin-producing Escherichia coli from healthy Australian slaughter-age sheep. J. Clin. Microbiol. 39:2017-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, E. J., R. M. Robins-Browne, E. V. O'Loughlin, V. Bennett-Wood, J. Bourke, P. Henning, G. G. Hogg, J. Knight, H. Powell, and D. Redmond. 2001. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological, and epidemiological features. Arch. Dis. Child. 85:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrix, R. W., M. C. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 12.James, C. E., K. N. Stanley, H. E. Allison, H. J. Flint, C. S. Stewart, R. J. Sharp, J. R. Saunders, and A. J. McCarthy. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karn, J., S. Brenner, L. Barnett, and G. Cesareni. 1980. Novel bacteriophage lambda cloning vector. Proc. Natl. Acad. Sci. USA 77:5172-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keskimaki, M., R. Ikaheimo, P. Karkkainen, F. Scheutz, Y. Ratiner, R. Puohiniemi, and A. Siltonen. 1997. Shiga toxin-producing Escherichia coli serotype OX3:H21 as a cause of hemolytic-uremic syndrome. Clin. Infect. Dis. 24:1278-1279. [DOI] [PubMed] [Google Scholar]
- 15.Keskimaki, M., M. Saari, T. Heiskanen, and A. Siitonen. 1998. Shiga toxin-producing Escherichia coli in Finland from 1990 through 1997: prevalence and characteristics of isolates. J. Clin. Microbiol. 36:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokai-Kun, J. F., A. R. Melton-Celsa, and A. D. O'Brien. 2000. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J. Biol. Chem. 275:3713-3721. [DOI] [PubMed] [Google Scholar]
- 17.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melton-Celsa, A. R., J. E. Rogers, C. K. Schmitt, S. C. Darnell, and A. D. O'Brien. 1998. Virulence of Shiga toxin-producing Escherichia coli (STEC) in orally infected mice correlates with the type of toxin produced by the infecting strain. Jpn. J. Med. Sci. Biol. 51(Suppl.):S108-S114. [DOI] [PubMed] [Google Scholar]
- 19.Melton-Celsa, A. R., J. F. Kokai-Kun, and A. D. O'Brien. 2002. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol. Microbiol. 43:207-215. [DOI] [PubMed] [Google Scholar]
- 20.Midgley, J., and P. Desmarchelier. 2001. Pre-slaughter handling of cattle and Shiga toxin-producing Escherichia coli (STEC). Lett. Appl. Microbiol. 32:307-311. [DOI] [PubMed] [Google Scholar]
- 21.Mizutani, S., N. Nakazono, and Y. Sugino. 1999. The so-called chromosomal verotoxin genes are actually carried by defective prophages. DNA Res. 6:141-143. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parma, A. E., M. E. Sanz, J. E. Blanco, J. Blanco, M. R. Vinas, M. Blanco, N. L. Padola, and A. I. Etcheverria. 2000. Virulence genotypes and serotypes of verotoxigenic Escherichia coli isolated from cattle and foods in Argentina. Importance in public health. Eur. J. Epidemiol. 16:757-762. [DOI] [PubMed] [Google Scholar]
- 24.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton, A. W., J. C. Paton, and P. A. Manning. 1993. Polymerase chain reaction amplification, cloning and sequencing of variant Escherichia coli Shiga-like toxin type II operons. Microb. Pathog. 15:77-82. [DOI] [PubMed] [Google Scholar]
- 26.Piérard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piérard, D., D. Stevens, L. Moriau, H. Lior, and S. Lauwers. 1997. Isolation and virulence factors of verocytotoxin-producing Escherichia coli in human stool samples. Clin. Microbiol. Infect. 3:531-540. [DOI] [PubMed] [Google Scholar]
- 28.Pradel, N., V. Livrelli, C. De Champs, J. B. Palcoux, A. Reynaud, F. Scheutz, J. Sirot, B. Joly, and C. Forestier. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran, V., M. A. Hornitzky, K. A. Bettelheim, M. J. Walker, and S. P. Djordjevic. 2001. The common ovine Shiga toxin 2-containing Escherichia coli serotypes and human isolates of the same serotypes possess a Stx2d toxin type. J. Clin. Microbiol. 39:1932-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rüssmann, H., E. Kothe, H. Schmidt, S. Franke, D. Harmsen, A. Caprioli, and H. Karch. 1995. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J. Med. Microbiol. 42:404-410. [DOI] [PubMed] [Google Scholar]
- 31.Sandhu, K. S., R. C. Clarke, K. McFadden, A. Brouwer, M. Louie, J. Wilson, H. Lior, and C. L. Gyles. 1996. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol. Infect. 116:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephan, R., and L. E. Hoelzle. 2000. Characterization of Shiga toxin type 2 variant B-subunit in Escherichia coli strains from asymptomatic human carriers by PCR-RFLP. Lett. Appl. Microbiol. 31:139-142. [DOI] [PubMed] [Google Scholar]
- 34.Tatusova, T. A., and T. L. Madden. 1999. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 35.Teel, L. D., A. R. Melton-Celsa, C. K. Schmitt, and A. D. O'Brien. 2002. One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70:4282-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyler, S. D., W. M. Johnson, H. Lior, G. Wang, and K. R. Rozee. 1991. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 29:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner, P. L., D. W. Acheson, and M. K. Waldor. 1999. Isogenic lysogens of diverse Shiga toxin 2-encoding bacteriophages produce markedly different amounts of Shiga toxin. Infect. Immun. 67:6710-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willshaw, G. A., H. R. Smith, D. Roberts, J. Thirlwell, T. Cheasty, and B. Rowe. 1993. Examination of raw beef products for the presence of Vero cytotoxin producing Escherichia coli, particularly those of serogroup O157. J. Appl. Bacteriol. 75:420-426. [DOI] [PubMed] [Google Scholar]