Abstract
It is well known that neuropsychological impairment can be associated with chronic epilepsy. This review suggests that a broad lifespan perspective of cognition in epilepsy should include consideration of: a) neurobiological factors that antedate the first seizure and influence cognition, b) epilepsy-related factors that influence brain growth and cognitive development after epilepsy is diagnosed and treated, c) clinical epilepsy and other risk factors associated with poor cognitive prognosis in the context of chronic pharmacoresistant epilepsy, and d) the modifiable and non-modifiable risk factors that influence cognitive aging in the general population.
Neuropsychological impairment is an important comorbidity of chronic epilepsy (1). A long and rich history of research has characterized relationships between cognitive status and a variety of clinical epilepsy factors, including etiology, age of onset, seizure type and severity, duration, antiepileptic medications, and other factors (2–7). In addition, modal cognitive profiles have been derived for several syndromes of epilepsy, and efforts have been undertaken to identify the shared versus unique cognitive abnormalities evident across epilepsy syndromes (1, 8, 9).
The nature, timing, and course of cognitive impairment in epilepsy are issues of substantial concern, particularly the degree to which chronic, medication-resistant epilepsy may lead to progressive cognitive impairment (10). While evidence of an association between persistent epilepsy and cognitive impairment has been reported (4), the early cognitive substrate or cognitive base upon which subsequent chronic epilepsy exerts its effects is an important consideration. Controlled studies of children and adolescents with chronic epilepsy, but with substantially fewer years of recurrent seizures than the typical chronic adult population, have demonstrated that these patients may exhibit significant neuropsychological impairment (11–15), suggesting the influence of an early adverse neurodevelopmental impact on cognition.
Cognitive Problems May Antedate the Diagnosis of Epilepsy
As investigators seek to determine the onset and timing of neurocognitive disruptions, children with new-onset epilepsy can help provide an early window on the process. To date, a small number of studies have examined cognition in children with new-onset epilepsy (16–21). Four of the six studies identified cognitive impairments at epilepsy onset; the mixed results may be attributable, at least in part, to the variable age ranges, test batteries, and epilepsy characteristics. Our own investigation of healthy children and children with recent onset of idiopathic localization-related and primary generalized epilepsy reveal a pattern of mild generalized cognitive difficulties that are evident approximately 10 months after seizure onset (Figure 1). Interesting are reports of academic underachievement prior to and/or at the onset of idiopathic epilepsy (18,22,23), suggestive of an antecedent neurobiological insult of uncertain etiology. Psychiatric or behavioral as well as academic problems may antedate the diagnosis of epilepsy not only in children (18, 22, 24–27) but also in adults (28, 29) with epilepsy. The mechanisms underlying this effect remain to be determined and are an important issue for future research. In that these antecedent insults can be observed even in children with idiopathic epilepsy, without abnormalities in overall brain volumes (16), the possibility exists that factors associated with underlying epileptogenesis leading to the onset of seizures may play a role (30).
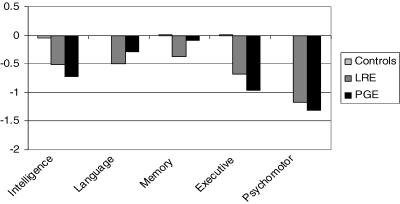
FIGURE 1.
Both the localization-related epilepsy (LRE) and primary generalized epilepsy (PGE) groups perform significantly worse than controls in intelligence, executive function, and psychomotor speed. LRE, but not PGE, differs from controls in language and memory. There are no significant differences between LRE and PGE groups. Y-axis shows adjusted z-scores (reprinted with permission from Brain 2006;129(Pt 10):2609–2619).
Structural Brain Abnormality
One factor that may underlie cognitive pathology in children with epilepsy is structural brain abnormality. Quantitative MRI volumetrics have been used to characterize the nature and pattern of brain abnormality in adults with epilepsy, especially temporal lobe epilepsy (31–34). Volumetric anomalies in adults are of clinical consequence, as demonstrated by their relationship with impaired cognition (35–42). In contrast, examination of the relationship between volumetric abnormalities and cognition are rare in the pediatric epilepsy literature and the structure-function relationships are limited to IQ. The studies, to date, have involved only children with chronic epilepsy, and the findings reveal abnormalities in cerebrum, cerebellum, and hippocampus (43–47). A recent voxel-based, morphometric investigation of children with chronic temporal lobe epilepsy found a distributed pattern of abnormality in temporal and extratemporal lobe gray matter (48), similar to that reported in the adults with temporal lobe epilepsy (49–54). Studies on volumetric abnormalities and cognition represent an area that needs future investigation.
Progressive Cognitive Impairment
Recent investigations have focused on the cumulative neurobiological burden associated with chronic epilepsy and the risk of progressive cognitive impairment (10). In addition, interest is growing in lifespan models of the neuropsychology of epilepsy (55,56). As mentioned, characterization of neuropsychological status at or near the onset of epilepsy can provide insight into the cognitive substrate upon which the effects of medically intractable seizure, medications, and other potentially adverse seizure-related factors may accumulate. It is becoming increasingly clear that this early substrate is not unaffected—the abnormalities often more diffuse than anticipated, with comparable effects across broad syndromes of epilepsy. An important clinical implication of these findings is the need to identify and remediate early neurobehavioral problems, especially in light of the possible adverse long-term psychosocial prognosis, even of persons with remitted childhood epilepsy (57). Children with academic problems at epilepsy onset are an especially important group to target in that prospective research (58) has demonstrated that a history of learning problems is a major marker for poor long-term psychosocial outcome.
The consequences of recurring seizures and antiepileptic treatment on normal cognitive development and brain growth must be more comprehensively characterized. Neurodevelopmental trajectories may be altered; perhaps, a more pronounced alteration occurs in younger children who are undergoing the greatest cognitive and brain growth compared with older children and adolescents—a hypothesis which remains to be fully tested (59,60). The presence of even static cognitive impairments in childhood and adolescence may have lifespan implications. Research on the general population has shown that lower childhood intelligence level at age 11 is associated with the risk of adverse cognitive outcomes decades later, while higher childhood intelligence is associated with better cognitive outcomes and protective effects (61, 62).
Similarly, long-term prospective investigations of normal aging have demonstrated that cognitive abnormalities in midlife may antedate or serve as harbingers of adverse cognitive outcomes decades later (63, 64). Thus, it may be important to optimize mentation and reduce the modifiable risk factors for adverse cognitive aging identified in the general population in order to maximize cognitive health in aging persons with epilepsy. The status of mentation in elders with unremitted epilepsy remains to be fully characterized, but the findings, to date, are not favorable (65). The degree to which early (childhood) or later (middle age) fixed cognitive abnormalities set the stage for greater than age-associated cognitive changes remains to be determined.
Cognition and Temporal Lobe Epilepsy
Temporal lobe epilepsy has been of special interest from a neuropsychological perspective, as it is a common syndrome, frequently with onset in childhood or adolescence and a prolonged and intractable course (66,67). Cognitive function among these patients is typically characterized by significant memory impairment (68), although it is now known that more diffuse neuropsychological impairments may be observed as well (69, 70). Specifically, a mean pattern of generalized cognitive dysfunction has been described, with poorer performance compared with controls across all tested cognitive domains, including memory (71). While informative, characterization of such an average or typical neuropsychological profile of patients with chronic temporal lobe epilepsy does not provide insight into the possible distinct groupings or cognitive typologies that may exist within the overall group.
A Taxonomic Approach to Cognitive Evaluation Presentation and Course
A yet untapped approach to understanding cognitive morbidity in epilepsy is taxonomic in nature. This approach involves assessing whether empirically derived groupings of patients with similar profiles of cognitive function can be identified either within or across epilepsy syndromes. Taxonomies facilitate reliable clustering of individuals into meaningful groups, could provide a different way of characterizing, and, thinking about cognitive morbidity in epilepsy, as well set the stage for further investigation of clinical and neurobiological correlates. To date, taxonomic approaches rarely have been used to advance the understanding of the neurobehavioral complications of various epilepsy syndromes (72). That is, rather than grouping patients on the basis of clinical seizure characteristics (e.g., seizure frequency) and examining the relationship between these seizure characteristics and cognition, a grouping of patients is derived based solely on the pattern of performance across several cognitive domains.
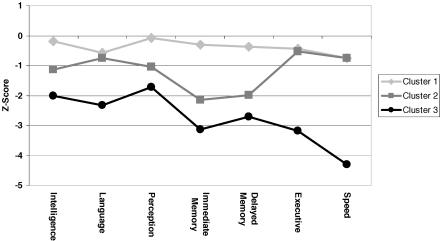
An example of the potential utility of this approach is the recent application of cluster analysis to a large sample of patients with temporal lobe epilepsy, which identified distinct cognitive subgroups or phenotypes (73). Two aspects of those findings are briefly presented here. First, from a neuropsychological perspective, three distinct cognitive profiles were uncovered: (a) minimally impaired, (b) memory impaired, and (c) memory, executive, and speed impaired (Figure 2).
FIGURE 2.
Cognitive profiles in temporal lobe epilepsy (reprinted with permission from Journal of the International Neuropsychological Society[in press]).
Cluster 1 (Minimally Impaired) consisted of approximately half (47%) of the temporal lobe epilepsy subjects; they exhibited the most intact cognition of the three cluster groups. That said, their performance across several cognitive domains, including language, immediate and delayed memory, executive function, and psychomotor speed domains was significantly worse than that of the controls. While statistically significant, the pattern was one of mild but discernable cognitive dysfunction.
Cluster 2 (Predominantly Memory Impaired) consisted of 27% of the patient sample. These subjects exhibited marked impairments in immediate and delayed memory as well as significantly poorer performance than controls across all other cognitive domains. Thus, memory was the most striking cognitive abnormality, and it occurred in the context of a mild, generalized depression of overall cognitive performance compared with controls.
Cluster 3 (Generalized Impairment) consisted of 29% of the temporal lobe epilepsy subjects. They exhibited the poorest cognition across all domains compared with controls and significantly poorer performance across all cognitive domains compared to both Clusters 1 and 2. The most striking impairments in this group fell in the areas of executive function and cognitive/psychomotor speed. Thus, an underlying taxonomy characterized by the nature, pattern, and severity of evident cognitive complications can be identified.
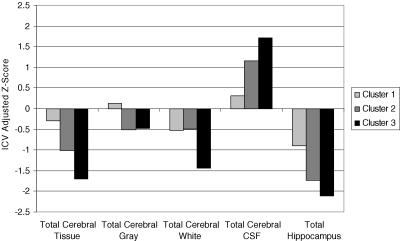
Second, validation of these cognitive phenotypes was provided by examining profiles of demographic features (e.g., age), clinical seizure features (e.g., duration of epilepsy, antiepileptic drug polytherapy), and brain volumetrics (e.g., segmented whole brain and lobar tissue volumes, CSF, and hippocampus). In brief summary, the volumetric findings paralleled the cognitive findings (Figure 3). The most intact group (Cluster 1) showed significant abnormality in hippocampal volume with minimal change in other morphometric measurements. As the degree of cognitive impairment increased (Cluster 2 to 3), a pattern of corresponding volumetric abnormality was demonstrated (including greater hippocampal atrophy), culminating in Cluster 3 for which there was evidence of widespread volumetric abnormality.
FIGURE 3.
MRI volumetric findings in cognitive profile groups (reprinted with permission from Journal of the International Neuropsychological Society[in press]).
In addition, the most cognitively impaired group (Cluster 3) was older, had the longest duration of epilepsy, and took more medications than the other groups, especially compared with Cluster 1. Meaningful but statistically nonsignificant trends in regard to other clinical seizure features also were found. For instance, the Cluster 3 group had the highest proportion of patients with histories of more than 50 lifetime-generalized tonic–clonic seizures, status epilepticus, and severe initial precipitating injuries. Thus, Cluster 3 appears to be the group most likely to have incurred both an earlier neurodevelopmental insult and a more protracted and severe course of epilepsy.
Cross-sectional studies, as valuable as they may be, cannot provide insight into the prospective course of the disorder. Of considerable interest, but still controversial, is the degree to which abnormalities in mental status may progress over the duration of the disorder. The issue of cognitive progression in temporal lobe epilepsy is important, as curative surgical treatments exist but are frequently delayed (66, 74, 75). Patients with medication-resistant temporal lobe epilepsy often present with considerable cognitive and behavioral handicap when finally referred for surgical consideration; unfortunately, sometimes after decades of unsuccessful medical management (76, 77).
Prospective cognitive studies of patients with epilepsy date back to the early part of the last century (4), but these are often characterized by rather limited assessment of cognition (often just IQ), the inclusion of mixed seizure types, varying test–retest intervals, lack of control groups, and other methodological shortcomings. More common are cross-sectional studies (70, 78, 79) that, while informative, suffer from the obvious limitation of providing an indirect evaluation of neuropsychological change over time, cohort effects, and other methodological problems that prevent a clear and unequivocal characterization of the cognitive course of epilepsy (1). A recent review (4) concluded that progressive cognitive decline does occur in a proportion of patients and appears to be associated with markers of a difficult epilepsy course (e.g., number of lifetime generalized tonic–clonic seizures).
In summary, the neurobehavioral status of persons with epilepsy is affected by factors unassociated with seizures or their treatment per se—given that there is evidence of abnormalities prior to the onset of the disorder, factors that are associated with the course or consequences of the disorder and its treatment, as well as general neurobiological factors that affect cognition in all of us. The literature on cognition and epilepsy is largely one of characterization and description, not treatment or remediation—a glaring omission in such a long and rich tradition.
References
- 1.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 2.Saling MM, Berkovic SF, O'Shea MF, Kalnins RM, Darby DG, Bladin PF. Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J Clin Exp Neuropsychol. 1993;15:608–618. doi: 10.1080/01688639308402582. [DOI] [PubMed] [Google Scholar]
- 3.Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004;45:54–63. doi: 10.1111/j.0013-9580.2004.33403.x. [DOI] [PubMed] [Google Scholar]
- 4.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(suppl 1):S21–S24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Helmstaedter C, Kurthen M. Memory and epilepsy: characteristics, course, and influence of drugs and surgery. Curr Opin Neurol. 2001;14:211–216. doi: 10.1097/00019052-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jones-Gotman M. Clinical neuropsychology and neocortical epilepsies. Adv Neurol. 2000;84:457–462. [PubMed] [Google Scholar]
- 7.Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- 8.Lassonde M, Sauerwein HC, Jambaque I, Smith ML, Helmstaedter C. Neuropsychology of childhood epilepsy: pre- and postsurgical assessment. Epileptic Disord. 2000;2:3–13. [PubMed] [Google Scholar]
- 9.Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, Bleasel AF, Bye AM. Intelligence in childhood epilepsy syndromes. Epilepsy Res. 2003;53:139–150. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 10.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 11.Roeschl-Heils A, Bledowski C, Elger CE, Heils A, Helmstaedter C. Neuropsychological functioning among 32 patients with temporal lobe epilepsy and their discordant siblings. Epilepsia. 2002;43(suppl 7):85. [Google Scholar]
- 12.Smith ML, Elliott IM, Lach L. Cognitive skills in children with intractable epilepsy: comparison of surgical and nonsurgical candidates. Epilepsia. 2002;43:631–637. doi: 10.1046/j.1528-1157.2002.26101.x. [DOI] [PubMed] [Google Scholar]
- 13.Germano E, Gagliano A, Magazu A, Sferro C, Calarese T, Mannarino E, Calamoneri F. Benign childhood epilepsy with occipital paroxysms: neuropsychological findings. Epilepsy Res. 2005;64:137–150. doi: 10.1016/j.eplepsyres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, Hermann B. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–731. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 16.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006 doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 17.Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol. 1983;14:438–444. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 18.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”—a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 19.Kolk A, Beilmann A, Tomberg T, Napa A, Talvik T. Neurocognitive development of children with congenital unilateral brain lesion and epilepsy. Brain Dev. 2001;23:88–96. doi: 10.1016/s0387-7604(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 20.Stores G, Williams PL, Styles E, Zaiwalla Z. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child. 1992;67:1330–1337. doi: 10.1136/adc.67.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams J, Bates S, Griebel ML, Lange B, Mancias P, Pihoker CM, Dykman R. Does short-term antiepileptic drug treatment in children result in cognitive or behavioral changes? Epilepsia. 1998;39:1064–1069. doi: 10.1111/j.1528-1157.1998.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 22.Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, Shinnar S. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 23.McNelis AM, Johnson CS, Huberty TJ, Austin JK. Factors associated with academic achievement in children with recent-onset seizures. Seizure. 2005;14:331–339. doi: 10.1016/j.seizure.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 25.Dunn DW, Harezlak J, Ambrosius WT, Austin JK, Hale B. Teacher assessment of behaviour in children with new-onset seizures. Seizure. 2002;11:169–175. doi: 10.1053/seiz.2001.0612. [DOI] [PubMed] [Google Scholar]
- 26.Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- 27.Jones J, Watson ER, Caplan R, Koehn M, Sheth R, Seidenberg M, Hermann BP. Psychiatric co-morbidity in children with newly diagnosed epilepsy. Epilepsia. 2004;47(suppl 7):353. [Google Scholar]
- 28.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–249. [PubMed] [Google Scholar]
- 29.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 30.Cortez MA, Perez Velazquez JL, Snead OC., 3rd Animal models of epilepsy and progressive effects of seizures. Adv Neurol. 2006;97:293–304. [PubMed] [Google Scholar]
- 31.Cendes F. Progressive hippocampal and extrahippocampal atrophy in drug resistant epilepsy. Curr Opin Neurol. 2005;18:173–177. doi: 10.1097/01.wco.0000162860.49842.90. [DOI] [PubMed] [Google Scholar]
- 32.Bernasconi A. Quantitative MR imaging of the neocortex. Neuroimaging Clin N Am. 2004;14:425–436. doi: 10.1016/j.nic.2004.04.013. viii. [DOI] [PubMed] [Google Scholar]
- 33.Koepp MJ, Duncan JS. Epilepsy. Curr Opin Neurol. 2004;17:467–474. doi: 10.1097/01.wco.0000137539.91738.2b. [DOI] [PubMed] [Google Scholar]
- 34.Kuzniecky RI, Knowlton RC. Neuroimaging of epilepsy. Semin Neurol. 2002;22:279–288. doi: 10.1055/s-2002-36647. [DOI] [PubMed] [Google Scholar]
- 35.Baxendale SA, van Paesschen W, Thompson PJ, Connelly A, Duncan JS, Harkness WF, Shorvon SD. The relationship between quantitative MRI and neuropsychological functioning in temporal lobe epilepsy. Epilepsia. 1998;39:158–166. doi: 10.1111/j.1528-1157.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 36.Baxendale SA, Sisodiya SM, Thompson PJ, Free SL, Kitchen ND, Stevens JM, Harkness WF, Fish DR, Shorvon SD. Disproportion in the distribution of gray and white matter: neuropsychological correlates. Neurology. 1999;52:248–252. doi: 10.1212/wnl.52.2.248. [DOI] [PubMed] [Google Scholar]
- 37.Martin R, Dowler R, Gilliam F, Faught E, Morawetz R, Kuzniecky R. Cognitive consequences of coexisting temporal lobe developmental malformations and hippocampal sclerosis. Neurology. 1999;53:709–715. doi: 10.1212/wnl.53.4.709. [DOI] [PubMed] [Google Scholar]
- 38.Burneo JG, Bilir E, Faught E, Morawetz R, Knowlton RC, Martin R, Kuzniecky RI. Significance of fornix atrophy in temporal lobe epilepsy surgery outcome. Arch Neurol. 2003;60:1238–1242. doi: 10.1001/archneur.60.9.1238. [DOI] [PubMed] [Google Scholar]
- 39.Dow C, Seidenberg M, Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav. 2004;5:919–925. doi: 10.1016/j.yebeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Hermann B, Seidenberg M, Sears L, Hansen R, Bayless K, Rutecki P, Dow C. Cerebellar atrophy in temporal lobe epilepsy affects procedural memory. Neurology. 2004;63:2129–2131. doi: 10.1212/01.wnl.0000145774.89754.0c. [DOI] [PubMed] [Google Scholar]
- 41.Griffith HR, Pyzalski RW, Seidenberg M, Hermann BP. Memory relationships between MRI volumes and resting PET metabolism of medial temporal lobe structures. Epilepsy Behav. 2004;5:669–676. doi: 10.1016/j.yebeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Seidenberg M, Geary E, Hermann B. Investigating temporal lobe contribution to confrontation naming using MRI quantitative volumetrics. J Int Neuropsychol Soc. 2005;11:358–366. [PubMed] [Google Scholar]
- 43.Lawson JA, Cook MJ, Bleasel AF, Nayanar V, Morris KF, Bye AM. Quantitative MRI in outpatient childhood epilepsy. Epilepsia. 1997;38:1289–1293. doi: 10.1111/j.1528-1157.1997.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 44.Lawson JA, Nguyen W, Bleasel AF, Pereira JK, Vogrin S, Cook MJ, Bye AM. ILAE-defined epilepsy syndromes in children: correlation with quantitative MRI. Epilepsia. 1998;39:1345–1349. doi: 10.1111/j.1528-1157.1998.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 45.Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Burns L, McAnally L, Pereira J, Bye AM. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia. 2000;41:1540–1545. doi: 10.1111/j.1499-1654.2000.001540.x. [DOI] [PubMed] [Google Scholar]
- 46.Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Bye AM. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia. 2000;41:1456–1462. doi: 10.1111/j.1528-1157.2000.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 47.Lawson JA, Cook MJ, Vogrin S, Litewka L, Strong D, Bleasel AF, Bye AM. Clinical, EEG, and quantitative MRI differences in pediatric frontal and temporal lobe epilepsy. Neurology. 2002;58:723–729. doi: 10.1212/wnl.58.5.723. [DOI] [PubMed] [Google Scholar]
- 48.Cormack F, Gadian DG, Vargha-Khadem F, Cross JH, Connelly A, Baldeweg T. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. Neuroimage. 2005;27:635–643. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Woermann FG, Free SL, Koepp MJ, Ashburner J, Duncan JS. Voxel-by-voxel comparison of automatically segmented cerebral gray matter—A rater-independent comparison of structural MRI in patients with epilepsy. Neuroimage. 1999;10:373–384. doi: 10.1006/nimg.1999.0481. [DOI] [PubMed] [Google Scholar]
- 50.Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- 51.Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004;61:1379–1384. doi: 10.1001/archneur.61.9.1379. [DOI] [PubMed] [Google Scholar]
- 54.Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005;25:1016–1021. doi: 10.1016/j.neuroimage.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 55.Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- 56.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 57.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 58.Camfield C, Camfield P, Smith B, Gordon K, Dooley J. Biologic factors as predictors of social outcome of epilepsy in intellectually normal children: a population-based study. J Pediatr. 1993;122:869–873. doi: 10.1016/s0022-3476(09)90009-9. [DOI] [PubMed] [Google Scholar]
- 59.Bjornaes H, Stabell K, Henriksen O, Loyning Y. The effects of refractory epilepsy on intellectual functioning in children and adults A longitudinal study. Seizure. 2001;10:250–259. doi: 10.1053/seiz.2000.0503. [DOI] [PubMed] [Google Scholar]
- 60.Neyens LG, Aldenkamp AP, Meinardi HM. Prospective follow-up of intellectual development in children with a recent onset of epilepsy. Epilepsy Res. 1999;34:85–90. doi: 10.1016/s0920-1211(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 61.Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- 62.Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 63.Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D'Agostino RB. The ‘preclinical phase’ of probable Alzheimer's disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 64.Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 65.Martin RC, Griffith HR, Faught E, Gilliam F, Mackey M, Vogtle L. Cognitive functioning in community dwelling older adults with chronic partial epilepsy. Epilepsia. 2005;46:298–303. doi: 10.1111/j.0013-9580.2005.02104.x. [DOI] [PubMed] [Google Scholar]
- 66.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–652. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 67.Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 68.Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–453. doi: 10.1016/S0079-6123(02)35041-6. [DOI] [PubMed] [Google Scholar]
- 69.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 70.Oyegbile T, Hansen R, Magnotta V, O'Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- 71.Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 72.Paradiso S, Hermann BP, Somes G. Patterns of academic competence in adults with epilepsy: a cluster analytic study. Epilepsy Res. 1994;19:253–261. doi: 10.1016/0920-1211(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 73.Hermann B, Seidenberg M, Leem EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. doi: 10.1017/S135561770707004X. in press. [DOI] [PubMed] [Google Scholar]
- 74.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 75.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 76.Trevathan E, Gilliam F. Lost years: delayed referral for surgically treatable epilepsy. Neurology. 2003;61:432–433. doi: 10.1212/wnl.61.4.432. [DOI] [PubMed] [Google Scholar]
- 77.Burneo JG, McLachlan RS. When should surgery be considered for the treatment of epilepsy? CMAJ. 2005;172:1175–1177. doi: 10.1503/cmaj.045118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999;67:44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Helmstaedter C, Elger CE. The phantom of progressive dementia in epilepsy. Lancet. 1999;354:2133–2134. doi: 10.1016/s0140-6736(99)03542-4. [DOI] [PubMed] [Google Scholar]