Abstract
There is a significant association between maternal cigarette smoking during pregnancy and greater subsequent risk of smoking in female offspring. In animal models, prenatal nicotine exposure causes persistent alterations in cholinergic and monoaminergic systems, both of which are important for nicotine actions underlying tobacco addiction. Accordingly, the current study was conducted to determine if there is a cause-and-effect relationship between prenatal nicotine exposure and nicotine self-administration starting in adolescence. Pregnant rats were administered nicotine (6 mg/kg/day) by osmotic minipump infusion throughout gestation and then, beginning in adolescence and continuing into adulthood, female offspring were given access to nicotine via a standard operant IV self-administration procedure (0.03 mg/kg/infusion). Gestational nicotine exposure did not alter the initial rate of nicotine self-administration. However, when animals underwent one week of forced abstinence and then had a second opportunity to self-administer nicotine, the prenatally-exposed animals showed a significantly greater rate of self-administration than did the controls. Prenatal nicotine exposure causes increased nicotine self-administration, which is revealed only when the animals are allowed to experience a period of nicotine abstinence. This supports a cause-and-effect relationship between the higher rates of smoking in the daughters of women who smoke cigarettes during pregnancy and implicates a role for nicotine in this effect. Our results further characterize the long-term liabilities of maternal smoking but also point to the potential liabilities of nicotine-based treatments for smoking cessation during pregnancy.
Keywords: Nicotine, Self-administration, Prenatal, Adolescent, Prenatal Nicotine Exposure
Introduction
Tobacco smoking during pregnancy despite the known health risks is still quite prevalent, 25% of all pregnancies in the United States (Ernst et al. 2001). Maternal smoking has been shown in many studies to be associated with a variety of adverse events in the offspring including low birth weight, sudden infant death syndrome, conduct disorder, cognitive impairment such as attention deficit hyperactivity syndrome and learning impairment, development of obesity as well as increased rates of smoking (Ernst et al. 2001; Levin and Slotkin 1998; Moolchan et al. 2000; Weissman et al. 1999). It is now clear that nicotine itself damages the developing brain through its effects on neural cell replication, differentiation and apoptosis, evoking lasting deficits in synaptic function, thus providing a mechanistic link between maternal smoking and adverse behavioral outcomes (reviews, (Levin and Slotkin 1998; Slotkin 1992; 1998; 1999; 2004)). Because smoking typically begins in adolescence (Nelson et al. 1995; Pierce and Gilpin 1996), recent attention has turned to identification of the biological differences that demarcate the response to nicotine in the adolescent brain. In general, the literature support the idea that there is a biobehavioral basis for the greater susceptibility of adolescents to effects of drugs of abuse (Laviola et al. 2003; Spear 2000), including with nicotine greater and more persistent nicotinic acetylcholine receptor upregulation than in adults, outright neuronal loss in brain regions responsible for learning, memory and mood, and more pronounced alterations in synaptic activity upon withdrawal of nicotine (Abreu-Villaça et al. 2003a; Abreu-Villaça et al. 2003b; Abreu-Villaça et al. 2003c; Slotkin 2002; Slotkin et al. 2006; Trauth et al. 2000; Trauth et al. 2001; Trauth et al. 1999). Adolescent rats were found to show fewer acute somatic signs of nicotine withdrawal than adults when mecamylamine was administered (O'Dell et al. 2004a). This diminished negative consequence of intermittent nicotine use may also facilitate continued use. When access to nicotine is started in rats during adolescence, there is a substantially higher level of self-administration than in adult rats (Levin et al. 2003a).
Superimposed on the greater susceptibility of adolescents to nicotine dependence and addiction, there are subpopulations that are especially vulnerable, most notably those whose mothers smoked during pregnancy (Cornelius et al. 2000; Kandel et al. 1994; Niaura et al. 2001; Weissman et al. 1999). Interestingly, there appears to be a robust gender difference in this relationship, with females showing a stronger association between prenatal tobacco exposure and subsequent smoking in adolescence and adulthood (Kandel et al. 1994; Weissman et al. 1999).
Although a strict cause-and-effect relationship between maternal smoking during pregnancy and subsequent smoking by the offspring is difficult to establish by epidemiological studies alone, a number of recent evaluations in animals support the idea that prenatal nicotine exposure programs the subsequent response to nicotine, enhancing the likelihood of dependence by augmenting the neuronal loss caused by adolescent nicotine, lowering tonic cholinergic synaptic reactivity and enhancing the deficits in synaptic function evoked by withdrawal from nicotine administered in adolescence (Abreu-Villaça et al. 2004a; Abreu-Villaça et al. 2004b; Slotkin et al. 2006). Here, too, the evidence supports a gender difference, with females showing greater hippocampal cell loss and synaptic inhibition during withdrawal (Abreu-Villaça et al. 2003c; Xu et al. 2002; Xu et al. 2003). Adolescent onset nicotine self-administration in female rats continues to be greater than adult onset self-administration even when those adolescents grow into adulthood (Levin et al. 2003a). Accordingly, in the present study utilizing female rats, we modeled intravenous nicotine self-administration to evaluate the effect of gestational nicotine exposure on subsequent voluntary nicotine self-administration beginning in adolescence and continuing into adulthood. Importantly, we also considered the role of nicotine abstinence (withdrawal), an aspect critical to both adolescent smoking and the effects of maternal smoking on tobacco use in the offspring. Subsets of adolescents, preferentially female, show the onset of nicotine dependence and loss of autonomy very early in the initiation of tobacco use, even after just a few cigarettes, and these effects are associated with enhanced withdrawal symptoms (DiFranza et al. 2002a; DiFranza et al. 2000; DiFranza et al. 2002b; Jacobsen et al. 2005; Jacobsen et al. 2006). Interestingly, the smoking withdrawal induced cognitive impairment of deficits in visuospatial memory was significantly greater in adolescents whose mothers smoked during pregnancy compared with withdrawn adolescent smokers whose mothers did not smoke during pregnancy (Jacobsen et al. 2005; Jacobsen et al. 2006). Thus, in addition to evaluating nicotine self-administration in animals with and without gestational nicotine exposure, we assessed the response to subsequent sessions of nicotine self-administration after a period of abstinence.
Acute drug challenges were given to determine if differences in the neural bases of nicotine self-administration could be detected. Acute nicotine challenge just prior to the nicotine self-administration session was made to determine if priming the nicotine response would differentially increase self-administration in the rats with a history of prenatal nicotine exposure. The monoamine oxidase inhibitor (MAOI) phenelzine was administered as a challenge because of the recent finding that MAOI treatment greatly increases motivation to self-administer nicotine (Guillem et al. 2005). The principal goal of this study was to determine which aspects of nicotine self-administration might be altered as a persisting legacy of prenatal nicotine intoxication in an animal model so that the finding of increased tobacco smoking addition in the offspring of mothers who smoked during pregnancy can be better understood and treated.
Methods
Prenatal nicotine treatment
All studies were carried out with the approval of the Duke University Institutional Animal Care and Use Committee, in accordance with the declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC) were shipped on gestational day 2 by climate-controlled truck (total transit time < 1 h), housed individually and allowed free access to food and water. On gestational day 4, before implantation of the embryo in the uterine wall, each animal was quickly anesthetized with ether, a 3 × 3 cm area on the back was shaved, and an incision made to permit s.c. insertion of type 2ML2 Alzet osmotic minipumps. Pumps were prepared with nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in bacteriostatic water adjusted to pH 6.0 (Abbott Laboratories, N. Chicago, IL), to deliver an initial dose rate of 6 mg/kg of nicotine (calculated as free base) per day. The incision was closed with wound clips and the animals were permitted to recover in their home cages. Control animals were implanted with minipumps containing only the water and an equivalent concentration of sodium bitartrate, adjusted to pH 6.0. It should be noted that the pump, marketed as a 2-week infusion device, actually takes 17.5 days to be exhausted completely (information supplied by the manufacturer) and thus the nicotine infusion terminates during GD21. Maternal plasma nicotine levels achieved with this administration model are in the same range of blood levels seen in heavy smokers of 2–3 packs/day (25–60 ng/ml) as characterized previously (Isaac and Rand 1972; Levin and Slotkin 1998; Lichtensteiger et al. 1988; Murrin et al. 1985; Slotkin 1992; 1998; 1999; 2004).
Parturition occurred during gestational day 22, which was also taken as postnatal day 0. After birth, pups were randomized within treatment groups and litter sizes were culled to 10 (5 males and 5 females) to ensure standard nutrition. Randomization was repeated every few days to distribute differential effects of maternal caretaking equally among all litters; cross-fostering, by itself, has no impact on neurochemical or behavioral effects of nicotine exposure (Ribary and Lichtensteiger 1989). Animals were weaned on PN21 and then housed singly.
Nicotine self-administration
Prior to commencing nicotine self-administration, the day-night cycle was reversed (lights on 18:00–6:00) so that animals were in their active (nocturnal) phase during behavioral testing. During this time, the rats had unrestricted access to water, and were fed approximately 20 g of chow daily. After a 3-day acclimation period, behavioral training began in the age range of 25–29 days and food consumption was restricted to approximately 16 g of chow per day to maintain the rats at approximately 85% of their free-feeding weights adjusted for growth. Testing began in the age range of days 40–46 in order to commence nicotine self-administration within the adolescent period.
For behavioral training and self-administration, rats were placed in dual lever test chambers (Med Associates, Georgia VT). Each chamber was equipped with a tone generator, house light, cue light above each lever, and a metal tether to cover the drug delivery line. A Pentium computer programmed with MED-PC software controlled experimental events and data collection. Each catheter was connected to a High Speed Micro-Liter Syringe Pump (Med Associates), with polyethylene tubing and a Huber needle to access the port (Instech-Solomon, Plymouth Meeting, PA). During each session, the rats wore Covance infusion harnesses (Instech-Solomon) that were connected to stainless steel tethers, which protected the drug delivery lines.
Solutions of nicotine bitartrate were prepared weekly in pyrogen-free glassware in sterilized isotonic saline. The dose used for self-administration (0.03 mg/kg/infusion) was calculated as a function of the nicotine base weight. The pH of the solutions was adjusted to 7.0 using NaOH and then the solutions were passed through a Nalgene filter (Nalgene Nunc International, Rochester, NY) for sterilization. Between sessions, all solutions were kept refrigerated in the dark to prevent the decomposition of nicotine.
Catheters were flushed daily, before the sessions began, with a 0.3 ml solution containing 100U/ml heparinized saline (Baxter Health Corporation, Deerfield, IL). When sessions were over, the nicotine remaining in the ports was drawn out and replaced by a sterile lock consisting of heparinized saline 500U/ml with 8 mg/kg gentamicin (American Pharmaceutical Partners, Schaumburg, IL). In determining the gentamicin dose, the volume of the sterile lock infusion was adjusted to the weight of each animal.
The rats were divided into four cohorts with at least two control and two prenatal nicotine treated rats in each to balance the capacity to do the catheter implantation surgeries over days 39–43 (average age at surgery was 41 days). Cohort was included as a factor in the statistical analysis of nicotine self-administration.
Initially, the rats were hand- trained to press the levers for food pellet reinforcers. They were given daily tutoring sessions, lasting 15 minutes, for 8 days. During these sessions, correct responses were rewarded by the trainer’s pressing a button, which caused immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 sec. Half the animals were reinforced for responding on the right lever and half for responding on the left. The cue light over the correct lever was illuminated while the light over the incorrect lever was covered (under the tutor program, both cue lights are turned on, but only one cue light is exposed, to show which lever is correct.). The tutoring sessions were followed by 3 daily pellet sessions on a FR-1 schedule, with feedback tone, lasting 45 minutes. This program activated the correct lever and only the cue light above that lever was illuminated. So, for the first 3 sessions, lever pressing was paired with delivery of food pellets, but, in the sessions that followed, nicotine was given as a sole reinforcer.
After the food reinforcement sessions, rats had catheters surgically implanted into the jugular vein to enable them to receive nicotine infusions. They then began nicotine self-administration at 40–46 days of age, evaluated over a period of 4 weeks. Two levers were available to be pressed in every session of nicotine self-administration and only one caused the delivery of nicotine, whereas the other did not, thus serving as a control. Pressing the lever on the active side resulted in the activation of the feedback tone for 0.5 sec and the immediate delivery of one 50-μl infusion of nicotine in less than 1 sec. Each infusion was immediately followed by a one-minute period in which the cue lights went out and responses were recorded but not reinforced. The sessions lasted for 45 min.
Immediately after this phase, the rats were tested for nicotine self-administration after an acute challenge with 0.07 mg/kg of nicotine bitartrate (dose measured as free base). The s.c. injections were given 10 minutes before the test session in a volume of 1 ml/kg of sterile saline. This nicotine dose was given in a counterbalanced order with an injection of the saline vehicle. Then the animals were withdrawn from drug access for a period of 1 week without testing, during which time the rats were left undisturbed in their home cages, but their catheters were kept open.
After the abstinent period, animals were once again tested for nicotine self-administration for an ensuing five 45-min. sessions at an average of 82 days of age. Three rats could not be tested during this phase because of blocked catheters. Because this left no controls in cohort four this cohort was removed from the analysis leaving N=6 controls and N=5 prenatal nicotine exposed rats. In addition to retesting nicotine self-administration, we further examined the response to an acute challenge with the monoamine oxidase inhibitor phenelzine (0, 2 and 4 mg/kg), injected i.p. 1 hour before the self-administration session, in a volume of 10 ml/kg of sterile saline, with the acute treatment delivered in a counterbalanced design.
Statistical analysis
Data are presented as means and standard errors, with differences between prenatal treatment groups assessed by analysis of variance; cohort (the age at which nicotine self-administration began) was included as a factor in the statistical analysis. An alpha level of p<0.05 was used as a cutoff for statistical significance. Data collected during the week of nicotine self-administration and challenge drug dose were evaluated at multiple times for each subject and were included as repeated measures.
Results
The effects of prenatal nicotine exposure on maternal weight gain, litter size, neonatal viability and postnatal growth have all appeared in previous publications with this model (Levin and Slotkin 1998; Slotkin 1992; 1998; 1999; 2004). By weaning, there were no weight differences between the control and nicotine-exposed offspring (data not shown). Initial training for self-administration of food pellets was not significantly affected by prenatal nicotine exposure. The control rats self-administered 110±16 pellets/session (mean±sem) while rats prenatally exposed to nicotine self-administered averaged 119±5.
There was no significant main effect of prenatal nicotine exposure in the initial phase of nicotine self-administration of nicotine over the four weeks from adolescence into adulthood. Both the prenatal nicotine and control groups averaged just over 10 infusions per session (Fig. 1). After four weeks of nicotine self-administration the impact of acute challenge with nicotine was assessed. Acute nicotine (0.07 mg/kg) challenge did not have a significant effect on nicotine self-administration nor did it significantly interact with the prenatal nicotine exposure condition.
Figure 1.
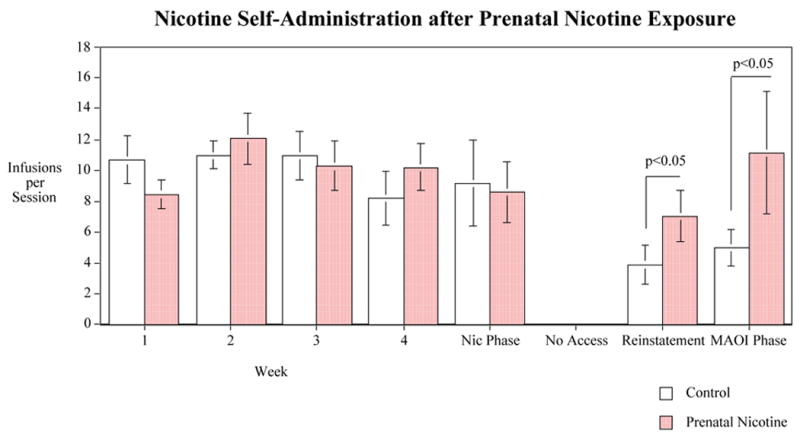
Nicotine IV self-administration in female offspring after prenatal chronic nicotine self-administration, infusions per session (mean±sem). Control Rats N=8 and Prenatal Nicotine Exposed Rats N=12 except for the phase after the abstinence and when the with the monoamine oxidase inhibitor (MAOI) phenelzine when control N=6 and prenatal nicotine N=5.
Then, the rats had one week of abstinence in which they did not have access to nicotine for self-administration. Upon reinstatement of access to nicotine, there was a significant (F(1,12)=6.03, p<0.05) main effect of prenatal nicotine exposure on later nicotine self-administration. As shown on the right hand side of figure 1, after the week of withdrawal the control rats descended to 3.9±1.3 infusions per session while the rats prenatally exposed to nicotine maintained self-administration of 7.0±1.7 infusions/session.
The final phase of testing included challenge with the MAO inhibitor phenelzine, which did not cause any detectable effect on nicotine self-administration, nor did it significantly interact with the prenatal nicotine exposure effect. Again, there was a significant (F(1,5)=14.03, p<0.05) main effect of prenatal nicotine exposure with the rats prenatally exposed to nicotine (11.1±2.3 infusions/session) continuing to have increased nicotine self-administration relative to controls (5.0±1.3 infusions/session).
Discussion
In the current study, we found that prenatal nicotine exposure results in a persisting increase in nicotine self-administration, which becomes clearly apparent only after an intervening period of nicotine abstinence, which appeared to be critical for the expression of the difference in nicotine self-administration in the rats exposed to nicotine prenatally vs. controls. Although both control and prenatally-exposed adolescent rats showed high rates of nicotine self-administration, the control rats did not maintain this rate in adulthood, exhibiting a marked decrease from the adolescent level as has been seen before (Levin et al. 2003b). In contrast, the rats with prenatal nicotine exposure showed a persistence of nicotine self-administration at the adolescent level. This type of long-lasting effect of prenatal nicotine may make relapse after smoking cessation more likely in the offspring of women who smoked during pregnancy.
In earlier work, we found that female rats self-administer nicotine at a higher rate in adolescence than in adulthood (Levin et al. 2003b), an effect also seen here in the fall-off in the number of infusions per session in the control group (animals that did not receive prenatal nicotine) between the first period of self-administration starting in adolescence and the second period in adulthood. The group that did have a history of prenatal nicotine exposure did have a different response to nicotine in adulthood, particularly in the continuation of nicotine self-administration after a period of withdrawal. The prenatal nicotine appears to have permanently altered future response to changes in nicotine exposure. Even so, the effect of prenatal nicotine on adolescent self-administration is not straightforward, since we did not observe any initial effect, in keeping with an earlier report for female mice with oral nicotine self-administration (Klein et al. 2003). Rather, the increase emerged only after the animals had undergone a period of abstinence, pointing to a potential critical role of withdrawal in establishing a persistent change in nicotine intake. Indeed, a number of studies point to the importance of withdrawal as a feature that distinguishes many of the neurochemical aspects of nicotine administration in the adolescent from that in the adult, including specific defects in cholinergic and monoaminergic synaptic signaling and behavioral response (Abreu-Villaça et al. 2003b; Levin et al. 1993; Levin et al. 1996; O'Dell et al. 2004b; Slotkin et al. 2006; Trauth et al. 2000; Trauth et al. 2001).
In the current study, the drug challenges of nicotine and the MAOI phenelzine administered before the nicotine self-administration session did not significantly influence nicotine self-administration in either the prenatal nicotine exposed or control rats. It may be the case that other doses or dosing regimens may be necessary to demonstrate effects of these challenges. But further research would be necessary to determine if there are effects of these treatments.
Our study focused on females in light of the stronger relationship of maternal smoking to subsequent smoking by female offspring (Kandel and Udry 1999; Kandel et al. 1994) but it would certainly be valuable to perform parallel assessments in males. In the oral self-administration model, adolescent male mice ordinarily show a lower nicotine intake than females, but after prenatal nicotine exposure, that pattern was reversed, effectively feminizing the male response (Klein et al. 2003). Indeed, prenatal nicotine exposure feminizes several aspects of neurobehavioral performance (Lichtensteiger and Schlumpf 1985), and accordingly, a detailed future elaboration of gender differences in the combined effects of prenatal and adolescent nicotine administration would likely prove quite valuable.
Nevertheless, what is most critical here is the parallelism between these findings and those in adolescent smokers. These include a higher rate of smoking in daughters of women who smoked during pregnancy (Kandel and Udry 1999; Kandel et al. 1994), the existence of subsets of adolescents who show rapid onset of nicotine dependence and withdrawal symptoms upon abstinence (DiFranza et al. 2002a; DiFranza et al. 2000; DiFranza et al. 2002b; Jacobsen et al. 2005), and the specific role of prenatal exposure via maternal smoking to that subset (Jacobsen et al. 2006). Our data thus strengthen the view that the effects of maternal smoking on subsequent smoking prevalence in the offspring reflect basic biologic changes in the developing brain that alter the response to nicotine in adolescence and adulthood. In particular, the increase in withdrawal effects after chronic nicotine in the offspring with nicotine exposure during development may make it more difficult to remain abstinent after smoking cessation attempts. Recognition of the importance of withdrawal as a factor initiating successively higher nicotine intake not only provides insight into the dynamics of adolescent smoking but also can guide future attempts to improve amelioration strategies. As just one example, recent findings point to cognitive deterioration with regard to visuospatial memory after smoking abstinence in teenagers whose mothers smoked during pregnancy, whereas abstinence in offspring of nonsmokers showed improved cognition (Jacobsen et al. 2006). Strategies aimed at offsetting cognitive impairment might then prove especially useful in this subpopulation.
The model used in the current study produced nicotine blood levels of a heavy smoker. Clearly significant effects were seen in terms of increased nicotine self-administration after a withdrawal period. The question of the relative risk of lower level nicotine exposure on self-administration remains to be characterized. This study and others show that a particularly vulnerable effect of prenatal nicotine follows nicotine withdrawal later in the life of the offspring. This will aid in focusing further investigation with lower doses.
The current results reinforce the concern about the blanket use of nicotine replacement therapy for smoking cessation in pregnancy. Nicotine itself is a neuroteratogen and therefore contributes in a major way to the neurobehavioral deficits associated with smoking in pregnancy and potentially to other deleterious outcomes such as Sudden Infant Death Syndrome (Levin and Slotkin 1998; Slotkin 1992; 1998; 1999; 2004). Continuous nicotine delivery, akin to that achieved with the transdermal nicotine patch, actually achieves higher concentrations of nicotine in fetal brain than in maternal blood (Sarasin et al. 2003). It should be kept in mind though that the typical blood levels achieved by nicotine skin patches are lower than those produced by the dosing in the current study. The dose-risk relationship needs further study to fully characterize the impact of lower nicotine exposure levels on risk of the offspring for smoking. Nevertheless, the current study does suggest that this approach to smoking cessation may not alleviate the increased risk of tobacco addiction in the offspring. Given the fact that nicotine replacement therapy does not appear to increase cessation rates significantly in pregnant smokers, or may be marginally effective at best (Kapur et al. 2001; Wisborg et al. 2000), it may be more useful to rely on alternative therapies for smoking cessation in pregnancy.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (DA015756) awarded to E.D.L. and a grant from Philip Morris USA Inc. and Philip Morris International awarded to T.A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003a;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003b;988:164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Slotkin TA. Does prenatal nicotine sensitize the brain to nicotine-induced neurotoxicity in adolescence? Neuropsychopharmacology. 2004a;29:1440–1450. doi: 10.1038/sj.npp.1300443. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004b;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Res. 2003c;979:114–128. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tobacco Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- DiFranza J, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30-month follow-up data from the DANDY study. Tobacco Control. 2002a;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tobacco Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (development and assessment of nicotine dependence in youths) study. Arch Pediatr Adolesc Med. 2002b;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador MLS. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. The Journal of Neuroscience. 2005;25:8593– 8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac PF, Rand MJ. Cigarette smoking and plasma levels of nicotine. Nature. 1972;236:308–310. doi: 10.1038/236308a0. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiat. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Westerveld M, Mencl WE, Pugh KR. Visuospatial memory deficits during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1300981. in press. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters' smoking: nicotine or testosterone exposure? Am J Pub Health. 1999;89:1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur B, Hackman R, Selby P, Klein J, Koren G. Randomized, double-blind, placebo-controlled trial of nicotine replacement therapy in pregnancy. Curr Ther Res - Clin Exp. 2001;62:274–278. [Google Scholar]
- Klein LC, Stine MM, Pfaff DW, Vandenbergh DJ. Maternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6J mice. Nicotine Tobacco Res. 2003;5:117–124. doi: 10.1080/14622200307257. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Levin E, Rezvani A, Montoya D, Rose J, Swartzwelder H. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003a;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003b;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. In: Slikker W, Chang LW, editors. Handbook of Developmental Neurotoxicology. Academic Press; San Diego: 1998. pp. 587–615. [Google Scholar]
- Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Dev Brain Res. 1996;97:207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Schlumpf M. Prenatal nicotine affects fetal testosterone and sexual dimorphism of saccharin preference. Pharmacol Biochem Behav. 1985;23:439–444. doi: 10.1016/0091-3057(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Ernst M, Henningfield JE. A review of tobacco smoking in adolescents: treatment implications. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:682–93. doi: 10.1097/00004583-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Zeng W. Nicotine administration during pregnancy and its effect on striatal development. Neurosci Abs. 1985;11:69. [Google Scholar]
- Nelson DE, Giovino GA, Shopland DR, Mowery PD, Mills SL, Eriksen MP. Trends in cigarette smoking among US adolescents, 1974 through 1991. Am J Public Health. 1995;85:34–40. doi: 10.2105/ajph.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, Buka S. Maternal transmission of nicotine dependence: psychiatric, neurocognitive and prenatal factors. Am J Addict. 2001;10:16–29. doi: 10.1080/105504901750160420. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004a;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann NY Acad Sci. 2004b;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Gilpin E. How long will today's new adolescent smoker be addicted to cigarettes? Am J Pub Health. 1996;86:253–256. doi: 10.2105/ajph.86.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- Sarasin A, Schlumpf M, Müller M, Fleischmann I, Lauber ME, Lichtensteiger W. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod Toxicol. 2003;17:153–162. doi: 10.1016/s0890-6238(02)00119-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Prenatal exposure to nicotine: What can we learn from animal models? In: Zagon IS, Slotkin TA, editors. Maternal Substance Abuse and the Developing Nervous System. Academic Press; San Diego: 1992. pp. 97–124. [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the response to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1300988. in press. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: effects on cholinergic systems in rat brain regions. Brain Res. 2000;873:18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiat. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol. 2000;96:967–971. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Cousins MM, Slikker W, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Tate CA, Garcia SJ, Slikker W, Slotkin TA. Sex-selective hippocampal alterations after adolescent nicotine administration: effects on neurospecific proteins. Nicotine Tobacco Res. 2003;5:955–960. doi: 10.1080/14622200310001615321. [DOI] [PubMed] [Google Scholar]


