Abstract
We show that intraepithelial cell neutralization of HIV by IgA antibodies to internal viral proteins can occur during antibody transcytosis from the basolateral to the apical surface. Polarized epithelial cells expressing the polymeric immunoglobulin receptor (pIgR) were transfected with HIV proviral DNA, and IgA was added to the basolateral side. Transcytosing IgA antibodies against Gag and RT significantly inhibited HIV replication as assessed by infection of HeLa-CD4-LTR/β-Gal cells and direct p24 assay. Consistent with intracellular neutralization, colocalization of the internal virus proteins and their IgA antibodies was demonstrated by confocal microscopy. Thus, at least in the context of infections of polarized epithelia, antibody-mediated neutralization may not be restricted to viral surface antigens.
Keywords: Immunodeficiency diseases, Viral infection, Mucosa, Antibodies
Most heterosexual, homosexual and vertical infections with HIV occur through contact with a mucosal surface (Smith and Wahl, 2005), which acts as a gateway to the body. Suggested mechanisms of entry include viral transcytosis across epithelial cells or their tight junctions, infection of lymphoid cells through breaches in the epithelial lining, and infection of epithelial cells (Amerongen et al., 1991; Bomsel, 1997; Fantini et al., 1992; Fantini et al., 1991; Han et al., 2000; Hu et al., 2000; Lagaye et al., 2001; Moore et al., 2002; Moore et al., 2003; Qureshi et al., 1995; Spira et al., 1996).
The protective functions of the principal class of mucosal antibody, IgA, depend on pIgR-mediated endocytosis at the basolateral surface of epithelial cells and subsequent transcytosis. This process makes it possible for IgA to (a) block viral adherence or (b) bind virus in the lamina propria or in an epithelial cell and transport it into the lumen (Bomsel et al., 1998; Mazanec et al., 1995; Mazanec et al., 1992; Mazanec et al., 1993; Yan et al., 2002). Function (b) is referred to in general as “antigen excretion”, or in the particular case of viruses as “virus excretion” (Kaetzel et al., 1991; Robinson et al., 2001; Yan et al., 2002). On the other hand, we have used the term “intracellular neutralization” to describe antibody that inhibits virus production through an intracellular action (Mazanec et al., 1995; Mazanec et al., 1992; Mazanec et al., 1993; Yan et al., 2002). Such intraepithelial cell neutralization has been shown for IgA antibodies against Sendai virus, measles, influenza, rotavirus and HIV (Burns et al., 1996; Feng et al., 2002; Fujioka et al., 1998; Huang et al., 2005; Mazanec et al., 1992; Mazanec et al., 1993; Schwartz-Cornil et al., 2002; Yan et al., 2002).
Resistance to HIV has been correlated with the presence of specific mucosal IgA in uninfected sex workers and sexual partners of infected individuals in some but not all studies (Beyrer et al., 1999; Buchacz et al., 2001; Devito et al., 2002; Devito et al., 2000; Dorrell et al., 2000; Kaul et al., 2001; Kaul et al., 1999; Mazzoli et al., 1999; Mazzoli et al., 1997; Skurnick et al., 2002), and anti-HIV IgA able to neutralize T-cell line-adapted and primary HIV isolates has been collected from both infected humans, even though anti-HIV IgA responses tend not to be prominent (Burnett et al., 1994; Devito et al., 2000; Kozlowski et al., 1995; Mestecky et al., 2004; Moja et al., 2000; Wu et al., 2003), and mucosally immunized mice (Akagi et al., 2003; Bukawa et al., 1995; VanCott et al., 1998). Earlier we demonstrated that IgA antibodies against HIV envelope protein can mediate intracellular neutralization (Huang et al., 2005). The neutralization is thought to result from the fusion of transcytotic vesicles containing IgA antibody with vesicles containing envelope protein that have budded off the secretory pathway that is followed by proteins synthesized in the rough endoplasmic reticulum and destined for cell surface expression or export. In contrast to envelope proteins and the secretory pathway, the subcellular trafficking of internal viral proteins that are synthesized on free cytoplasmic ribosomes is less well understood. Here we show that the less variant internal HIV proteins Gag and RT can also be targets for antibody-mediated intracellular neutralization. The results have implications for the design of mucosal vaccines.
Murine IgA monoclonal antibodies (MAb) against RT and the structural protein p24 (Gag) were employed. The anti-Gag hybridoma, 183-H12-5C, which produces IgG1 (Chesebro et al., 1992), was obtained from the NIH AIDS Research and Reagent Program, and an IgA MAb switch variant was selected after repeated cycles of limiting dilution and spontaneous isotype switching (Boot et al., 1988). RT IgG and IgA hybridomas were produced by immunizing mice with RT protein (kindly provided by Stuart Le Grice, NIH). Production, purification, and characterization of MAbs were performed as described (Yan et al., 2002). IgA anti-gp120 was described in (Huang et al., 2005). The percent of oligomeric IgA, the form capable of binding to the pIgR, was 84% and 68% for the RT and Gag IgAs respectively.
Transport of RT and Gag IgA
The ability of the MAbs to transcytose a polarized epithelial membrane was tested with a monkey kidney cell line, Vero C1008, stably transfected to express human pIgR (Huang et al., 1999; Yan et al., 2002). Membrane polarization was tested by monitoring electrical resistance between apical and basal chambers. Transcytosis was assessed after adding 100 μg/mL MAb to the basolateral chamber. Apical supernatants were collected and analyzed by ELISA for Ig content. The amount of anti-RT IgA transported at 4, 8, and 12 h was 149±19, 433±25, and 557±37 ng, and for anti-Gag IgA was 73±13, 145±21, and 237±15 ng. In contrast, the maximum amount of IgG, which does not bind to pIgR, that reached the apical chamber at 12 h was 26±2 ng.
Intracellular HIV neutralization
The ability of the MAbs to neutralize HIV inside polarized epithelial cells was assayed as in (Huang et al., 2005). Briefly, polarized pIgR+ Vero cells were transfected with 2 μg of proviral HIV DNA (pKS242) with a 4 h incubation before the cells were washed and fresh medium added apically and either medium or MAb added to the basolateral chamber for 18 h. Then cells were washed and fresh media added. After a 12 h incubation the apical and basolateral media were collected. Monolayers were washed to remove extracellular virus, and cells were collected via scraping, freeze-thawed and centrifuged to create cell lysate. HeLa (CD4-LTR/β-Gal) cells expressing human CD4 and the HIV long terminal repeat fused to a LacZ reporter gene (Kimpton and Emerman, 1992) were infected with apical supernatant, basal medium, or cell lysate, and the blue infected cells were counted 48 h after infection (Huang et al., 2005).
Virus titers measured by the above blue cell assay were decreased 38% and 40% (p<0.005) in the apical supernatants by RT and Gag IgA and by 36% and 35% (p<0.005) in the cell lysates compared to controls without antibody (Table 1). Virus titers in the basal samples were too low to quantify (data not shown). The IgAs capable of mediating this neutralization were internalized by the pIgR since neither pIgR− cells with the same IgAs nor pIgR+ cells with anti-RT or anti-Gag IgG showed neutralization. Irrelevant IgA MAb (anti-measles virus hemagglutinin) did not neutralize, indicating the intraepithelial neutralization was immunologically specific (Table 1).
Table 1.
Intracellular neutralization of HIV by transcytosing IgA against internal HIV-1 proteins.a
| Apical Supernatant
|
Cell Lysate
|
|||
|---|---|---|---|---|
| MAb (150μg/mL) | Blue Cell # | % Virus Reductionb | Blue Cell # | % Virus Reductionb |
| pIgR+ Cells | ||||
| Control | 136 ± 16 | 1797 ± 194 | ||
| RT-IgA | 84 ± 23 | 38c | 1143 ± 200 | 36c |
| RT-IgG | 133 ± 20 | 3 | 1851 ± 197 | 0 |
| Gag-IgA | 81 ± 18 | 40c | 1176 ± 128 | 35c |
| Gag-IgG | 129 ± 17 | 5 | 1777 ± 164 | 1 |
| Irrelevant IgA | 131 ± 27 | 4 | 1871 ± 357 | 0 |
| pIgR− Cells | ||||
| Control | 152 ± 12 | 1943 ± 134 | ||
| RT-IgA | 155 ± 33 | 0 | 1872 ± 127 | 4 |
| Gag-IgA | 159 ± 32 | 0 | 1933 ± 137 | 1 |
Polarized monolayers of pIgR+ or pIgR− Vero C1008 cells were transfected with HIV proviral DNA for 4 h and then exposed to MAbs basolaterally for 18 h. HIV levels in apical supernatants and cell lysates 30 h post transfection were determined by infecting target HeLa cells (“blue cell” assay). Data are means from eight data points ± SEM pooled from four experiments.
Compared to the no antibody control.
Result significantly different (p<0.005) from the no antibody control according to a two-tailed t-test.
Concentration dependence of neutralization
Intracellular neutralization as a function of IgA antibody concentration is shown in Table 2. For both anti-RT and anti-Gag IgA, the reduction in virus titers decreased with lower antibody concentrations. This was true for both apical supernatants and cell lysates. A higher antibody concentration, 300 μg/mL, reduced virus production similarly to the 150 μg/mL concentration (data not shown).
Table 2.
IgA antibody concentration dependence of intracellular virus neutralization and comparison to conditioned media a
| Apical Supernatant
|
Cell Lysate
|
|||
|---|---|---|---|---|
| IgA MAb (μg/mL) | Blue Cell # | % Virus Reductionb | Blue Cell # | % Virus Reductionb |
| Control | 156 ± 26 | 1781 ± 123 | ||
| Anti-RT (150) | 96 ± 21 | 39 c | 1075 ± 125 | 40c |
| Anti-RT (50) | 115 ± 14 | 26c | 1336 ± 252 | 25c |
| Anti-RT (20) | 126 ± 34 | 19c | 1674 ± 133 | 6c |
| Control | 145 ± 21 | 1814 ± 76 | ||
| Anti-Gag (150) | 100 ± 16 | 31c | 1330 ± 158 | 27c |
| Anti-Gag (50) | 114 ± 16 | 21c | 1489 ± 122 | 18c |
| Anti-Gag (20) | 140 ± 23 | 4 | 1808 ± 202 | 1 |
| Control | 137 ± 15 | 1776 ± 43 | ||
| Anti-RT (150) | 82 ± 14 | 40c | 1086 ± 99 | 39c |
| Anti-Gag (150) | 80 ± 20 | 42c | 1032 ± 138 | 42c |
| C.M.1- Anti-RTd | 125 ± 33 | 9 | 1707 ± 114 | 4 |
| C.M.1- Anti-GAGd | 125 ± 20 | 9 | 1774 ± 124 | 2 |
| C.M.2- Anti-RTe | 1717 ± 159 | 3 | ||
| C.M.2- Anti-GAGe | 1774 ± 124 | 0 | ||
Experiments as in Table 1 except that only pIgR+ epithelial cells were used. In some cases conditioned medium (C.M.) was added to the apical chamber or cell lysate without MAb having been added to the basolateral surface. Data are means from eight data points ± SEM pooled from four experiments.
Compared to no antibody control.
Result significantly different (p≤0.03) from the no antibody control.
C.M. 1 - apical supernatant with transcytosed IgA (see text).
C.M. 2 - IgA-transcytosing cell lysate (see text).
Confirmation that neutralization is intraepithelial
To verify that the neutralization observed was an intracellular event, two conditioned media (C.M.) were prepared (Huang et al., 2005). To test if neutralization could be due to transcytosed IgA in the apical compartment, C.M.1 was made from apical supernatant of uninfected monolayers exposed basolaterally to MAb. C.M.1 was then added apically to infected monolayers not exposed to antibody basolaterally for the final 12 h of incubation before collection as usual. The results in Table 2 show that the apical supernatant viral titers for C.M.1 of anti-RT and anti-Gag IgAs both had a 9% (p>0.14) decrease compared to the significant decrease of 40–42% (p<0.005) when anti-RT and anti-Gag IgAs were added basolaterally. Note that these MAbs against internal HIV proteins lack conventional neutralizing activity, even at 50 μg/ml (data not shown). C.M.1 also did not decrease viral titers in cell lysates (4% versus 39% for transcytosing anti-RT (p<0.005) and 2% versus 42% for transcytosing anti-Gag (p<0.005)). The finding that transcytosing IgA antibodies reduced virus titers in apical supernatants and cell lysates significantly more than previously transcytosed IgA antibodies in C.M.1 supports intraepithelial neutralization.
The other conditioned medium, C.M.2, was used to show that neutralization did not result from the freeze-thaw cycle releasing HIV and free antibody that subsequently complexed. C.M.2 was generated from uninfected monolayers exposed basolaterally to antibody, whose cells were then collected and lysed. C.M.2 was used as the collection medium for infected cell monolayers that were not exposed to MAb basolaterally. C.M.2 containing anti-RT and anti-Gag yielded virus reductions of 3% and 0% (Table 2) versus 39% and 42% for the transcytosing antibodies in the standard assay. These results further support the interpretation that the virus neutralization observed was due to IgA antibody binding viral antigen while the antibody was transversing the cell.
Our data do not directly address the intracellular site where transcytosing IgA antibody meets Gag and RT, both of which are synthesized on cytoplasmic ribosomes. Nevertheless, because both IgA and Gag utilize endosomes and multivesicular bodies in their cellular trafficking (Apodaca et al., 1994; Gibson et al., 1998; Nydegger et al., 2003; Ono and Freed, 2004; Spearman, 2006; von Schwedler et al., 2003) it seems reasonable to suggest a confluence of such vesicles as a likely point of intersection.
Effect on p24 synthesis
To assess more directly the effect of transcytosing IgA antibody on virus synthesis, p24 was measured by ELISA in apical supernatant, basal medium and cell lysate (Huang et al., 2005). Concentrations of p24 in apical and basal media and cell lysates were reduced by 11%, 18%, and 11% respectively by anti-RT IgA compared to the no antibody control (all p’s ≤ 0.01) (Table 3). For anti-Gag IgA the reductions were 10%, 19%, and 11% (all p’s<0.02). That p24 was decreased in all three compartments by both anti-Gag and anti-RT IgA antibody confirms that the reduced yield of virus observed was not a result of intracellular virus being diverted to the apical compartment.
Table 3.
Direct measurement of p24 concentrations.a
| Apical Supernatant
|
Basal Medium
|
Cell Lysate
|
||||
|---|---|---|---|---|---|---|
| IgA MAb (150μg/mL) | p24 (ng/mL) | % Virus Reductionb | p24 (ng/mL) | % Virus Reductionb | p24 (ng/mL) | % Virus Reductionb |
| Control | 7.0 ± 0.9 | 7.7 ± 0.9 | 29.9 ± 4.0 | |||
| Anti-RT | 6.2 ± 1.4 | 11c | 6.3 ± 0.5 | 18c | 26.7 ± 4.2 | 11c |
| Anti-Gag | 6.3 ± 1.4 | 10c | 6.2 ± 1.7 | 19c | 26.7 ± 2.3 | 11c |
Experiments as in Table 1 except that p24 concentrations were measured. MAbs were added for 18 h to the basolateral surface of polarized monolayers of pIgR+ Vero C1008 cells that had been transfected with HIV proviral DNA for 4 h. HIV levels in apical supernatants, basolateral media and cell lysates 30 h post transfection were determined by p24 ELISA assay. Data are means from 11 data points ± SEM pooled from seven experiments.
Compared to the no antibody control.
Mean virus titer significantly less than control (p<0.02).
Intracellular colocalization of viral antigen and antibody
Two-color immunofluorescence confocal microscopy was used to visualize the interaction of transcytosing IgA antibody with viral protein inside transfected epithelial cells (Fig.1). Monolayers were treated as above, but after 18–24 h exposure to antibody basolaterally the monolayers were washed to remove free antibody, fixed, permeabilized, and stained (Huang et al., 2005). HIV proteins gp120, Gag, and RT were stained red with rhodamine, and IgA antibodies were stained green with fluorescein (Huang et al., 2005). The gp120 protein was used for comparison since it was previously demonstrated to colocalize with transcytosing IgA anti-gp120 (Huang et al., 2005). Merged images of IgA antibody and Gag or RT protein show colocalization in all three cell planes, apical, middle and basal. Irrelevant IgA did not show colocalization with the HIV proteins (RT protein shown).
Fig. 1.
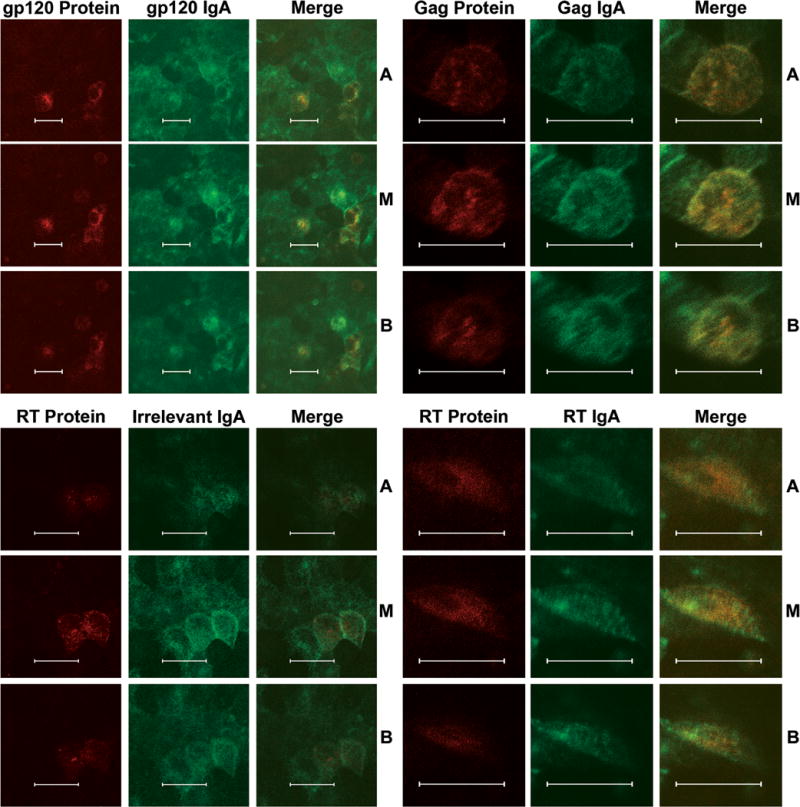
Intracellular colocalization of IgA antibodies and HIV proteins. When transfected, 5–10% of the cells produce virus and the Gag and RT IgAs neutralize 30–40% of that virus. Apical (A), middle (M), and basal (B) horizontal sections through transfected cell monolayers were imaged. Each has a red (rhodamine) channel for virus protein, a green (fluorescein) channel for IgA antibody, and merged red and green channels. Colocalization of red and green signals creates a yellow-orange color. IgA antibodies and their HIV antigen showed colocalization in A, M and B sections whereas irrelevant IgA showed little to no colocalization. (Bar = 20μm)
Even though direct evidence for infection of mucosal epithelial cells under natural conditions in humans is lacking, because transmission of HIV usually occurs across mucosal surfaces, the observations that IgA antibodies against both envelope protein (Huang et al., 2005) and internal proteins (the present study) can inhibit viral replication inside epithelial cells may be relevant to vaccine strategies for HIV, as well as other viruses. In our in vitro system, anti-Gag and anti-RT IgA antibodies were less effective than anti-gp120 antibodies. However, there have been difficulties in developing effective vaccines with envelope protein antigens due to shielding by glycosylation and high mutability. Therefore, the ability of mucosal IgA antibodies to target the non-glycosylated and relatively invariant internal antigens of HIV may be worth considering in the design of mucosal vaccines.
More generally, studies of virus neutralization by antibodies have traditionally emphasized viral surface antigens. In contrast, the present and related studies argue that in certain contexts, such as mucosal epithelia, antibodies to the internal antigens of virus particles can play significant roles in host defense.
Acknowledgments
We thank the Confocal Microscopy Core in the Comprehensive Cancer Center of Case School of Medicine/University Hospitals of Cleveland (P30 CA43703-12) for use of their facility. This research was supported by NIH grants AI-36359 and CA-43703.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi T, Kawamura M, Ueno M, Hiraishi K, Adachi M, Serizawa T, Akashi M, Baba M. Mucosal immunization with inactivated HIV-1-capturing nanospheres induces a significant HIV-1-specific vaginal antibody response in mice. J Med Virol. 2003;69(2):163–172. doi: 10.1002/jmv.10279. [DOI] [PubMed] [Google Scholar]
- Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4(8):760–765. [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125(1):67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Artenstein AW, Rugpao S, Stephens H, VanCott TC, Robb ML, Rinkaew M, Birx DL, Khamboonruang C, Zimmerman PA, Nelson KE, Natpratan C. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J Infect Dis. 1999;179(1):59–67. doi: 10.1086/314556. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3(1):42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9(2):277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- Boot JH, Geerts ME, De Groot ER, Aarden LA. Murine monoclonal isotype switch variants. Detection with rat monoclonal antibodies in ELISA and isolation by sequential sublining. J Immunol Methods. 1988;106(2):195–202. doi: 10.1016/0022-1759(88)90197-4. [DOI] [PubMed] [Google Scholar]
- Buchacz K, Parekh BS, Padian NS, van der Straten A, Phillips S, Jonte J, Holmberg SD. HIV-specific IgG in cervicovaginal secretions of exposed HIV-uninfected female sexual partners of HIV-infected men. AIDS Res Hum Retroviruses. 2001;17(18):1689–1693. doi: 10.1089/08892220152741388. [DOI] [PubMed] [Google Scholar]
- Bukawa H, Sekigawa K, Hamajima K, Fukushima J, Yamada Y, Kiyono H, Okuda K. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat Med. 1995;1(7):681–685. doi: 10.1038/nm0795-681. [DOI] [PubMed] [Google Scholar]
- Burnett PR, VanCott TC, Polonis VR, Redfield RR, Birx DL. Serum IgA-mediated neutralization of HIV type 1. J Immunol. 1994;152(9):4642–4648. [PubMed] [Google Scholar]
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272(5258):104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito C, Hinkula J, Kaul R, Kimani J, Kiama P, Lopalco L, Barass C, Piconi S, Trabattoni D, Bwayo JJ, Plummer F, Clerici M, Broliden K. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30(4):413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo JJ, Plummer F, Clerici M, Broliden K. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14(13):1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- Dorrell L, Hessell AJ, Wang M, Whittle H, Sabally S, Rowland-Jones S, Burton DR, Parren PW. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. Aids. 2000;14(9):1117–1122. doi: 10.1097/00002030-200006160-00008. [DOI] [PubMed] [Google Scholar]
- Fantini J, Yahi N, Baghdiguian S, Chermann JC. Human colon epithelial cells productively infected with human immunodeficiency virus show impaired differentiation and altered secretion. J Virol. 1992;66(1):580–585. doi: 10.1128/jvi.66.1.580-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Yahi N, Chermann JC. Human immunodeficiency virus can infect the apical and basolateral surfaces of human colonic epithelial cells. Proc Natl Acad Sci U S A. 1991;88(20):9297–9301. doi: 10.1073/pnas.88.20.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Lawton JA, Gilbert J, Kuklin N, Vo P, Prasad BV, Greenberg HB. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J Clin Invest. 2002;109(9):1203–1213. doi: 10.1172/JCI14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Emancipator SN, Aikawa M, Huang DS, Blatnik F, Karban T, DeFife K, Mazanec MB. Immunocytochemical colocalization of specific immunoglobulin A with sendai virus protein in infected polarized epithelium. J Exp Med. 1998;188(7):1223–1229. doi: 10.1084/jem.188.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J Cell Biol. 1998;143(1):81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Ventura CL, Black KP, Cummins JE, Jr, Hall SD, Jackson S. Productive human immunodeficiency virus-1 infection of epithelial cell lines of salivary gland origin. Oral Microbiol Immunol. 2000;15(2):82–88. doi: 10.1034/j.1399-302x.2000.150203.x. [DOI] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74(13):6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YT, Miller CJ, Wong V, Fujioka H, Nedrud JG, Lamm ME. Replication and budding of simian immunodeficiency virus in polarized epithelial cells. Virology. 1999;257(1):24–34. doi: 10.1006/viro.1999.9637. [DOI] [PubMed] [Google Scholar]
- Huang YT, Wright A, Gao X, Kulick L, Yan H, Lamm ME. Intraepithelial cell neutralization of HIV-1 replication by IgA. J Immunol. 2005;174(8):4828–4835. doi: 10.4049/jimmunol.174.8.4828. [DOI] [PubMed] [Google Scholar]
- Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88(19):8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R, Plummer F, Clerici M, Bomsel M, Lopalco L, Broliden K. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15(3):431–432. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- Kaul R, Trabattoni D, Bwayo JJ, Arienti D, Zagliani A, Mwangi FM, Kariuki C, Ngugi EN, MacDonald KS, Ball TB, Clerici M, Plummer FA. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13(1):23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66(4):2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski P, Buckheit RW, Jr, Jackson S. Neutralization of HIV infection by serum IgA antibodies. Adv Exp Med Biol. 1995;371B:1027–1030. [PubMed] [Google Scholar]
- Lagaye S, Derrien M, Menu E, Coito C, Tresoldi E, Mauclere P, Scarlatti G, Chaouat G, Barre-Sinoussi F, Bomsel M. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J Virol. 2001;75(10):4780–4791. doi: 10.1128/JVI.75.10.4780-4791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69(2):1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A. 1992;89(15):6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14(9):430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- Mazzoli S, Lopalco L, Salvi A, Trabattoni D, Lo Caputo S, Semplici F, Biasin M, Bl C, Cosma A, Pastori C, Meacci F, Mazzotta F, Villa ML, Siccardi AG, Clerici M. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999;180(3):871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi ML, Tofani N, Biasin M, Villa ML, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3(11):1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, Sabbaj S, Mulligan MJ, Goepfert PA. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20(9):972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- Moja P, Tranchat C, Tchou I, Pozzetto B, Lucht F, Desgranges C, Genin C. Neutralization of human immunodeficiency virus type 1 (HIV-1) mediated by parotid IgA of HIV-1-infected patients. J Infect Dis. 2000;181(5):1607–1613. doi: 10.1086/315420. [DOI] [PubMed] [Google Scholar]
- Moore JS, Hall SD, Jackson S. Cell-associated HIV-1 infection of salivary gland epithelial cell lines. Virology. 2002;297(1):89–97. doi: 10.1006/viro.2002.1469. [DOI] [PubMed] [Google Scholar]
- Moore JS, Rahemtulla F, Kent LW, Hall SD, Ikizler MR, Wright PF, Nguyen HH, Jackson S. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology. 2003;313(2):343–353. doi: 10.1016/s0042-6822(03)00283-6. [DOI] [PubMed] [Google Scholar]
- Nydegger S, Foti M, Derdowski A, Spearman P, Thali M. HIV-1 egress is gated through late endosomal membranes. Traffic. 2003;4(12):902–910. doi: 10.1046/j.1600-0854.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- Ono A, Freed EO. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J Virol. 2004;78(3):1552–1563. doi: 10.1128/JVI.78.3.1552-1563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi MN, Barr CE, Seshamma T, Reidy J, Pomerantz RJ, Bagasra O. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J Infect Dis. 1995;171(1):190–193. doi: 10.1093/infdis/171.1.190. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Blanchard TG, Levine AD, Emancipator SN, Lamm ME. A mucosal IgA-mediated excretory immune system in vivo. J Immunol. 2001;166(6):3688–3692. doi: 10.4049/jimmunol.166.6.3688. [DOI] [PubMed] [Google Scholar]
- Schwartz-Cornil I, Benureau Y, Greenberg H, Hendrickson BA, Cohen J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J Virol. 2002;76(16):8110–8117. doi: 10.1128/JVI.76.16.8110-8117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnick JH, Palumbo P, DeVico A, Shacklett BL, Valentine FT, Merges M, Kamin-Lewis R, Mestecky J, Denny T, Lewis GK, Lloyd J, Praschunus R, Baker A, Nixon DF, Stranford S, Gallo R, Vermund SH, Louria DB. Correlates of nontransmission in US women at high risk of human immunodeficiency virus type 1 infection through sexual exposure. J Infect Dis. 2002;185(4):428–438. doi: 10.1086/338830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Wahl SM. Immunobiology of Mucosal HIV-1 Infection. In: Mestecky J, et al., editors. Mucosal Immunology. 3 ed. Academic Press; San Diego, California: 2005. pp. 1199–1211. [Google Scholar]
- Spearman P. Cellular cofactors involved in HIV assembly and budding. Curr Opin HIV AIDS. 2006 doi: 10.1097/01.COH.0000221592.49412.1f. In Press. [DOI] [PubMed] [Google Scholar]
- Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183(1):215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanCott TC, Kaminski RW, Mascola JR, Kalyanaraman VS, Wassef NM, Alving CR, Ulrich JT, Lowell GH, Birx DL. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J Immunol. 1998;160(4):2000–2012. [PubMed] [Google Scholar]
- von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell. 2003;114(6):701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Hall S, Jackson S. Tropism-restricted neutralization by secretory IgA from parotid saliva of HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2003;19(4):275–281. doi: 10.1089/088922203764969474. [DOI] [PubMed] [Google Scholar]
- Yan H, Lamm ME, Bjorling E, Huang YT. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J Virol. 2002;76(21):10972–10979. doi: 10.1128/JVI.76.21.10972-10979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


