Abstract
Filamentous ascomycetous fungi possess many histidine kinases and two conserved response regulators, Ssk1p and Skn7p, in their two-component signaling systems. We previously reported that the fungus unique group III histidine kinase regulates high-osmolarity adaptation and iprodione/fludioxonil fungicide sensitivity by controlling the phosphorylation of Hog1-type mitogen-activated protein kinase (MAPK) in filamentous ascomycetes. Here, we have characterized the response regulator genes ChSsk1 and ChSkn7 in the southern corn leaf blight fungus Cochliobolus heterostrophus. Both ChSsk1- and ChSkn7-disrupted mutants showed little sensitivity to high-osmolarity stress and moderate resistance to the iprodione/fludioxonil fungicides. The phosphorylation of Hog1-type MAPK BmHog1p induced by high-osmolarity stress and fungicide treatments was only regulated by ChSsk1p, indicating that ChSkn7p has roles in high-osmolarity adaptation and fungicide sensitivity that are independent from the activation of BmHog1p. The Chssk1 Chskn7 double mutants clearly showed higher sensitivity to osmolar stress and higher resistance to fungicides than the single mutants. The dose responses of the double mutants fit well with those of the group III histidine kinase-deficient strain. These results suggest that in filamentous ascomycetes, the Ssk1- and Skn7-type response regulators control high-osmolarity adaptation and fungicide sensitivity additively with differential mechanisms under the regulation of the group III histidine kinase. This study provides evidence that filamentous fungi have a unique two-component signaling system that is different from that of yeast and is responsible for high-osmolarity adaptation and fungicide sensitivity.
In many procaryotes, two-component signal transduction systems are used to sense and adapt to various environmental stresses (4, 15, 27, 32). Two-component signaling systems typically consist of sensor histidine kinases and response regulators (39). Sensor histidine kinases respond to environmental stresses by regulating the phosphorylation of response regulator proteins, which in turn control the expression pattern of the stress response genes. For example, in Escherichia coli, the high-osmolarity stress-signaling pathway consists of the osmosensor histidine kinase EnvZp and the response regulator OmpRp (13). EnvZp regulates the phosphorylation state of OmpRp as the osmolarity of the cellular environment changes. The activated OmpRp then acts as a transcription factor by controlling the expression pattern of genes that encode outer membrane proteins (13, 21).
Similar two-component systems can be found in fungal and plant eukaryotes (1, 9, 26). In eukaryotic cells, two-component systems usually contain a histidine kinase, a response regulator, and an additional signal protein known as a histidine-containing phosphotransfer protein (Hpt). The Hpt acts as an intermediary signal molecule between the sensor histidine kinase and the response regulator (28, 33).
Saccharomyces cerevisiae possesses a single histidine kinase and an Hpt, called Sln1p and Ypd1p, respectively, as well as two response regulators, Ssk1p and Skn7p. Sln1p is known to act as a transmembrane osmosensor (28). Under normal conditions, autophosphorylated Sln1p converts Ypd1p to its phosphorylated form, which relays the phosphate to Ssk1p. The phosphorylated Ssk1p is inactive. Conversely, under conditions of high osmolarity, Sln1p is not phosphorylated, which activates the HOG1 mitogen-activated protein kinase (MAPK) cascade, thereby regulating the expression pattern of high-osmolarity response genes (28). Thus, Ssk1p is a key regulator for osmoadaptation in yeast. The role of the other response regulator protein Skn7p in high-osmolarity stress adaptation is less clear. It has been proposed that Skn7p is activated by hypo-osmolar stress rather than hyper-osmotic conditions (37) and that it is involved in cell wall integrity (5, 6) and the cell cycle (3, 22).
In contrast to yeast, filamentous fungi possess many putative histidine kinase genes (8). The putative histidine kinases fall into 11 classes (8), of which group III is particularly well characterized. Neurospora crassa Nik1 (also known as Os-1: a group III histidine kinase) mutants demonstrate high-osmolarity sensitivity (25). Cochliobolus heterostrophus cells that contain mutations in the group III histidine kinase dic1 gene also show sensitivity to high-osmolarity stress (43). Interestingly, several group III histidine kinase mutants also demonstrate resistance to dicarboximide fungicides and phenylpyrrole fungicides (2, 25, 43). Our previous studies reported that Hog1-type MAPK is phosphorylated by the control of group III histidine kinase under conditions of high-osmolarity stress and fungicide treatment (17, 44). These results suggest that the group III histidine kinase regulates the phosphorylation of Hog1-type MAPK in response to high-osmolarity stress and that fungicides that mimic this stress induce abnormal phosphorylation of Hog1-type MAPK through the group III histidine kinase. Thus, the group III histidine kinase appears to be a key protein in the high-osmolarity stress response and fungicide activity of filamentous fungi. However, except for components of the Hog1-type MAPK cascade, the downstream proteins of the group III histidine kinase are not known.
Genome sequence analyses have revealed that filamentous fungi possess two putative response regulator genes that are apparent orthologues of Ssk1 and Skn7 in yeast. Both C. heterostrophus and Gibberella moniliformis were shown to contain the additional potential response regulator gene Rec1 (ChRec1 and GmRec1, respectively) (8). These response regulators may act as the downstream signaling protein of the two-component histidine kinase signaling system with the group III histidine kinase. However, to date, few response regulator genes from filamentous fungi have been characterized.
In the present study, we found that, in situations of high-osmolarity stress adaptation and fungicide activity, the Ssk1-type response regulator ChSsk1p and the Skn7-type response regulator ChSkn7p act additively downstream of the group III histidine kinase Dic1p. Here, we describe how both ChSsk1 and ChSkn7 are involved in high-osmotic and fungicide stress responses.
MATERIALS AND METHODS
Strains and growth conditions.
The C. heterostrophus HITO7711 (MAT1-2) and MASHIKI2-2 (MAT1-1) strains (35) were used as wild types for the transformation and crossing experiments, respectively. The group III histidine kinase dic1-deficient strain E4509 was obtained by chemical mutagenesis of C. heterostrophus HITO7711 conidia (42). All C. heterostrophus cultures were maintained on complete medium agar (CMA) at 27°C. Liquid complete medium (CM; CMA minus agar) was used to extract DNA and RNA. To prepare the vegetative mycelia for analysis of phosphorylation of Hog1-related MAPKs, C. heterostrophus strains were also incubated in CM.
Plasmid construction and fungal transformation.
To construct the vector pCBmSsk1 for disruption of the ChSsk1 gene in C. heterostrophus (GenBank accession no. AY456027), a DNA fragment (approximately 600 bp) that did not contain part of the 5′ or 3′ coding region of the gene was amplified by PCR using two primers: ChSsk1-f1 (5′-TCCTCAACACCGGACAGCTAC-3′) and ChSsk1-r1 (5′-GATCTCTTTTGTTGCTTCGAGAC-3′). The amplified fragment was introduced to the pCB1004 plasmid (7) containing the HPH gene. To construct the pCBmSkn7 vector for disruption of the ChSkn7 gene in C. heterostrophus (GenBank accession no. AY456028), an approximately 850-bp fragment that did not contain part of the 5′ or 3′ coding region of the gene was amplified by PCR using two primers, ChSkn7-f1 (5′-TCGTTCTGGAAGTATGCAG-3′) and ChSkn7-r1 (5′-TGTGCCCTAGCAAAGTCTG-3′), and introduced into the pCB1004 plasmid. Fungal protoplasts were prepared using the method outlined by Tanaka et al. (36). Transformation experiments were performed using a method described previously (30). Briefly, a 5-μl aliquot of plasmid DNA (1 mg/ml) was mixed with 5 μl of 50 mM spermidine-HCl. A protoplast suspension (5 × 107 protoplasts/100 μl) in STC (1.2 M sorbitol, 10 mM CaCl2, 10 mM Tris-HCl [pH 7.5]) was added to the DNA solution and incubated for 20 min at room temperature before 1 ml of PTC solution (60% [wt/vol] polyethylene glycol dissolved in STC) was added to the suspension and incubated for another 20 min at room temperature. After removing the PTC solution by centrifugation, the protoplasts were resuspended in 100 μl of STC and mixed with 10 ml of CMA containing 1.2 M sucrose. The resulting protoplasts were regenerated and germinated on an agar plate, and the plate was overlaid with 10 ml of CMA containing 200 μg/ml (wt/vol) of hygromycin B and incubated. The resulting transformants were reinoculated onto CMA containing 100 μg/ml hygromycin B.
Southern blot analysis.
DNA digestion and gel electrophoresis were conducted according to standard methods (29). To confirm homologous recombination events, Southern blot analysis was performed using DNA probes that were obtained by PCR amplification using ChSsk1-f1 as the forward primer and ChSsk1-r1 as the reverse primer for the ChSsk1 probe (PSSK1) and ChSkn7-f1 as the forward primer and ChSkn7-r1 as the reverse primer for the ChSkn7 probe (PSKN7).
Crossing.
The Chssk1 Chskn7 double-mutant strain was obtained by sexual hybridization. Crossing was performed according to the method described by Ueyama and Tsuda (38). Mature pseudothecia were harvested after 3 weeks of incubation. Asci were isolated aseptically from several pseudothecia and then crushed in sterilized water and analyzed according to the method described by Taga et al. (34). Each ascospore was allowed to germinate on a small agar block on CMA and incubated at 27°C. After mycelial growth was confirmed, single ascospore isolates were cultured on CMA slants. The offspring with hygromycin resistance regenerated from a recombinant ditype ascus were selected as double mutants, and their genotypes were confirmed by Southern hybridization.
Growth inhibition testing of mutants.
The fungicides iprodione (Rovral WP, 50% active ingredient [a.i.]; Aventis Crop Sci.) and fludioxonil (Saphire flowable, 20% a.i.; Novartis Agro) were obtained commercially and added to media from 1,000-fold-concentrated stock solutions in 70% ethanol (iprodione) or dimethyl sulfoxide (fludioxonil). Sensitivity of the mutants to the chemicals was evaluated by plotting the dose-response curve for colonial growth using the plate dilution method. Mycelial disks (6-mm diameter) were cut with a sterilized cork borer from the margin of 1-week-old colonies, and each disk was placed upside down on a series of CMA plates containing 0.01 to 400 μg/ml iprodione (a.i.), 0.01 to 100 μg/ml fludioxonil (a.i.), 50 to 1,400 mM KCl, and 100 to 2,800 mM sorbitol. The dose-response curve was determined 4 days after incubation at 27°C by plotting the percent decrease in colony diameter against the log concentration of the chemicals. Each experiment was performed in triplicate. Resistance or sensitivity of single ascospore isolates to dicarboximide and phenylpyrrole fungicides was determined by evaluating mycelial growth on CMA with 100 μg/ml of iprodione and fludioxonil added, respectively.
Western blot analysis.
The phosphorylation of Hog1-related MAPK in C. heterostrophus was examined using a Western blot analysis with an anti-dually phosphorylated p38 antibody (Cell Signaling Technology). Total protein samples were isolated as follows: mycelia of each strain were incubated for 2 days in CM at 25°C and then filtered through 4-layer gauze. The mycelia were then ground with a mortar and pestle to a fine powder under liquid nitrogen. Ice-chilled buffer containing protease and phosphatase inhibitors (50 mM Tris-HCl [pH 7.5], 1% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X-100, 0.1% [wt/vol] sodium dodecyl sulfate, 50 mM NaF, 5 mM sodium pyrophosphate, 0.1 mM sodium vanadate, protease inhibitor cocktail set [1 tablet per 50 ml; Roche]) was added to the powder, and the suspensions were sonicated for 20 s to promote solubility. After centrifuging the suspensions at 8,000 × g for 3 min, the resulting supernatants were separated on 10% polyacrylamide gels and blotted onto nitrocellulose membranes (Millipore). The concentration of total proteins was calculated using a BCA protein assay reagent (Pierce), and 50 μg of protein was loaded into each well. Anti-Hog1p antibody was purchased from Santa Cruz Biotechnology (C-terminal anti-Hog1p), and antibody binding was visualized using an ECL Plus Western blotting detection reagent (Amersham Biosciences) after the binding of a horseradish peroxidase-conjugated secondary antibody.
RESULTS
Disruption of the ChSsk1 and ChSkn7 genes of C. heterostrophus.
To investigate the role of the two response regulator genes ChSsk1 and ChSkn7, a gene disruption strategy was used. To disrupt the ChSsk1 gene, the targeted insertion vector pCBmSsk1, which contained a hygromycin B phosphotransferase (HPH) gene cassette and a part of the ChSsk1 coding region, was constructed and introduced into the wild-type strain (see Fig. S1A in the supplemental material). Similarly, to disrupt the ChSkn7 gene, the targeted insertion vector pCBmSkn7 containing an HPH gene cassette and a part of the ChSkn7 coding region was constructed and introduced into the wild-type strain (see Fig. S1C in the supplemental material).
Targeted insertion of ChSsk1 in the transformants was investigated by Southern blot analysis (see Fig. S1B in the supplemental material). Genomic DNA was isolated from the wild-type HITO7711 and two transformants, DSSK101 and DSSK201. The isolated genomic DNA was digested with SalI and EcoRI and hybridized with the probe (see Fig. S1A in the supplemental material). The results indicated that HITO7711 contained 1.5-kb SalI and 6.0-kb EcoRI fragments (see Fig. S1B, lane 1, in the supplemental material). These fragments were not seen in the transformants. The transformants did, however, contain other fragments (see Fig. S1B, lanes 2 and 3, in the supplemental material) that were consistent with the expected length for the targeted insertion event. We therefore concluded that ChSsk1 was disrupted in the transformants DSSK101 and DSSK201.
Targeted insertion of ChSkn7 in the transformants was also confirmed by Southern blot analysis (see Fig. S1D in the supplemental material). The results showed that HITO7711 contained 2.1-kb SmaI and 1.5-kb EcoRV fragments (see Fig. S1D, lane 1, in the supplemental material) that were not found in the transformants. The transformants contained other fragments (see Fig. S1D, lanes 2 and 3, in the supplemental material) that were consistent with the expected length for the targeted insertion event. Thus, we concluded that ChSkn7 was disrupted in the transformants DSKN101 and DSKN201.
Osmosensitivity and fungicide resistance.
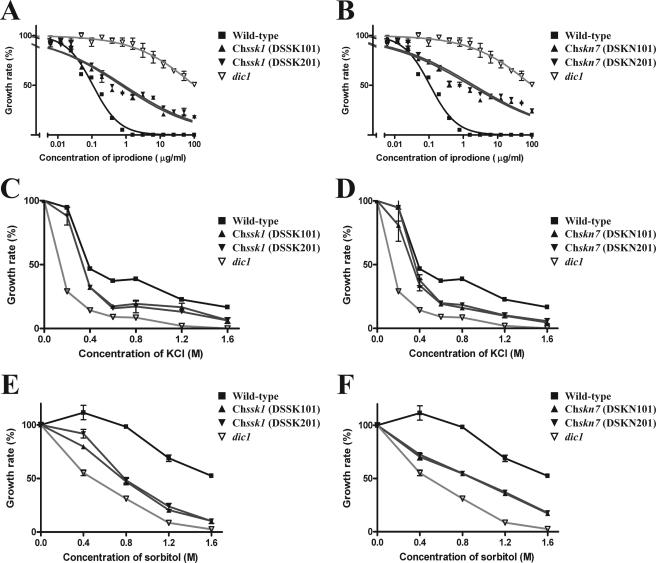
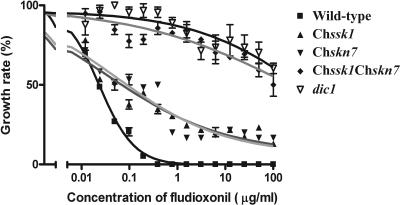
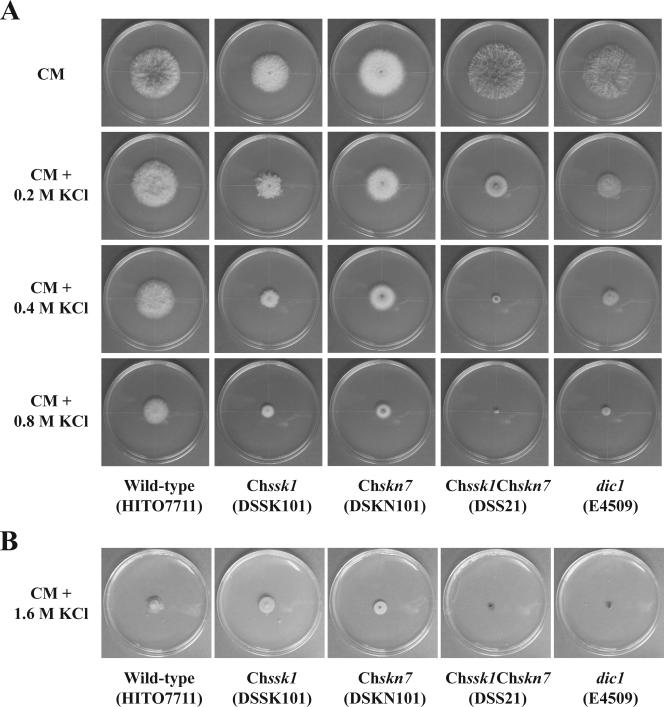
To investigate the role of the response regulator ChSsk1p in osmoadaptation and fungicide sensitivity, growth inhibition tests were conducted. Results for the Chssk1 mutant strains, the group III histidine kinase dic1-deficient strain, and wild-type strain are shown in Fig. 1. The Chssk1 mutant strains demonstrated moderate resistance to dicarboximide fungicide (Fig. 1A); however, the degree of resistance was clearly lower than that of the dic1-deficient strain. When the phenylpyrrole fungicide fludioxonil was added to the medium, Chssk1 mutant strains showed moderate resistance, but again, the degree of the fungicide resistance was clearly lower than that of the dic1-deficient strain (Fig. 2).
FIG. 1.
Sensitivity to the dicarboximide fungicide iprodione, KCl, and sorbitol in strains of C. heterostrophus with disrupted response regulator genes. (A) Sensitivity to iprodione in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSsk1-disrupted strains DSSK101 and DSSK201. (B) Sensitivity to iprodione in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSkn7-disrupted strains DSKN101 and DSKN201. (C) Sensitivity to KCl in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSsk1-disrupted strains DSSK101 and DSSK201. (D) Sensitivity to KCl in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSkn7-disrupted strains DSKN101 and DSKN201. (E) Sensitivity to sorbitol in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSsk1-disrupted strains DSSK101 and DSSK201. (F) Sensitivity to sorbitol in the wild-type strain HITO7711, the dic1-deficient strain E4509, and the ChSkn7-disrupted strains DSKN101 and DSKN201. Error bars represent standard errors of the means (n = 3).
FIG. 2.
Sensitivity to the phenylpyrrole fungicide fludioxonil in the wild-type strain HITO7711, the dic1-deficient strain E4509, the Chssk1 mutant strain DSSK101, the Chskn7 mutant strain DSKN101, and the Chssk1 Chskn7 double-mutant strain DSS21. Error bars represent standard errors of the means (n = 3).
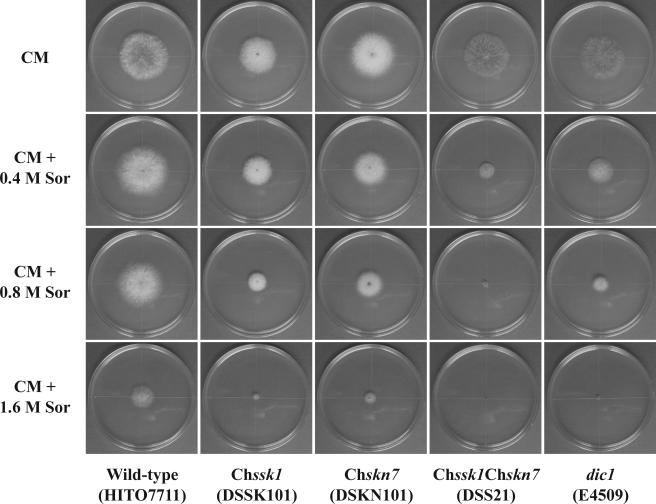
In the osmotic stress experiments, the Chssk1 mutant strains showed some sensitivity to KCl (Fig. 1C), but the degree was lower than that of the dic1-deficient strain. Similarly, Chssk1 mutant strains showed less sensitivity to sorbitol than the dic1-deficient strain (Fig. 1E).
Similar to the Chssk1 mutant strains, the Chskn7 mutant strains showed moderate resistance to iprodione (Fig. 1B) and fludioxonil (Fig. 2) and some sensitivity to KCl and sorbitol (Fig. 1D and F), but the degree was clearly lower than that of the dic1-deficient strain. These results suggest that the two response regulator proteins, ChSsk1p and ChSkn7p, contribute to osmoadaptation and fungicide sensitivity in C. heterostrophus.
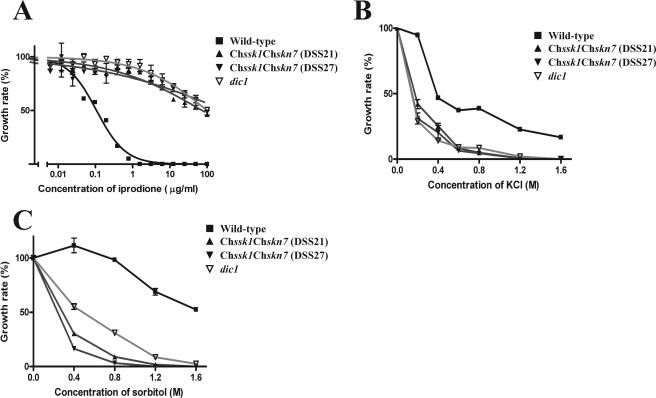
To investigate the relationship between ChSsk1 and ChSkn7, a Chssk1 Chskn7 double-mutant strain was created by sexual hybridization. Growth responses of the double-mutant strain to dicarboximide fungicide and osmotic stress are shown in Fig. 3. The double-mutant cells showed levels of resistance to dicarboximide fungicide similar to those of the dic1-deficient strain (Fig. 3A and 4). The degree of fungicide resistance was clearly higher than that of the Chssk1 and Chskn7 single-mutant strains. Similarly, when fludioxonil was added to the medium, the double-mutant strain showed higher resistance than either single-mutant strain (Fig. 2). These results suggest that ChSsk1p and ChSkn7p contribute to the sensitivity to these fungicides in an additive manner.
FIG. 3.
Sensitivities to the dicarboximide fungicide iprodione, KCl, and sorbitol in the wild-type strain HITO7711, the group III histidine kinase dic1-deficient strain E4509, and the Chssk1 Chskn7 double-mutant strains DSS21 and DSS27. (A) Sensitivity to iprodione. (B) Sensitivity to KCl. (C) Sensitivity to sorbitol. Error bars represent standard errors of the means (n = 3).
FIG. 4.
Sensitivity to the dicarboximide fungicide iprodione in the wild-type strain HITO7711, the Chssk1 mutant strain DSSK101, the Chskn7 mutant strain DSKN101, the Chssk1 Chskn7 double-mutant strain DSS21, and the group III histidine kinase dic1-deficient strain E4509.
The additive effects were also observed in the high-osmotic stress experiments. That is, the Chssk1 Chskn7 double-mutant strain showed hypersensitivity to KCl, similar to the dic1-deficient strain (Fig. 3B and 5), and again, the degree of sensitivity was clearly higher than that of the single-mutant strains. Indeed, the double-mutant strains and dic1-deficient strain could not grow in medium containing 1.6 M KCl even when left for 2 weeks, whereas both the Chssk1 and Chskn7 single-mutant strains grew to some degree (Fig. 5B). Similar results were seen in the experiment using medium containing sorbitol (Fig. 3C and 6); however, the double-mutant strain showed slightly higher sensitivity than the dic1-deficient strain.
FIG. 5.
Sensitivity to KCl in the wild-type strain HITO7711, the Chssk1 mutant strain DSSK101, the Chskn7 mutant strain DSKN101, the Chssk1 Chskn7 double-mutant strain DSS21, and the dic1-deficient strain E4509. (A) Sensitivity to KCl after 3 days. (B) Sensitivity to the medium containing 1.6 M KCl after 2 weeks.
FIG. 6.
Sensitivity to sorbitol in the wild-type strain HITO7711, the Chssk1 mutant strain DSSK101, the Chskn7 mutant strain DSKN101, the Chssk1 Chskn7 double-mutant strain DSS21, and the dic1-deficient strain E4509.
Again, these results suggest that ChSsk1p and ChSkn7p contribute to osmoadaptation and sensitivity to fungicides in an additive manner.
Phosphorylation of Hog1-related MAPK.
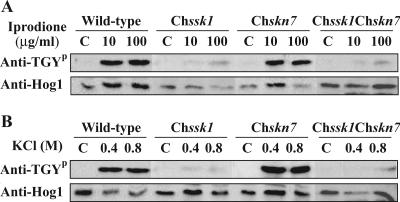
In the yeast S. cerevisiae, high-osmolarity stress induced activation of Hog1p MAPK through the histidine kinase Sln1p and the response regulator Ssk1p (28). In C. heterostrophus, Hog1-related MAPK BmHog1p was phosphorylated by high-osmolarity stress and dicarboximide fungicide treatment via the histidine kinase Dic1p (17, 44). To confirm whether the two response regulator proteins ChSsk1p and ChSkn7p are related to the activation of BmHog1p, the phosphorylation of BmHog1p was analyzed in the Chssk1 mutant strain, the Chskn7 mutant strain, and the Chssk1 Chskn7 double-mutant strain (Fig. 7). In the Chssk1 mutant strain, the phosphorylation of BmHog1p induced by the high-osmolarity stress and fungicide treatments was clearly reduced compared to the wild-type strain. In the Chskn7 mutant strain, however, phosphorylation of BmHog1p was similar to that of the wild-type strain. In the Chssk1 Chskn7 double-mutant strain, the phosphorylation of BmHog1p was again clearly reduced compared to the wild-type control. No differences in the level of phosphorylation were observed between the Chssk1 single-mutant strain and the Chssk1 Chskn7 double-mutant strain. These results suggest that ChSsk1p regulates the phosphorylation of BmHog1p under conditions of high-osmolarity stress and fungicide treatment and that ChSkn7p is not associated with the activation of BmHog1p.
FIG. 7.
Phosphorylation of C. heterostrophus BmHog1p MAPK induced by osmotic stress and fungicides. (A) BmHog1p phosphorylation in the wild-type strain, the Chssk1 mutant strain, the Chskn7 mutant strain, and the Chssk1 Chskn7 double-mutant strain. Mycelia of the strains HITO7711 (wild type), DSSK101 (Chssk1), DSKN101 (Chskn7), and DSS21 (Chssk1 Chskn7) were used for analysis. Prepared mycelia of the strain tested were incubated in CM with or without 10 and 100 μg/ml iprodione for 10 min. The cells were harvested, and total protein extracts were prepared as described in Materials and Methods. Protein samples (50 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes for Western blot analysis. Phosphorylated BmHog1p was detected using anti-dually phosphorylated p38 antibody (Anti-TGYp). The total amount of BmHog1p was measured using anti-Hog1 C terminus antibody (anti-Hog1). (B) Phosphorylation of the C. heterostrophus BmHog1p MAPK by iprodione in the wild-type strain, Chssk1 mutant strain, Chskn7 mutant strain, and Chssk1 Chskn7 double-mutant strain. Prepared mycelia of the strains tested were incubated in CM with or without 0.4 and 0.8 M KCl for 10 min.
Morphological changes caused by dicarboximide fungicide.
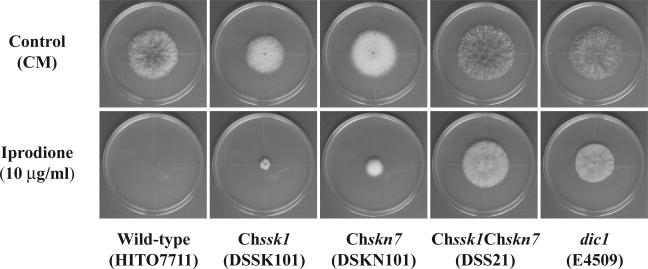
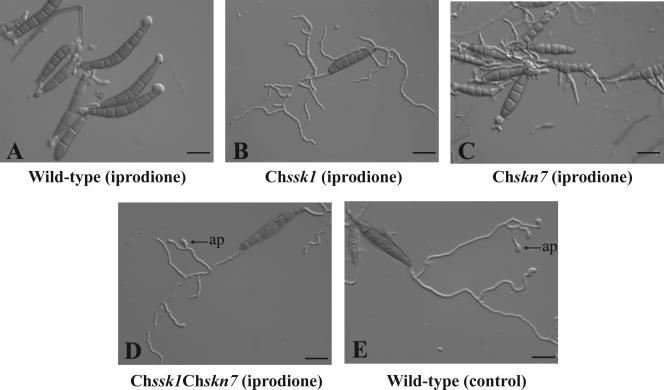
Dicarboximide and phenylpyrrole fungicides are known to cause abnormal morphology in fungal cells. Morphological changes caused by dicarboximide fungicide were compared in the Chssk1 mutant, the Chskn7 mutant, the Chssk1 Chskn7 double-mutant, and the wild-type strains (Fig. 8). When the wild-type strain was incubated in the presence of fungicide, conidial cells were slightly inflated, abnormal germ tubes developed, swelling bulbously and pyriformly, and hyphal extensions were stunted (Fig. 8A). When the Chskn7 mutant strain was exposed to fungicide, conidial cells were often inflated, leading to the breakage of conidial pods and thereby allowing for lateral germination. The geminated cells developed somewhat extended, swollen hyphae (Fig. 8C). In contrast, hyphal swelling was rarely observed in the Chssk1 mutant and Chssk1 Chskn7 double-mutant strains that were exposed to fungicides (Fig. 8B and D); however, hyphal development was reduced in the Chssk1 mutant cells. Therefore, it is likely that the fungicides affect hyphal development in Chssk1 and Chskn7 mutants and that only ChSsk1p is involved in hyphal swelling caused by fungicides.
FIG. 8.
Effect of iprodione treatment on the C. heterostrophus wild-type strain, the Chssk1 mutant strain, the Chskn7 mutant strain, and the Chssk1 Chskn7 double-mutant strain. (A) Wild-type strain incubated in CM containing 10 μg/ml iprodione for 6 h at 25°C. (B) Chssk1 mutant DSSK101 incubated in CM containing 10 μg/ml iprodione for 6 h at 25°C. (C) Chskn7 mutant DSKN101 incubated in CM containing 10 μg/ml iprodione for 6 h at 25°C. (D) Chssk1 Chskn7 double-mutant strain DSS21 incubated in CM containing 10 μg/ml iprodione for 6 h at 25°C. (E) Untreated wild-type strain. No differences were observed between the untreated wild-type strain and the untreated mutant strains (data not shown). ap, appressorium. Scale bars, 50 μm.
DISCUSSION
The response regulator protein is a key element in the two-component signaling system. It governs output responses by its level of phosphorylation, which is under the control of an upstream regulator histidine kinase (39). Two conserved response regulator proteins homologous to Ssk1 and Skn7 in S. cerevisiae have been identified in several fungal species (8). However, the specific responses that these proteins govern have only been characterized in some yeast species (2, 10, 11, 18, 24, 28, 31). In the present study, we examined the responses and thereby characterized the roles of the response regulators in the filamentous fungus C. heterostrophus. Chssk1 mutants showed increased sensitivity to osmolar stress and moderate iprodione and fludioxonil resistance, implying that ChSsk1 plays a role in osmotic adaptation and fungicide sensitivity. While the role of Ssk1p in the two-component signaling system and the high-osmolarity glycerol pathway has been well characterized in yeast, only one functional analysis has been conducted on the Ssk1p homologue in filamentous fungi. In that report, an Aspergillus nidulans ssk1 (sskA) mutant showed an osmosensitive phenotype and a deficiency of Hog1p (HogAp) phosphorylation (14). Our data suggest that the Ssk1-type response regulator has roles in high-osmolarity adaptation and in the mode of action of fungicides used against filamentous fungi. Our results also indicate that the other response regulator, ChSkn7, plays a role in osmotic adaptation and moderate fungicide resistance.
Until now, the Skn7-type response regulator had not been characterized in any filamentous fungi. In human pathogenic yeast Cryptococcus neoformans, CnSkn7p was shown to be involved in fungicide sensitivity, sodium resistance, and oxidative stress response (2, 40). In our study, Chskn7 mutants showed sensitivity to sorbitol as well as KCl, implying that ChSkn7p regulates adaptation under general osmolarity stress in addition to changes in sodium cation concentration. Skn7-type response regulators have been reported to govern oxidative stress responses in some yeasts (2, 40). We observed that C. heterostrophus Chskn7 mutants showed sensitivity to hydrogen peroxide (our unpublished observation).
Both the ChSsk1p and ChSkn7p response regulators were involved in high-osmolarity adaptation and fungicide sensitivity. The two proteins, however, showed different mechanistic functions in the response pathway. The disruption of the ChSsk1 gene prevented the phosphorylation of BmHog1p in both the high-osmolarity stress and fungicide treatments, whereas the Chskn7 mutation did not affect the phosphorylation of BmHog1p. Distinct morphological observations of the Chssk1 and Chskn7 mutants compared to wild-type cells after the application of fungicides also indicated a difference in function between the two response regulators. The wild-type strain developed heavily swollen hyphae with inflated cells, and mycelial growth was strongly inhibited by the fungicides, whereas both of the mutants showed partial restricted growth of hyphae, indicating incomplete fungicidal activity. In addition, only the Chskn7 mutant developed swollen hyphae and inflated cells similar to those of the wild type, and the Chssk1 mutant did not. It is known that iprodione and fludioxonil treatment of N. crassa and C. heterostrophus causes abnormal accumulations of cellular glycerol, resulting in cell inflation and hyphal swelling (45; our unpublished data). These results suggest that only ChSsk1p controls BmHog1p phosphorylation, which, under conditions of osmotic and fungicide stress, seems to result in the accumulation of cellular glycerol. Moreover, ChSkn7p appears to have other roles in high-osmolarity adaptation and fungicide sensitivity that are independent of the activation of Hog1p.
One of the key findings in this study is that the Ssk1-type and Skn7-type response regulators work additively in the osmolarity and fungicide stress responses in C. heterostrophus. The phenotypes of the Chssk1, Chskn7, and dic1 mutants were comparable but not identical. Dic1p is the histidine kinase responsible for conferring osmotic adaptation and fungicide sensitivity in C. heterostrophus (43, 44). All phenotypic characteristics of the Chssk1 and Chskn7 mutants were also observed in the dic1 mutants. In contrast to the Chssk1 and Chskn7 single mutants, the Chssk1 Chskn7 double-mutant cells clearly showed higher resistance to the fungicides than either single-mutant strain alone. Furthermore, the double-mutant strains were much more sensitive to the osmotic stress than the single-mutant strains. The dose-response curve of the Chssk1 Chskn7 double mutant to high osmolarity and fungicide exposure paralleled that of the dic1 mutant. These results imply that the Ssk1-type and Skn7-type response regulators work additively in response to osmolarity and fungicide stress. This is the first such report for fungi.
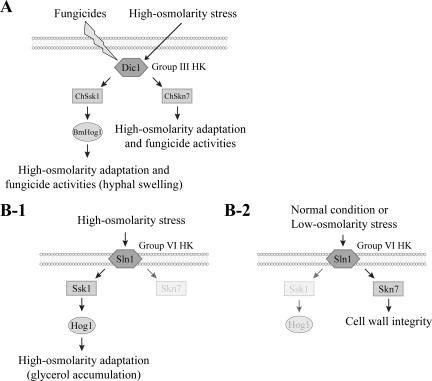
Given these results, we propose that C. heterostrophus has two pathways for the osmolar adaptation and fungicide sensitivity: “Dic1p-ChSsk1p-BmHog1p” and “Dic1p-ChSkn7p” (Fig. 9). These two pathways contribute to high-osmolarity stress adaptation and fungicide sensitivity additively under the control of Dic1p (Fig. 9A). In S. cerevisiae, the response pathway for osmotic adaptation is well characterized and Ssk1p is a key response regulator that is activated under high-osmolarity conditions, thereby mediating signals from the histidine kinase Sln1p to the HOG1-MAPK pathway in response to hyperosmotic stress (Fig. 9B-1) (28). Skn7p, the other response regulator, is also believed to accept signals from Sln1p. However, its specific role in acclimatization to a high-osmolarity environment is unclear. Skn7p is thought to play a role in cell wall integrity under normal and hypo-osmotic conditions (Fig. 9B-2) (5, 6, 37). Therefore, it is believed that these two response regulators are involved in different aspects of osmotic adaptation in yeast (19). Our results indicate that ChSsk1p works in the same manner in C. heterostrophus as in S. cerevisiae in that it causes HOG1-MAPK to activate the pathway for glycerol genesis, which is likely a major coping mechanism for fungal cells under high-osmolarity stress conditions. In contrast to yeast, the fungal Skn7p homologue appears to work in the same aspect of hyperosmotic adaptation as Ssk1p, acting under the control of the histidine kinase in a high-osmolarity environment. ChSkn7 is probably situated in a HOG1-independent pathway and regulates the expression of genes that are also necessary for hyperosmotic adaptation.
FIG. 9.
Model illustration of the two-component signal pathways in C. heterostrophus and S. cerevisiae. (A) Two-component signal pathway involved in the high-osmolarity adaptation and fungicidal activities in C. heterostrophus. (B-1) Two-component signal pathway involved in high-osmolarity adaptation in S. cerevisiae. (B-2) Two-component signal pathway involved in low-osmolarity adaptation in S. cerevisiae.
Our results bring new insight into the mode of action of dicarboximide and phenylpyrrole fungicides. After almost 30 years of research, the mode of action of dicarboximides had remained uncertain. However, recent evidence suggests that dicarboximides and phenylpyrroles interfere with the osmotic signal transduction pathway consisting of histidine kinase and MAPK cascades (16, 17, 44). It was previously known that hyperactivation of Hog1 MAPK caused growth arrest and lethality in yeast (28, 41) and that the phenylpyrrole and dicarboximide fungicides caused the improper activation of Hog1-type MAPKs in some filamentous fungi (17, 44, 45). Many previously developed dicarboximide- and phenylpyrrole-resistant mutant fungi in the field and laboratory contained mutations in the group III histidine kinase (12, 20, 25). When exposed to dicarboximide and phenylpyrrole fungicides, these mutants invariably lacked activated Hog1-type MAPKs (44). Furthermore, the yeast S. cerevisiae normally exhibits tolerance to dicarboximides and phenylpyrroles; however, introduction of the group III histidine kinase gene into the yeast cell resulted in phenotypic alterations such that the transformants were no longer tolerant to these fungicides (23). These observations suggest that the group III histidine kinase is likely a primary target of these fungicides or a mediator of their fungicidal action and that the main mode of action of these fungicides is the abnormal phosphorylation of the Hog1-type MAPK controlled by the group III histidine kinase. Our results indicate that improper signal mediation by the “group III histidine kinase-Skn7p” pathway, which will cause abnormal expression of the genes necessary for hyperosmotic adaptation, along with the “group III histidine kinase-Ssk1p” pathway, is required for the full activity of these fungicides.
A recent report by Bahn et al. (2) suggested the involvement of both response regulator homologues, CnSsk1p and CnSkn7p, in the phenylpyrrole sensitivity of the basidiomycotic yeast C. neoformans. The authors did not address the relationship between the group III histidine kinase (Tco1p) and CnSkn7p. However, previous reports have suggested that the group III histidine kinase is responsible for phenylpyrrole fungicidal activity and that Tco1p relays the stimulus of the fungicide to the downstream response regulators CnSsk1p and CnSkn7p in C. neoformans. Our results, combined with those reported by Bahn et al., imply that the two conserved response regulators, Ssk1p and Skn7p, participate additively in the mode of action of dicarboximide and phenylpyrrole fungicides downstream of the group III histidine kinase in both ascomycotic and basidiomycotic fungi.
In filamentous fungi, including C. heterostrophus, N. crassa, Magnaporthe grisea, and Botrytis cinerea, the group III histidine kinase is responsible not only for fungicide sensitivity but also for hyperosmotic adaptation. These fungi have an Sln1p homologue, which is categorized as a group VI histidine kinase and is the sole histidine kinase in S. cerevisiae. In S. cerevisiae, Sln1p is considered to be an osmosensor required in mediating environmental cues to operate the pathways governing osmotic adaptation. However, the role of the Sln1p homologues in filamentous fungi is unclear. Mutants with disrupted fungal Sln1 homologues did not show any apparent phenotypic changes with respect to osmosensitivity (14; our unpublished observation). These results suggest that the group III histidine kinase replaced the function of Sln1p in an osmoadaptation system in the filamentous fungi. In conclusion, our study provides evidence that filamentous fungi have a unique two-component signaling system consisting of a group III histidine kinase and two response regulators that act additively to regulate hyperosmotic adaptation and sensitivity to dicarboximide or phenylpyrrole fungicides.
Supplementary Material
Acknowledgments
This work was supported in part by grants-in-aid (11760035 and 15380104) from JSPS and the Center of Excellence for Microbial Process Development Pioneering Future Production Systems (COE program of the Ministry of Education, Culture, Sports, Science and Technology of Japan).
Footnotes
Published ahead of print on 8 December 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alex, L. A., K. A. Borkovich, and M. I. Simon. 1996. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc. Natl. Acad. Sci. USA 93:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahn, Y. S., K. Kojima, G. M. Cox, and J. Heitman. 2006. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 17:3122-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouquin, N., A. L. Johnson, B. A. Morgan, and L. H. Johnston. 1999. Association of the cell cycle transcription factor Mbp1 with the Skn7 response regulator in budding yeast. Mol. Biol. Cell 10:3389-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourret, R. B., K. A. Borkovich, and M. I. Simon. 1991. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu. Rev. Biochem. 60:401-441. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. L., S. North, and H. Bussey. 1993. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall-glucan assembly, encodes a product with domains homologous to prokaryotic two component regulators and to heat shock transcription factors. J. Bacteriol. 175:6908-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. L., H. Bussey, and R. C. Stewart. 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J. 13:5186-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, A. M., J. A. Sweigard, and B. Valent. 1994. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41:22. [Google Scholar]
- 8.Catlett, N. L., O. C. Yoder, and B. G. Turgeon. 2003. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot. Cell 2:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, C., S. F. Kwok, A. B. Bleecker, and E. M. Meyerowitz. 1993. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulator. Science 262:539-544. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan, N., D. Inglis, E. Roman, J. Pla, D. Li, J. A. Calera, and R. Calderone. 2003. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot. Cell 2:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottarel, G. 1997. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics 147:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, W., R. E. Beever, S. L. Parkes, P. L. Weeds, and M. D. Templeton. 2002. An osmosensing histidine kinase mediates dicarboximide fungicide resistance in Botryotinia fuckeliana (Botrytis cinerea). Fungal Genet. Biol. 36:187-198. [DOI] [PubMed] [Google Scholar]
- 13.Forst, S., J. Delgado, and M. Inouye. 1989. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc. Natl. Acad. Sci. USA 16:6052-6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa, K., Y. Hoshi, T. Maeda, T. Nakajima, and K. Abe. 2005. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 56:1246-1261. [DOI] [PubMed] [Google Scholar]
- 15.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. American Society for Microbiology, Washington, DC.
- 16.Irmler, S., H. Rogniaux, D. Hess, and C. Pillonel. 2006. Induction of OS-2 phosphorylation in Neurospora crassa by treatment with phenylpyrrole fungicides and osmotic stress. Pestic. Biochem. Physiol. 84:25-37. [Google Scholar]
- 17.Kojima, K., Y. Takano, A. Yoshimi, C. Tanaka, T. Kikuchi, and T. Okuno. 2004. Fungicide activity through activation of a fungal signaling pathway. Mol. Microbiol. 53:1785-1796. [DOI] [PubMed] [Google Scholar]
- 18.Krems, B., C. Charizanis, and K. D. Entian. 1996. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr. Genet. 29:327-334. [DOI] [PubMed] [Google Scholar]
- 19.Li, S., S. Dean, Z. Li, J. Horecka, R. J. Deschenes, and J. S. Fassler. 2002. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13:412-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, T. K., S. Renault, and C. P. Selitrennikoff. 2002. Molecular dissection of alleles of the osmotic-1 locus of Neurospora crassa. Fungal Genet. Biol. 35:147-155. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno, T., and S. Mizushima. 1987. Isolation and characterization of deletion mutants of ompR and envZ, regulatory genes for expression of the outer membrane proteins OmpC and OmpF in Escherichia coli. J. Biochem. 101:387-396. [DOI] [PubMed] [Google Scholar]
- 22.Morgan, B. A., N. Bouquin, G. F. Merrill, and L. H. Johnston. 1995. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 14:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motoyama, T., T. Ohira, K. Kadokura, A. Ichiishi, M. Fujimura, I. Yamaguchi, and T. Kudo. 2005. An Os-1 family histidine kinase from a filamentous fungus confers fungicide-sensitivity to yeast. Curr. Genet. 47:298-306. [DOI] [PubMed] [Google Scholar]
- 24.Nakamichi, N., H. Yamada, H. Aiba, K. Aoyama, R. Ohmiya, and T. Mizuno. 2003. Characterization of the Prr1 response regulator with special reference to sexual development in Schizosaccharomyces pombe. Biosci. Biotechol. Biochem. 3:547-555. [DOI] [PubMed] [Google Scholar]
- 25.Ochiai, N., M. Fujimura, T. Motoyama, A. Ichiishi, R. Usami, K. Horikoshi, and I. Yamaguchi. 2001. Characterization of mutants in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa. Pest Manag. Sci. 57:437-442. [DOI] [PubMed] [Google Scholar]
- 26.Ota, I. M., and A. Varshavsky. 1993. A yeast protein similar to bacterial two-component regulator. Science 262:566-569. [DOI] [PubMed] [Google Scholar]
- 27.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 28.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Shimizu, K., C. Tanaka, and M. Tsuda. 1997. Cloning of Brn1, a reductase gene involved in melanin biosynthesis in Cochliobolus heterostrophus. J. Gen. Appl. Microbiol. 43:145-450. [DOI] [PubMed] [Google Scholar]
- 31.Singh, P., N. Chauhan, A. Ghosh, F. Dixon, and R. Calderone. 2004. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect. Immun. 72:2390-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki, T., A. Imamura, C. Ueguchi, and T. Mizuno. 1998. Histidine-containing phosphotransfer (HPt) signal transducers implicated in His-to-Asp phosphorelay in Arabidopsis. Plant Cell Physiol. 39:1258-1268. [DOI] [PubMed] [Google Scholar]
- 34.Taga, M., H. Nagakubo, M. Tsuda, and A. Ueyama. 1978. Ascospore analysis of kasugamycin resistance in the perfect stage of Pyricularia oryzae. Phytopathology 68:815-817. [Google Scholar]
- 35.Tanaka, C., Y. Kubo, and M. Tsuda. 1991. Genetic analysis and characterization of Cochliobolus heterostrophus color mutants. Mycol. Res. 92:49-56. [Google Scholar]
- 36.Tanaka, C., M. Nakada, and M. Tsuda. 1992. Electrophoretic separation of chromosomes of some graminicolous fungi. Trans. Mycol. Soc. Jpn. 33:95-102. [Google Scholar]
- 37.Tao, W., R. J. Deschenes, and J. S. Fassler. 1999. Intracellular glycerol levels modulate the activity of Sln1p, a Saccharomyces cerevisiae two-component regulator. J. Biol. Chem. 274:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueyama, A., and M. Tsuda. 1975. Formation in culture of Cochliobolus miyabeanus, the perfect state of Helminthosporium oryzae. Ann. Phytopathol. Soc. Jpn. 41:434-440. [Google Scholar]
- 39.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 40.Wormley, F. L., Jr., G. Heinrich, J. L. Miller, J. R. Perfect, and G. M. Cox. 2005. Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect. Immun. 73:5022-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaakov, G., M. Bell, S. Hohmann, and D. Engelberg. 2003. Combination of two activating mutations in one HOG1 gene forms hyperactive enzymes that induce growth arrest. Mol. Cell. Biol. 23:4826-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimi, A., J. Imanishi, A. Gafur, C. Tanaka, and M. Tsuda. 2003. Characterization and genetic analysis of laboratory mutants of Cochliobolus heterostrophus resistant to dicarboximide and phenylpyrrole fungicides. J. Gen. Plant Pathol. 69:101-108. [Google Scholar]
- 43.Yoshimi, A., M. Tsuda, and C. Tanaka. 2004. Cloning and characterization of the histidine kinase gene Dic1 from Cochliobolus heterostrophus that confers dicarboximide resistance and osmotic adaptation. Mol. Genet. Genomics 271:228-236. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimi, A., K. Kojima, Y. Takano, and C. Tanaka. 2005. Group III histidine kinase is a positive regulator of Hog1-type mitogen-activated protein kinase in filamentous fungi. Eukaryot. Cell 4:1820-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J.-R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a Hog1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.