Abstract
The human fungal pathogen Candida albicans can undergo a morphological transition from a unicellular yeast growth form to a multicellular hyphal growth form. During hyphal growth, cell division is asymmetric. Only the apical cell divides, whereas subapical cells remain in G1, and cell surface growth is highly restricted to the tip of the apical cell. Hgc1, a hypha-specific, G1 cyclin-like protein, is essential for hyphal development. Here, we report, using indirect immunofluorescence, that Hgc1 is preferentially localized to the dividing apical cells of hyphae. Hgc1 protein is rapidly degraded in a cell cycle-independent manner, and the protein turnover likely occurs in both the apical and the subapical cells of hyphae. In addition to rapid protein turnover, the HGC1 transcript is also dynamically regulated during cell cycle progression in hyphal growth. It is induced upon germ tube formation in early G1; the transcript level is reduced during the G1/S transition and peaks again around the G2/M phase in the subsequent cell cycles. Transcription from the HGC1 promoter is essential for its apical cell localization, as Hgc1 no longer exhibits preferential apical localization when expressed under the MAL2 promoter. Using fluorescence in situ hybridization, the HGC1 transcript is detected only in the apical cells of hyphae, suggesting that HGC1 is transcribed in the apical cell. Therefore, the preferential localization of Hgc1 to the apical cells of hyphae results from the dynamic temporal and spatial control of HGC1 expression.
Asymmetric cell division and distribution of cell fate determinants are conserved mechanisms for generating cell diversity during development, ranging from unicellular bacteria and yeast to multicellular plants and animals. They are the primary mechanisms used by neuronal progenitor cells to generate neuronal diversity in Drosophila (43, 55). The asymmetric distribution of cell fate determinants also generates the different fates between mother and daughter cells in the yeast Saccharomyces cerevisiae for mating type switching in mother cells (10, 47) and degradation of cell wall material at septa by daughter cells (16). The development of swarmer and stalked cells in bacterial Caulobacter crescentus depends on asymmetric expression/localization of key developmental regulators and asymmetric cell division as well (19). Asymmetric cell division also happens during hyphal growth in Candida albicans, one of the most frequently isolated opportunistic fungal pathogens of humans. C. albicans can undergo a switch from a unicellular yeast growth form to a multicellular hyphal growth form in response to various environmental signals, and the unique ability of C. albicans to switch from yeast to hyphal growth is inherent to its pathogenicity (53). During hyphal growth, cell surface growth is highly restricted to the tip of the germ tube and of the apical cell, generating thin and long hyphal filaments with parallel sides along its entire length (50). Cell division is asymmetric; only the apical cell divides, whereas subapical cells often remain in G1. Cytokinesis occurs in the absence of cell separation, and cells remain attached after each cell division, producing linear filaments of elongated cells with no constrictions at the septal junctions. In addition, hyphal tip cells are vacuole poor and cytosol rich compared to the rest of the hyphal cells (6).
Multiple signaling pathways participate in the regulation of hyphal development and the expression of hypha-specific genes in response to different signals (53). Among them, the cyclic AMP/protein kinase A pathway plays a major role, as many mutants of the pathway are defective in hyphal development. A key component of the pathway is the adenylate cyclase Cdc35, which is essential for hyphal development (44). The cyclase activity is regulated by two G proteins, Ras1 and Gpa2, in C. albicans (21, 22, 38, 41, 45). Two transcription factors, Efg1 and Flo8, function downstream of the cyclic AMP-dependent protein kinase A pathway and are both essential for hyphal development and the induction of hypha-specific genes in response to a range of environmental signals (12, 34, 49). Another transcription factor, Tec1, whose expression requires Efg1 (30), is also necessary for hyphal development (46).
The cell cycle has been shown to play a role in hyphal development in C. albicans. Three G1 cyclins (Ccn1, Cln3, and Hgc1) and two B-type cyclins (Clb2 and Clb4) have been characterized for the cell division kinase Cdc28 in C. albicans. Ccn1, similar to Cln1/Cln2 of S. cerevisiae in its protein sequence and the timing of its expression at the G1/S transition, is not required for germ tube formation but is required for maintaining hyphal growth (35). Hgc1, homologous to Cln1/Cln2 of S. cerevisiae in its sequence and expressed specifically in hyphae, is required for hyphal morphogenesis (56). Repression of the CLN3 homolog, an essential gene in C. albicans, leads to hyphal growth under certain growth conditions (4, 13). Clb2 and Clb4, peaking at the G2/M transition, are negative regulators of polarized growth in C. albicans (9). Sol1, an inhibitor of the mitotic cyclin Cdc28 in C. albicans, can induce polarized growth when overexpressed (1). Repression of the polo-like kinase Cdc5 also induces hyphal growth (3). Cell cycle toxins, such as hydroxyurea (HU) and nocodazole (NOC), cause cell elongation as well (2, 5). Deletion of the CDC14 phosphatase gene results in impaired true hyphal growth (15). Recently, repression of Cdc28 has been shown to cause cell elongation and reduce the expression of several transcription factors required for hyphal development, including Efg1, Tec1, and Fkh2 (51).
HGC1, unlike most hypha-specific genes which encode cell wall proteins and secreted proteases, is the only hypha-specific gene found to be required for hyphal morphogenesis (56). Its expression in hyphae requires Cdc35, Efg1, Flo8, and Tec1 (8, 12, 56). In this report, we show that Hgc1 is preferentially localized to the apical cells of hyphae. Not only is the expression of HGC1 hypha specific, but its expression is also cyclic during the cell cycle. Furthermore, the HGC1 transcript is detected only in the apical cells of hyphae, and transcription from its own promoter is essential for Hgc1 localization to the apical cells in hyphae.
MATERIALS AND METHODS
Plasmid construction.
The HGC1p-myc-HGC1 plasmid was constructed by first cloning a 2.4-kb HGC1 PCR fragment, using primers P680 (5′CGACGCGTATGATAAATATAACTAAACCATTAACT) and P682 (5′GGGGTACCCTTTAATGAATCATATTACATAATCG), into the MluI-Kpn1 site of plasmid BP2 (31), a vector derived from BES116 (22) for genomic integration at the ADE2 locus. Then, a PCR product of the 3-kb upstream region of HGC1, using primers P798 (5′ATAAGAATGCGGCCGCTTAATGCATCAGTTTCTACTGAATAGC) and P801 (5′GCGACGCGTGCAGCTGGGCAGATCTGCATTATTAATATATGTTTATATGTGTATATGTG), was cloned into the NotI-MluI site, displacing the PCK1 promoter. The underlined sequences indicate MluI, PvuII, and BglII sites, respectively, and the ATG is italicized. Finally, a 13-myc PCR fragment from a modular vector designed by Longtine et al. (37) was cut with BamHI and BfaI and cloned into the BglII-PvuII site, downstream of the ATG and upstream of the MluI site.
The HGC1p-myc-HGC1(2T) plasmid was constructed by sequential replacement of the two potential cyclin-dependent-kinase phosphorylation sites Thr477 and Thr641 in Hgc1 with Gly by site-directed mutagenesis with fusion PCRs. The resulting mutant, HGC1T477G, T641G, was cloned into the MluI-Kpn1 site of the plasmid HGC1p-myc-HGC1, replacing the wild-type copy, and confirmed by ApaI digestion and DNA sequencing.
The MAL2p-myc-HGC1 plasmid was constructed by replacing the HGC1 promoter region (positions −3000 to −794 of HGC1) from the plasmid HGC1p-myc-HGC1 with a 550-bp NotI-Xba1 PCR fragment of the MAL2 promoter region. The use of the XbaI restriction site at position −794 upstream of the HGC1 ATG leaves an approximately 794-bp 5′ untranslated region in the MAL2p-myc-HGC1 construct.
GALp-myc-HGC1 was constructed by cloning a myc-HGC1 PCR fragment, with primers 5′CGGGATCCGCATCAAACCAATACCCAACAC and 5′ATAAGAATGCGGCCGCTTAATGAATCATATTACATAATCGAG from the HGC1p-myc-HGC1 plasmid, into the BamHI-NotI site of the plasmid pRS316-GAL1 for expression in S. cerevisiae (33).
All subclones were confirmed by DNA sequencing.
C. albicans strain construction.
The C. albicans and S. cerevisiae strains used in this study are listed in Table 1. HGC1 was deleted based on the method of Wilson et al. (54). Primers P687 (5′GGAAATCAAAATTCATAATCAAAGTCTTGCTGAATATGATTTAGACATTTATGATATTATGGTTAATTTGATTGAAACCGTTTTCCCAGTCACGACGTT) and P688 (5′TTAATGAATCATATTACATAATCGAGTTTTAGTATAAATAGGAGAATCATTTTCACTAATAGGTGTACCACTTGTGGAATTGTGAGCGGATA) were used to amplify C. albicans HIS1 and ARG4 from plasmids pGEM-HIS1 and pRS-ARG4ΔspeI (54), respectively. The PCR products were used to transform C. albicans strain BWP17, yielding hgc1/HGC1 heterozygous strains. The heterozygous strains were used for a second round of transformation, yielding an hgc1 homozygous deletion strain, HLY3435 (Table 1). The replacement of each HGC1 gene with a selectable marker was determined by PCR with primers P689 (5′CTTCCATACAAGAAGAGTCC) and P690 (5′GGATACTTTCCAGTAGTGTA).
TABLE 1.
Candida albicans and Saccharomyces cerevisiae strains
| Strain | Genotype | Source or reference |
|---|---|---|
| C. albicans | ||
| SC5314 | Wild type | 23 |
| BWP17 | ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 54 |
| HLY3577 | ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG ADE2/ade2::HGC1p-MYC-HGC1-URA3 | This study |
| HLY3578 | ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG ADE2/ade2::MAL2p-MYC-HGC1-URA3 | This study |
| HLY3588 | ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG ADE2/ade2::URA3 | This study |
| HLY3435 | hgc1::HIS1/hgc1::ARG4 ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | This study |
| HLY3443 | hgc1::HIS1/hgc1::ARG4 ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG ADE2/ade2::URA3 | This study |
| HLY3579 | hgc1::HIS1/hgc1::ARG4 ura3::1 imm434/ura3::1 imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG ADE2/ade2::HGC1p-MYC-HGC1-2T-URA3 | This study |
| SN148 | arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3::imm434/ura3::imm434 iro1::imm434/iro1::imm434 | 42 |
| HLY3586 | grr1::C.d.HIS1/grr1::C.m.LEU2 arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3::imm434/ura3::imm434 iro1::imm434/iro1::imm434 | This study |
| HLY3587 | grr1::C.d.HIS1/grr1::C.m.LEU2 arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3::imm434/ura3::imm434 iro1::imm434/iro1::imm434 ADE2/ade2::MAL2p-MYC-HGC1-URA3 | This study |
| S. cerevisiae | ||
| PY1 | MATa bar1 | 28 |
| PY288 | MATa cdc34-3 bar1 | 28 |
| PY170 | MATa cdc23-1 bar1 | 28 |
| HLY1377 | MATa grr1::LEU2 ura3-52 leu2::hisG trp1::his G | 14 |
A C. albicans grr1 mutant was generated according to the method of Noble et al. (42). The same primers, universal primers 2 and 5, were used to amplify internal deletion sequences of C. dubliniensis HIS1 and C. maltosa LEU2 from plasmids pSN52 and pSN40 (42), respectively. GRR1 primers 1 (5′CAGTATCAACTGACAATACTTTG) and 3 (5′cacggcgcgcctagcagcggGAGTTATGAATAATCTTGAGTTTG) and GRR1 primers 4 (5′gtcagcggccgcatccctgcGTGATAACAATTGCTGATTGAAT) and 6 (5′TATAGCACCTTCGCACTCGT) were used to amplify flanking sequences (sequences in lowercase are complementary to plasmid sequences for fusion PCR). The fusion PCR products were transformed sequentially into strain SN148 (42) to generate the grr1 deletion mutant HLY3586. The homozygous deletion was confirmed with upstream (5′CGTAATCAATCTCACACCGC) and downstream (5′GAGCTGTTATGGACCTTGAC) primers as well as internal forward (5′TAATTCAAGCACTAATATTACCG) and reverse (5′AGACAATCCTGTTCACCACG) primers.
HLY3577 (myc-HGC1), HLY3578, and HLY3588 were obtained by transforming BWP17 with AscI-digested plasmids HGC1p-myc-HGC1, MAL2p-myc-HGC1, and BES116 (22), respectively.
HYL3443 was a transformant of HLY3435 with an AscI-digested BES116 vector (22).
HLY3579 (myc-HGC1enh) was obtained as a transformant with enhanced and inducible hyphae by transforming HLY3435 with an AscI-digested HGC1p-myc-HGC1(2T) plasmid. Although the construct contains two point mutations at potential cyclin-dependent-kinase phosphorylation sites of Hgc1, the mutations do not contribute to the enhanced filamentation phenotype of HLY3579, as other transformants of this plasmid did not exhibit an enhanced filamentation phenotype. In addition, the two mutations do not change Hgc1 stability when cloned under the MAL2 promoter (our unpublished data).
HLY3587 is a transformant of HLY3586 with AscI-digested MAL2p-myc-HGC1.
Indirect immunofluorescence.
C. albicans cells were fixed by incubation in 3.7% formaldehyde for 45 min. Once fixed, samples were incubated in 2.5 μg/ml Zymolyase 100-T (Seikagaku, Tokyo, Japan) in 1.2 M sorbitol, pH 7.5, for 45 min to 1 h to fully digest the cell wall. Cells were then attached to poly-lysine-coated 12-well slides (Erie Scientific). Slides were incubated in 9E10 antibody (Covance) diluted 1:500 in phosphate-buffered saline plus 1% Tween plus 10 mg/ml bovine serum albumin (PBST) overnight at 4°C. Slides were washed in PBST three times and incubated for 2 h in fluorescein isothiocyanate (FITC)-labeled anti-mouse antibodies (Jackson Laboratory) diluted 1:500 in PBST. Samples were again washed with PBST and mounted with Prolong Gold antifade reagent (Invitrogen). A Zeiss Axioplan 2 microscope with a 100× objective and a digital camera were used for all image acquisition. To maintain relative intensity, myc-tagged and untagged images were captured with the same exposure time on the scope, and images were processed identically with Adobe Photoshop software.
Promoter shutdown assays.
C. albicans strains containing myc-Hgc1 under the regulation of the MAL2 promoter were grown in yeast extract-peptone plus 2% maltose (YPM) to early log phase to induce the expression of myc-Hgc1. Where indicated, 50 μM nocodazole or 200 mM hydroxyurea was added until the cells were arrested in G2/M or S phase, respectively. Glucose (2%) was added to shut off the promoter, and aliquots were collected after the times indicated. Myc-Hgc1 protein levels were analyzed via Western blotting. Myc-Hgc1 was detected on Western blots by using a horseradish peroxidase-conjugated anti-myc antibody (Roche).
S. cerevisiae strains harboring a plasmid with myc-Hgc1 under the regulation of the GAL1 promoter were grown at 30°C to early log phase in yeast extract-peptone plus 2% raffinose, at which time the expression of myc-Hgc1 from the GAL1 promoter was induced by adding 2% galactose. After 1 h, 2% glucose was added, and aliquots were collected after the times indicated. myc-Hgc1 protein levels were analyzed via Western blotting with a horseradish peroxidase-conjugated anti-myc antibody (Roche). In the case of temperature-sensitive mutants cdc34-3 and cdc23-1, cultures were shifted to the restrictive temperature of 37°C 30 min after the addition of 2% galactose. After an additional 30 min, 2% glucose was added, aliquots were taken, and myc-Hgc1 levels were analyzed via Western blotting.
Northern analysis.
Methods for RNA isolation and Northern blot hybridization were as previously described (30). Probes used for Northern hybridization were a 1.2-kb HGC1 PCR product (primers P681 [5′TTGGCGGGTTTGGTAATAGTGG] and P682 [5′GGGGTACCCTTAATGAATCATATTACATAATCG]), a CDC28 PCR product (primers P125 [5′GAAGGTGTACCTAGTACCGGC] and P126 [5′TCATACACCAACATTTGATCC]), a 0.9-kb HindIII-ClaI fragment of a CCN1 PCR product (primers P301 [5′GGGGTACCTACTATTACTCAGTCCATTCG] and P302 [5′ GGGGTACCATGTATAGCTAGAAATAACACC]), and a ClaI-SalI ACT1 fragment from plasmid p1595/3.
Elutriation.
C. albicans G1 cells were collected using centrifugal elutriation as described previously (25). Log phase cells were collected by centrifugation from an overnight culture grown in yeast extract-peptone plus 2% raffinose medium at 30°C. The cells were spun down, resuspended in water, and sonicated to resolve clumps before being loaded into a separation chamber spinning at 2,000 rpm in a Beckman JE-5.0 elutriation system. Unbudded G1 cells were collected, concentrated by centrifugation, and released in prewarmed yeast or hyphal-inducing media. Aliquots were taken for Northern analysis and visualization of chitin rings and nuclear DNA by microscopy as described by Hazan et al. (25).
Creation and labeling of double-stranded DNA probes against the HGC1 transcript.
Cy3-labeled double-stranded DNA probes were generated based on the method of Dernburg et al. (18). DNA of the HGC1 coding region was amplified via PCR in three parts by using genomic DNA from a wild-type strain. The three fragments were positions 1 to 501, 1 to 1442, and 144 to 2358 of the HGC1 open reading frame and were further digested with ApoI, MseI, and a cocktail of ApoI, AluI, HinfI, and HphI, respectively, to create fragments 50 to 200 bp in length. The 50- to 200-bp probes were combined and then 3′ labeled with Cy3 dCTP (Amersham Biosciences) by using the TdT terminal transferase reaction (New England Biolabs) for 1 hour.
FISH.
Fluorescence in situ hybridization (FISH) on C. albicans cells was adapted from the method used by Long et al. on S. cerevisiae (36). C. albicans cells were fixed by incubation in 3.7% formaldehyde for 45 min. Once fixed, samples were incubated in 2.5 μg/ml Zymolyase 100-T (Seikagaku, Tokyo, Japan) in 1.2 M sorbitol, pH 7.5, for 45 min to 1 h to fully digest the cell wall. Cells were then attached to poly-lysine-coated 12-well slides (Erie Scientific) and dehydrated overnight in 70% ethanol. Slides containing prepared cells were removed from the 70% ethanol and were washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 2× SSC-50% formaldehyde. The Cy3-labeled probe was boiled for 5 min to separate the double-stranded DNA. The probe was then hybridized to the HGC1 transcript by incubating the slides at 37°C overnight with 1 ng/ml labeled probe in 2× SSC, 50% formaldehyde, 10% dextran sulfate, and 0.02% bovine serum albumin, with 1 mg/ml calf thymus DNA. After hybridization, the slides were washed with 2× SSC and mounted with Prolong Gold antifade reagent (Invitrogen).
RESULTS
Hgc1 shows enhanced apical cell localization in hyphae.
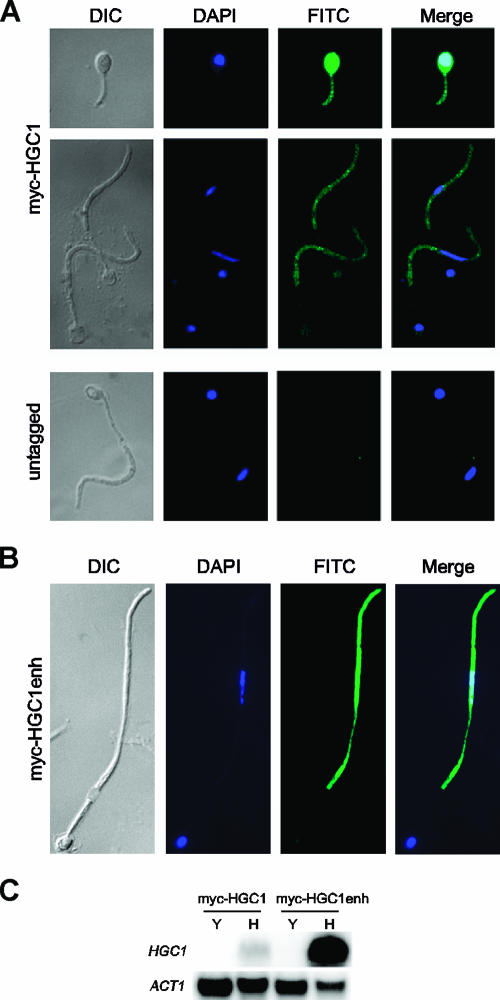
We identified HGC1 as a hypha-specific gene from a genome-wide transcriptional analysis of Efg1-, Flo8-, and Tec1-regulated genes (12; S. Lane and H. Liu, unpublished data), as reported by Zheng et al. (56). To gain insight into Hgc1 function and regulation, we localized Hgc1 by using indirect immunofluorescence. Hgc1 was tagged with 13-myc at the N terminus, and myc-HGC1 was placed under the control of its own promoter and integrated into the ADE2 locus in a wild-type C. albicans strain named myc-HGC1. The myc-HGC1 fusion was functional, as it was able to rescue the defect in hyphal morphogenesis when transformed into an hgc1 mutant (data not shown). myc-Hgc1 localization during hyphal development was visualized by indirect immunofluorescence using anti-myc antibodies. C. albicans myc-HGC1 cells were grown in Lee's medium at 37°C for hyphal formation. Germ tube formation was observed after 3 h of growth in Lee's medium, and Hgc1 displayed a general staining throughout the entire cell (Fig. 1A.). The general staining observed was specific to myc-Hgc1, as no background signal was detected in untagged cells under identical treatment and exposure (Fig. 1A) or the myc-HGC1 strain under yeast growth conditions (data not shown). After 6 h of growth in Lee's medium, when germ tubes had developed into hyphae with two cells, myc-Hgc1 was detected primarily in the actively dividing apical cells but not in the subapical cells (Fig. 1A). To ensure that the lack of signals in the subapical cells was not due to uneven digestion or staining procedure, we routinely included a control strain expressing a myc-tagged nuclear protein. This control strain persistently showed nuclear signals in the apical as well as subapical cells (data not shown), verifying that the absence of detectable myc-Hgc1 in the subapical cells was not due to technical problems. Our myc-Hgc1 immunofluorescence data suggest that the Hgc1 protein is primarily present in the dividing apical cells and not in subapical G1 cells.
FIG. 1.
Hgc1 is detected in the apical cells of hyphae via indirect immunofluorescence. (A) myc-HGC1 (HLY3577), a strain expressing myc-Hgc1 under the HGC1 promoter, was grown in Lee's media at 37°C for hyphal induction. Cells were fixed at 3 h and 6 h after induction and stained for myc-Hgc1 with 9E10 mouse antibodies and FITC-conjugated secondary antibodies. DNA was stained with DAPI. An untagged control (HLY3588) was included. (B) myc-HGC1enh (HLY3579), a strain with enhanced expression of myc-Hgc1 under the HGC1 promoter, was grown in YPD plus 10% serum at 37°C for 3 h. Cells were then fixed and treated as described above. (C) Northern blot analysis comparing HGC1 transcript levels in the myc-HGC1 and myc-HGC1enh strains. RNA was extracted from both strains after 2 h of growth in YPD at 25°C for yeast growth (Y) and in YPD plus 10% serum at 37°C for hyphal growth (H). Blots were probed with ACT1 as a loading control. DIC, differential interference contrast microscopy.
The apical cell localization of Hgc1 was further confirmed with another myc-HGC1 C. albicans transformant that we obtained (Fig. 1B). The strain was dimorphic and grew as the yeast form at 25°C in yeast extract-peptone-dextrose (YPD) but readily switched to hyphal growth mode even in YPD at 30°C (data not shown). When exposed to hypha-inducing conditions, such as YPD plus 10% serum at 37°C, cells were hyperfilamentous, forming especially long hyphae. We therefore named the strain myc-HGC1enh. In the myc-HGC1enh strain, myc-Hgc1 was under the HGC1 promoter and integrated at the ADE2 locus, and the myc-Hgc1 was the only source of Hgc1 in the strain. Consistent with its phenotype, Northern analysis showed that HGC1 was highly induced in the myc-HGC1enh cells under hyphal growth conditions, and the transcript levels in hyphae in the myc-HGC1enh strain were much higher than those in hyphae in the myc-HGC1 strain (Fig. 1C). The myc-Hgc1 protein level was also highly induced in the myc-HGC1enh strain under hyphal growth conditions (data not shown). Taking advantage of the highly inducible myc-Hgc1 protein, we visualized myc-Hgc1 localization in the myc-HGC1enh strain via indirect immunofluorescence. As expected, the immunofluorescence signal in the myc-HGC1enh cells was much stronger than that in the myc-HGC1 cells and was easily detected by indirect immunofluorescence. Importantly, the myc-Hgc1 signal was strongly detected in the apical cells of hyphae and not detected in the subapical cells (Fig. 1B). This further solidifies the conclusion that Hgc1 is primarily present in the apical cells of hyphae.
Hgc1 is rapidly degraded in both apical and subapical cells, independent of cell cycle regulation.
One possible explanation for the apical cell localization of Hgc1 in hyphae is that Hgc1 is preferentially degraded in the G1-arrested subapical cells but stable in the dividing apical cells. For example, the anaphase-promoting-complex (APC)-mediated ubiquitination and protein degradation are cell cycle dependent (20). APCCdh1 is activated at anaphase, and its activity persists to G1. A protein degraded by APCCdh1 is likely present in the dividing apical cells only because it is degraded in G1-arrested subapical cells. To examine whether this could be a mechanism for the asymmetric localization of Hgc1 in the apical cell, we examined Hgc1 stability in C. albicans. myc-HGC1 was cloned under the regulation of the MAL2 promoter and transformed into the BWP17 strain. After the MAL2 promoter was shut off by the addition of glucose, myc-Hgc1 was rapidly degraded under both yeast and hyphal growth conditions (Fig. 2A and data not shown). To address the effect of the cell cycle on myc-Hgc1 degradation, we performed promoter shutoff degradation assays on cells arrested in S phase with hydroxyurea and in the G2/M phase with nocodazole. After growth in 200 mM HU or 50 μM NOC for 3 h, >90% of the cells were arrested in S or G2/M phase, respectively, as examined by DAPI (4′,6′-diamidino-2-phenylindole) staining/microscopy (data not shown). When arrested in S or G2/M phase, myc-Hgc1 was degraded with dynamics similar to those of cycling cells (Fig. 2A). This suggests that the cell cycle does not have a significant impact on Hgc1 turnover, and therefore, preferential degradation in the subapical G1 cells is not likely the mechanism for the asymmetric distribution of Hgc1 between apical and subapical cells.
FIG. 2.
Hgc1 is rapidly degraded independent of the cell cycle. (A) Hgc1 stability is monitored by MAL2 promoter shutdown and Western blot analysis in C. albicans wild-type (WT) cells (HLY3578) or grr1 mutant cells (HLY3586) expressing myc-Hgc1 from the MAL2 promoter. Hgc1 stability is examined in cycling cells (Cyc) and in cells arrested with 50 μM nocodazole (+NOC) and 200 mM hydroxyurea (+HU). (B) Hgc1 stability is monitored by GAL1 promoter shutdown and Western blot analysis in the following S. cerevisiae strains, carrying the GAL1-myc-HGC1 plasmid: WT (PY1), cdc34-3 (PY288), cdc23-1 (PY170), and grr1 (HLY1377). α-myc-Hgc1, anti-myc-Hgc1; α-PSTAIRE, anti-PSTAIRE.
To determine which E2 ubiquitin-conjugating enzyme and E3 ubiquitin ligase are responsible for Hgc1 degradation, we expressed myc-HGC1 in Saccharomyces cerevisiae under the regulation of the GAL1 promoter. After the expression was turned off by the addition of glucose, Hgc1 was degraded rapidly in a wild-type S. cerevisiae strain (Fig. 2B). We then transformed the pGAL1-myc-HGC1 plasmid into various E2 mutants, including mms2, rad6, ubc4, ubc5, ubc7, ubc8, ubc10, ubc11, ubc12, ubc13, udf2, and cdc34, and an APC mutant, cdc23. By performing a promoter shutdown assay on these mutants, we found that Hgc1 was completely stabilized in the cdc34 mutant at the restrictive temperature and was degraded in all other E2 mutants examined (data not shown). In particular, Hgc1 degradation was also not affected in the cdc23 mutant at the restrictive temperature. Cdc34 is the E2 that functions together with a Skp1-Cullin-F-box (SCF) complex type of E3. The F-box protein allows for specific substrate recognition. Because S. cerevisiae G1 cyclins Cln1 and Cln2 are degraded via SCFGrr1-mediated ubiquitination, we examined the stability of Hgc1 in a grr1 mutant. As in the cdc34 mutant, Hgc1 was completely stabilized in the grr1 mutant. Therefore, Hgc1 degradation requires the SCFGrr1 protein in S. cerevisiae.
We then examined whether Grr1 is also required for the degradation of Hgc1 in C. albicans. C. albicans Grr1 has been shown to function in a manner similar to that of S. cerevisiae Grr1 and is responsible for the degradation of Ccn1, Cln3, and Hof1 in C. albicans (11, 32). We constructed a grr1 deletion mutant and transformed it with the pMAL2-myc-HGC1 construct. After promoter shutoff by the addition of glucose, myc-Hgc1 was degraded as rapidly in the grr1 mutant as in a wild-type strain (Fig. 2A), consistent with a recent report (32). Since Hgc1 was completely stabilized by the grr1 or cdc34 mutant but not other E2 mutants in S. cerevisiae, and because C. albicans Grr1 is a functional homolog of S. cerevisiae Grr1 (11, 32), it is possible that the C. albicans SCFGrr1 protein could still mediate the degradation of Hgc1, but in addition to SCFGrr1, another F-box protein for the SCF complex or a different set of E2 and E3 proteins could also degrade Hgc1 in C. albicans. The former scenario is a possibility, as C. albicans Cdc4 and S. cerevisiae Cdc4 are found to have different specificities for their targets despite the fact that each mediates the degradation of the transcription factor Gcn4 in the respective yeast (1). The potential Grr1-independent pathway is unlikely to undergo APC-mediated degradation, as myc-Hgc1 was still degraded in the grr1 mutant in the presence of HU or NOC (Fig. 2A). In addition, Hgc1 was not stabilized in the cdc23 mutant in S. cerevisiae (Fig. 2B). Our analyses of Hgc1 degradation in both S. cerevisiae and C. albicans suggest that Hgc1 degradation is not controlled by the cell cycle-regulated APC. Therefore, the asymmetric localization of Hgc1 is probably not caused by preferential degradation of Hgc1 in the subapical G1 cell. The dynamic turnover of Hgc1 during hyphal growth in C. albicans supports the hypothesis that Hgc1 is also rapidly degraded in the apical cells.
HGC1 transcription is dynamic throughout hyphal development and is affected by the cell cycle.
Since asymmetric Hgc1 localization is not likely regulated through preferential degradation in the subapical cells, we decided to determine whether HGC1 transcription or mRNA localization is restricted to the apical cells. HGC1 is expressed only in hyphal cells, and its induction is not restricted to a specific cell cycle phase, as HGC1 can be induced from cells treated with HU or NOC (56). We took a closer look at the level of HGC1 during the induction of germ tubes from synchronized G1 cells. Wild-type G1 yeast cells were isolated by centrifugal elutriation methods and released into YPD plus 10% serum at 37°C for hyphal growth. Cells were collected every 10 minutes for Northern analysis of HGC1. The same Northern blot was also probed with CCN1 for the timing of the G1/S transition and with CDC28 for a loading control. Cells from each time point were also stained with calcofluor white and DAPI to monitor the progression of the cell cycle. HGC1 expression was detected at all time points after hyphal induction (Fig. 3a), while no HGC1 expression was detected when elutriated G1 cells were released into YPD at 30°C for yeast growth (data not shown). This is consistent with the published data that HGC1 transcription is hypha specific (56). However, we also observed that the HGC1 transcript level displayed dynamic changes with several ups and downs during the time course. A huge initial burst in HGC1 expression was observed within 20 min upon hyphal induction. Then, the HGC1 transcript level fell when the CCN1 transcript level rose around the G1/S transition. Later in the time course, the HGC1 transcript level peaked again around the G2/M phase and then fell in the subsequent G1/S transition (Fig. 3B and C).
FIG. 3.
HGC1 expression is affected by the cell cycle. Wild-type (SC5314) G1 cells were collected by elutriation centrifugation and released into YPD plus 10% serum at 37°C for hyphal growth, and cells were collected every 10 min. (A) Northern blots were probed with HGC1, CCN1, and CDC28. (B) HGC1 and CCN1 expression were quantified relative to CDC28 expression. (C) Cells were stained with calcofluor white and DAPI to determine the percentages of cells with chitin rings and dividing nuclei, respectively. (D) Northern blots of wild-type (SC5314) asynchronous cells released into YPD plus 10% serum at 37°C. Cells were collected every 15 min.
To examine whether the initial burst and periodic pattern of HGC1 transcription were cell cycle associated and not coincidental, HGC1 transcription was analyzed during hyphal induction in YPD plus 10% serum at 37°C with asynchronous cells. An initial peak was observed at 15 min of induction with asynchronous cells (Fig. 3D), similar to the finding that the HGC1 transcript peaks at 10 min upon hyphal induction (8). Therefore, the initial burst in HGC1 transcription is cell cycle independent. This is consistent with the result that cells arrested in the cell cycle by HU or NOC are able to induce HGC1 expression (56), but the initial burst of HGC1 transcription was strongest with synchronous G1 cells (Fig. 3A). We did not detect any periodic pattern of HGC1 transcription during hyphal induction with asynchronous cells, supporting the fact that the observed periodic HGC1 transcript level during hyphal induction from synchronous G1 cells was associated with the cell cycle. Our Northern analyses, therefore, suggest that HGC1 transcription is not only hypha specific but also associated with cell cycle progress.
Enhanced apical localization of Hgc1 requires transcription from the HGC1 promoter.
Because HGC1 transcription is so highly regulated, transcription from the HGC1 promoter may be important for the apical cell localization of Hgc1. To examine this possibility, indirect immunofluorescence against myc-Hgc1 was carried out to compare the localization of myc-Hgc1 under the control of the HGC1 promoter to that of myc-Hgc1 under the control of the MAL2 promoter. Cells were exposed to Lee's media with maltose as the carbon source at 37°C for 6 h, so hyphae had reached the two-cell-stage of hyphal development. When expressed from the MAL2 promoter, Hgc1 was detected in both the actively dividing apical cell and the G1-arrested subapical cell with equal intensity (Fig. 4). This was not because Hgc1 was overexpressed from the MAL2 promoter, as the level of Hgc1 expression from the MAL2 promoter was comparable to that from the HGC1 promoter in the myc-HGC1 strain and lower than that in the myc-HGC1enh strain (data not shown). Therefore, apical Hgc1 localization is dependent on its transcription from the HGC1 promoter. This demonstrates that enhanced apical localization of Hgc1 is not regulated through differential Hgc1 degradation and suggests that transcription from the HGC1 promoter may be more active in the dividing apical cells than in the subapical cells.
FIG. 4.
Apical-specific localization of Hgc1 is promoter dependent. myc-Hgc1 localization by indirect immunofluorescence in cells expressing myc-Hgc1 from the HGC1 promoter (HLY3577) and cells expressing myc-Hgc1 from the MAL2 promoter (HLY3578) is shown. Both strains were grown in Lee's medium with 2% maltose as the carbon source for 6 h at 37°C. An untagged control (HLY3588) was included. DIC, differential interference contrast microscopy.
Transcription is detected only in the apical cells of hyphae.
The promoter swap experiment suggested that transcription from the HGC1 promoter was primarily in the apical cell. To validate this observation, FISH with FITC-labeled probes was used to directly visualize the HGC1 transcript in hyphae. The myc-HGC1enh strain was used in this experiment because its highly inducible HGC1 transcription gave a readily detectable signal by FISH. Cells were grown in YPD plus 10% serum at 37°C for hyphal induction, fixed, and probed for the HGC1 transcript. A “dot” signal was detected near the nucleus during germ tube development (Fig. 5A). The signal was specific to the HGC1 transcript, as it was not detected in the same strain in the yeast form (Fig. 5B) or in an hgc1 deletion strain under the same experimental conditions (Fig. 5C). When hyphae had reached the two-cell stage, the “dot” signal was detected only in the actively dividing apical cell, again near the nucleus, but was not detected in the G1-arrested subapical cell. Likewise, in hyphae with three cells, a FISH signal was detected only in the actively dividing apical cell. This provides direct evidence for our previous notion that transcription from the HGC1 promoter is enhanced in the apical cell.
FIG. 5.
HGC1 mRNA is visualized in C. albicans cells by using FISH. (A) myc-HGC1enh (HLY3579) cells were grown in YPD plus 10% serum at 37° for hyphal induction. Cells were fixed at 1, 3, and 5 h of induction during germ tube formation and when cells were entering the second and third cell division for FISH with FITC-labeled DNA probes against the HGC1 transcript. Results are shown for control FISH experiments with myc-HGC1enh (HLY3579) cells grown in YPD at 25°C for yeast growth (B) and hgc1 (HLY3443) cells grown in YPD plus 10% serum at 37° for 1.5 h (C). DIC, differential interference contrast microscopy.
DISCUSSION
We show in this report that the Hgc1 protein displays preferential localization in the actively dividing apical cells of hyphae. Hgc1 protein is rapidly degraded, probably through SCF-mediated ubiquitination. The protein turnover is not under the control of the cell cycle and occurs in both the apical and the subapical cells of hyphae. HGC1 transcription is also dynamically regulated. Not only is HGC1 transcribed specifically in hyphae (56), but we show here that its transcription is also regulated by the cell cycle and low during the G1/S transition. Furthermore, the HGC1 transcript is detected, using FISH, only in the dividing apical cells and not in the subapical cells. By replacing the HGC1 promoter with the MAL2 promoter, we demonstrate that the dynamic temporal and spatial regulation of HGC1 transcription is critical for the asymmetric localization of Hgc1 protein to the apical cell during hyphal development.
It has long been appreciated that the apical cells in hyphal filaments are different from subapical cells in many aspects, and the difference in cell division is a major aspect. Only the apical cells undergo cell division; the subapical cells are arrested in G1 for a period of time until a lateral bud or hypha forms. Cyclins and many cell cycle regulators, whose transcription and protein degradation are tightly regulated during the cell cycle, are likely present only in the dividing apical cells in hyphal filaments. For example, Cdc14p is detected only in the nucleus of the apical cell of the hypha, whereas no signal is detected in any of the G1-arrested subapical cells (15). Here, we show that HGC1 transcription is not only specific to hyphal cells but also regulated during the cell cycle program. Therefore, we speculate that integration of cell cycle regulation with the hyphal signaling pathway may give rise to the transcription of HGC1 in the apical cell.
Another major difference between apical and subapical cells during hyphal development is that much of the active growth happens in the apical cells, and new cell wall growth is highly restricted to the tips of hyphae. The actin cytoskeleton is highly polarized to the tip, where active Cdc42 and other polarity determinant proteins are localized (8, 17, 24, 39, 57). Active Cdc42 and the actin cytoskeleton not only are essential for hyphal morphogenesis (7, 24, 40, 52) but also play a role in maintaining hypha-associated transcription (8, 24). Whether active Cdc42 plays a role in keeping the hyphal transcriptional program active in the apical cell remains to be determined.
Two molecular mechanisms for asymmetric distribution or expression of mRNAs between mother and daughter cells have been elucidated in S. cerevisiae. One involves the transport of ASH1 mRNA along actin cables to the apices of daughter cells, where Ash1 protein is translated and imported into the daughter nuclei to repress the expression of the HO endonucleases, preventing mating-type switching in the daughter cells (10, 47). As in S. cerevisiae, Ash1 is preferentially localized to daughter cell nuclei in the yeast form of C. albicans and to the apical cell nuclei in growing hyphae (26). The fact that the HGC1 transcript is not concentrated to the tips of hyphae indicates that HGC1 mRNA is probably not transported on actin cables to the apical cells. Another mechanism involves the transcription of a group of early-G1 genes in the daughter cells through Cbk1- and Mob2-dependent activation and localization of Ace2 to the daughter nuclei at the end of mitosis and in early G1 (16, 27). A functional homolog of Ace2 in C. albicans is also localized to daughter yeast cells at the end of mitosis (29). However, Ace2 is localized in the nuclei of both apical and subapical cells in hyphae (29), and whether its transcriptional activity is limited to the apical cell of a hypha is unclear.
Is the transcription of any other hypha-specific gene restricted or enriched in the apical cell? Transcription from the HWP1 promoter is likely not restricted to the apical cell, as a green fluorescent protein under the control of the HWP1 promoter is expressed equally in the apical and subapical cells of hyphae (48). Even if the transcription is specific to the apical cell, the protein products will still be present in the subapical cells if the mRNA and/or protein products are stable. Therefore, for cell wall proteins like Hwp1 and Rbt5, they are likely present throughout the hyphal walls of both apical and subapical cells. Since different hypha-specific genes are transcribed with different tempo dynamics (8), we speculate that their transcripts may also differ in spatial distribution.
Acknowledgments
We thank W. A. Fonzi, A. P. Mitchell, S. M. Noble, and P. Kaiser for strains.
This work was supported by an NIH grant (GM55155) to H.L.
Footnotes
Published ahead of print on 15 December 2006.
REFERENCES
- 1.Atir-Lande, A., T. Gildor, and D. Kornitzer. 2005. Role for the SCFCDC4 ubiquitin ligase in Candida albicans morphogenesis. Mol. Biol. Cell 16:2772-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachewich, C., A. Nantel, and M. Whiteway. 2005. Cell cycle arrest during S or M phase generates polarized growth via distinct signals in Candida albicans. Mol. Microbiol. 57:942-959. [DOI] [PubMed] [Google Scholar]
- 3.Bachewich, C., D. Y. Thomas, and M. Whiteway. 2003. Depletion of a polo-like kinase in Candida albicans activates cyclase-dependent hyphal-like growth. Mol. Biol. Cell 14:2163-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachewich, C., and M. Whiteway. 2005. Cyclin Cln3p links G1 progression to hyphal and pseudohyphal development in Candida albicans. Eukaryot. Cell 4:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai, C., N. Ramanan, Y. M. Wang, and Y. Wang. 2002. Spindle assembly checkpoint component CaMad2p is indispensable for Candida albicans survival and virulence in mice. Mol. Microbiol. 45:31-44. [DOI] [PubMed] [Google Scholar]
- 6.Barelle, C. J., E. A. Bohula, S. J. Kron, D. Wessels, D. R. Soll, A. Schafer, A. J. Brown, and N. A. Gow. 2003. Asynchronous cell cycle and asymmetric vacuolar inheritance in true hyphae of Candida albicans. Eukaryot. Cell 2:398-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassilana, M., J. Blyth, and R. A. Arkowitz. 2003. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot. Cell 2:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassilana, M., J. Hopkins, and R. A. Arkowitz. 2005. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot. Cell 4:588-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensen, E. S., A. Clemente-Blanco, K. R. Finley, J. Correa-Bordes, and J. Berman. 2005. The mitotic cyclins Clb2p and Clb4p affect morphogenesis in Candida albicans. Mol. Biol. Cell 16:3387-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobola, N., R. P. Jansen, T. H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84:699-709. [DOI] [PubMed] [Google Scholar]
- 11.Butler, D. K., O. All, J. Goffena, T. Loveless, T. Wilson, and K. A. Toenjes. 2006. The GRR1 gene of Candida albicans is involved in the negative control of pseudohyphal morphogenesis. Fungal Genet. Biol. 43:573-582. [DOI] [PubMed] [Google Scholar]
- 12.Cao, F., S. Lane, P. P. Raniga, Y. Lu, Z. Zhou, K. Ramon, J. Chen, and H. Liu. 2006. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 17:295-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapa y Lazo, B., S. Bates, and P. Sudbery. 2005. The G1 cyclin Cln3 regulates morphogenesis in Candida albicans. Eukaryot. Cell 4:90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou, S., L. Huang, and H. Liu. 2004. Fus3-regulated Tec1 degradation through SCFCdc4 determines MAPK signaling specificity during mating in yeast. Cell 119:981-990. [DOI] [PubMed] [Google Scholar]
- 15.Clemente-Blanco, A., A. Gonzalez-Novo, F. Machin, D. Caballero-Lima, L. Aragon, M. Sanchez, C. R. de Aldana, J. Jimenez, and J. Correa-Bordes. 2006. The Cdc14p phosphatase affects late cell-cycle events and morphogenesis in Candida albicans. J. Cell Sci. 119:1130-1143. [DOI] [PubMed] [Google Scholar]
- 16.Colman-Lerner, A., T. E. Chin, and R. Brent. 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107:739-750. [DOI] [PubMed] [Google Scholar]
- 17.Crampin, H., K. Finley, M. Gerami-Nejad, H. Court, C. Gale, J. Berman, and P. Sudbery. 2005. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 118:2935-2947. [DOI] [PubMed] [Google Scholar]
- 18.Dernburg, A. F., and J. W. Sedat. 1998. Mapping three-dimensional chromosome architecture in situ. Methods Cell Biol. 53:187-233. [DOI] [PubMed] [Google Scholar]
- 19.Domian, I. J., K. C. Quon, and L. Shapiro. 1996. The control of temporal and spatial organization during the Caulobacter cell cycle. Curr. Opin. Genet. Dev. 6:538-544. [DOI] [PubMed] [Google Scholar]
- 20.Fang, G., H. Yu, and M. W. Kirschner. 1999. Control of mitotic transitions by the anaphase-promoting complex. Philos. Trans. R. Soc. Lond. B 354:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang, H. M., and Y. Wang. 2006. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol. Microbiol. 61:484-496. [DOI] [PubMed] [Google Scholar]
- 22.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazan, I., and H. Liu. 2002. Hyphal tip-associated localization of Cdc42 is F-actin dependent in Candida albicans. Eukaryot. Cell 1:856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazan, I., M. Sepulveda-Becerra, and H. Liu. 2002. Hyphal elongation is regulated independently of cell cycle in Candida albicans. Mol. Biol. Cell 13:134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inglis, D. O., and A. D. Johnson. 2002. Ash1 protein, an asymmetrically localized transcriptional regulator, controls filamentous growth and virulence of Candida albicans. Mol. Cell. Biol. 22:8669-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen, P., B. Nelson, M. D. Robinson, Y. Chen, B. Andrews, M. Tyers, and C. Boone. 2002. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics 162:1091-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaiser, P., R. A. Sia, E. G. Bardes, D. J. Lew, and S. I. Reed. 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12:2587-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, M. T., D. M. MacCallum, S. D. Clancy, F. C. Odds, A. J. Brown, and G. Butler. 2004. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol. Microbiol. 53:969-983. [DOI] [PubMed] [Google Scholar]
- 30.Lane, S., C. Birse, S. Zhou, R. Matson, and H. Liu. 2001. DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J. Biol. Chem. 276:48988-48996. [DOI] [PubMed] [Google Scholar]
- 31.Lane, S., S. Zhou, T. Pan, Q. Dai, and H. Liu. 2001. The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol. Cell. Biol. 21:6418-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, W. J., Y. M. Wang, X. D. Zheng, Q. M. Shi, T. T. Zhang, C. Bai, D. Li, J. L. Sang, and Y. Wang. 2006. The F-box protein Grr1 regulates the stability of Ccn1, Cln3 and Hof1 and cell morphogenesis in Candida albicans. Mol. Microbiol. 62:212-226. [DOI] [PubMed] [Google Scholar]
- 33.Liu, H., J. Krizek, and A. Bretscher. 1992. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics 132:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 35.Loeb, J. D., M. Sepulveda-Becerra, I. Hazan, and H. Liu. 1999. A G1 cyclin is necessary for maintenance of filamentous growth in Candida albicans. Mol. Cell. Biol. 19:4019-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long, R. M., D. J. Elliott, F. Stutz, M. Rosbash, and R. H. Singer. 1995. Spatial consequences of defective processing of specific yeast mRNAs revealed by fluorescent in situ hybridization. RNA 1:1071-1078. [PMC free article] [PubMed] [Google Scholar]
- 37.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 38.Maidan, M. M., L. De Rop, J. Serneels, S. Exler, S. Rupp, H. Tournu, J. M. Thevelein, and P. Van Dijck. 2005. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 16:1971-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, R., A. Walther, and J. Wendland. 2005. Ras1-induced hyphal development in Candida albicans requires the formin Bni1. Eukaryot. Cell 4:1712-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel, S., S. Ushinsky, B. Klebl, E. Leberer, D. Thomas, M. Whiteway, and J. Morschhauser. 2002. Generation of conditional lethal Candida albicans mutants by inducible deletion of essential genes. Mol. Microbiol. 46:269-280. [DOI] [PubMed] [Google Scholar]
- 41.Miwa, T., Y. Takagi, M. Shinozaki, C. W. Yun, W. A. Schell, J. R. Perfect, H. Kumagai, and H. Tamaki. 2004. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3:919-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble, S. M., and A. D. Johnson. 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4:298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prokopenko, S. N., and W. Chia. 2005. When timing is everything: role of cell cycle regulation in asymmetric division. Semin. Cell Dev. Biol. 16:423-437. [DOI] [PubMed] [Google Scholar]
- 44.Rocha, C. R., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12:3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Martinez, C., and J. Perez-Martin. 2002. Gpa2, a G-protein alpha subunit required for hyphal development in Candida albicans. Eukaryot. Cell 1:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweizer, A., S. Rupp, B. N. Taylor, M. Rollinghoff, and K. Schroppel. 2000. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 38:435-445. [DOI] [PubMed] [Google Scholar]
- 47.Sil, A., and I. Herskowitz. 1996. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell 84:711-722. [DOI] [PubMed] [Google Scholar]
- 48.Staab, J. F., Y. S. Bahn, and P. Sundstrom. 2003. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology 149:2977-2986. [DOI] [PubMed] [Google Scholar]
- 49.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudbery, P., N. Gow, and J. Berman. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol. 12:317-324. [DOI] [PubMed] [Google Scholar]
- 51.Umeyama, T., A. Kaneko, M. Niimi, and Y. Uehara. 2006. Repression of CDC28 reduces the expression of the morphology-related transcription factors, Efg1p, Nrg1p, Rbf1p, Rim101p, Fkh2p and Tec1p and induces cell elongation in Candida albicans. Yeast 23:537-552. [DOI] [PubMed] [Google Scholar]
- 52.Ushinsky, S. C., D. Harcus, J. Ash, D. Dignard, A. Marcil, J. Morchhauser, D. Y. Thomas, M. Whiteway, and E. Leberer. 2002. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell 1:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteway, M., and U. Oberholzer. 2004. Candida morphogenesis and host-pathogen interactions. Curr. Opin. Microbiol. 7:350-357. [DOI] [PubMed] [Google Scholar]
- 54.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, F., C. T. Kuo, and Y. N. Jan. 2006. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron 51:13-20. [DOI] [PubMed] [Google Scholar]
- 56.Zheng, X., and Y. Wang. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 23:1845-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng, X. D., Y. M. Wang, and Y. Wang. 2003. CaSPA2 is important for polarity establishment and maintenance in Candida albicans. Mol. Microbiol. 49:1391-1405. [DOI] [PubMed] [Google Scholar]