Abstract
A new in vitro system based on real-time PCR was developed for evaluation of human herpesvirus 8 susceptibility to antiviral agents. Cidofovir had the greatest inhibitory activity against HHV-8 (50% inhibitory concentration [IC50], 0.43 μM) followed by ganciclovir (2.61 μM), adefovir (18.00 μM), acyclovir (31.00 μM), and foscarnet (34.15 μM). The potential therapeutic efficacy for HHV-8 (i.e., peak serum drug level/IC50) is highest for cidofovir (167) and foscarnet (22).
The human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus, is a new member of the γ-herpesvirinae subfamily that has been associated with human immunodeficiency virus (HIV)- and non-HIV-related Kaposi's sarcoma (KS) as well as with multicentric Castleman's disease and primary effusion lymphoma (5, 6, 17, 25). Despite the decrease in HIV-related KS with the advent of highly active antiretroviral therapy, there is still an interest in evaluating strategies to inhibit the early stages of HHV-8 replication.
Several in vitro systems have been reported for determination of HHV-8 susceptibility to antiviral drugs (10, 16, 20, 22). Because most B cells are latently infected by the virus, inducing agents such as 12-O-tetradecanoylphorbol-13-acetate (TPA) or sodium butyrate have been used to promote lytic viral replication (23). Also, due to the absence of viral plaques in infected lymphoma cell lines, most susceptibility systems were designed to measure viral DNA synthesis. However, the existing assays are very cumbersome, as they require gel electrophoresis and isotopic hybridization. In this report, we describe the development of a rapid and objective in vitro susceptibility system for HHV-8 based on real-time quantitative PCR.
The BCBL-1 cell line, which is a B-cell line latently infected by HHV-8 (23), was kindly provided by Benoît Barbeau (Centre de Recherche en Infectiologie, Ste-Foy, Québec, Canada). The cells were maintained as described previously (16). On day 1, 10 ml of BCBL-1 cells at 2 × 105 cells/ml (in RPMI 1640 medium [Life Technologies, Burlington, Ontario, Canada] supplemented with 10% heat-inactivated fetal bovine serum) were pelleted at 250 × g for 10 min and then washed with 2 ml of phosphate-buffered saline. The cell pellet was then resuspended in an equal volume of medium with TPA (without TPA for the negative control) at a final concentration of 20 ng/ml in 25-cm2 flasks (BD Biosciences, Oakville, Ontario, Canada). Serial concentrations of antivirals, i.e., acyclovir (zovirax; GlaxoSmithKline), foscarnet (Sigma, Oakville, Ontario, Canada), ganciclovir (cytovene; Hoffman La Roche), cidofovir (vistide; Gilead Sciences, Foster City, Calif.), and adefovir (kindly provided by Tomas Cihlar; Gilead Sciences) were made in triplicate and added to culture medium. On day 2, 20 h after TPA stimulation, the cells were repelleted as described above, washed with phosphate-buffered saline, and resuspended in 10 ml of fresh medium containing the same antiviral drugs but without TPA. On day 4 (3 days after the addition of TPA), the same volume of medium containing the same concentrations of antivirals was added to the plates. On the last day (day 7), an aliquot of 1.5 ml of supernatant was removed from the culture for subsequent viral DNA extraction.
The supernatants were centrifuged (2,000 × g for 10 min) followed by treatment with 30 U of RNase-free DNase I for 30 min to remove unencapsidated HHV-8 viral DNA. DNA was extracted from 200 μl of treated supernatant using the QIAamp DNA blood mini kit (QIAGEN, Mississauga, Ontario, Canada) and then eluted in 100 μl of sterile water. The HHV-8 DNA load was determined by a previously described quantitative real-time PCR assay (3) using 5 μl of eluted DNA. Briefly, the competitive real-time PCR assay was performed in a LightCycler instrument (Roche Diagnostics, Laval, Québec, Canada) using primers designed to amplify the orf26 gene (6) and two sets of adjacent fluorogenic probes (one for the target and one for an internal control) to monitor the amplification reaction (3). Fluorescence measurements were performed at each cycle (during annealing) in the F2 (Red-640) and F3 (Red-705) channels, and a threshold cycle (Ct) value for each drug concentration was calculated by determining the point at which the fluorescence exceeded a threshold limit. A standard curve of the Ct values was generated using serial 10-fold dilutions of an external HHV-8 standard from which the Ct values of the different drug concentrations were interpolated. The drug 50% inhibitory concentration (IC50) value was defined as the antiviral concentration that reduced DNA synthesis by 50% compared to TPA-induced controls without drug. Presence of PCR-inhibitory substances was assessed in each sample by verifying that the Ct of the internal control was less than 30.
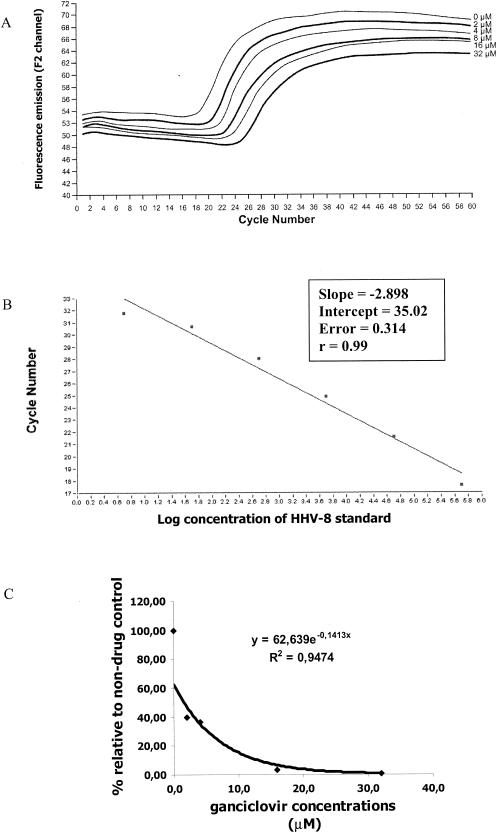
Under our conditions of TPA stimulation, approximately 1.5 × 106 viral genome equivalents were detected in 5 μl of eluted DNA, which corresponds to 3.0 × 107 copies per 200 μl of initial cell culture supernatant. A typical susceptibility experiment showing the reduction of HHV-8 DNA synthesis by ganciclovir is shown in Fig. 1. Cidofovir had the lowest IC50 value (0.43 ± 0.27 μM), followed by ganciclovir (2.61 ± 1.42 μM), adefovir (18.00 ± 6.36 μM), acyclovir (31.00 ± 9.00 μM), and foscarnet (34.15 ± 1.87 μM) (Table 1). The highest therapeutic ratios (peak serum concentration/IC50 value) were seen for cidofovir (167) and foscarnet (22), whereas adefovir (dipivoxil) had the lowest ratio (0.004) (Table 1).
FIG. 1.
Real-time PCR assay for evaluation of HHV-8 susceptibility to ganciclovir. (A) Amplification plots showing threshold cycle (Ct) values obtained for the HHV-8 ORF26 gene in presence of serial concentrations (from 0 to 32 μM) of ganciclovir. (B) Standard curve of the real-time PCR assay generated using serial 10-fold dilutions of an external HHV-8 standard and from which the Ct values of the different drug concentrations were interpolated. (C) Calculation of the ganciclovir IC50 value based on relative viral load for each drug concentration compared to induced BCBL-1 cells with no drug.
TABLE 1.
Susceptibility of HHV-8 to various antiviral agents
| Drug | Standard dosinga | Peak serum level (μM) (reference) | IC50 (μM)b | Therapeutic ratioc |
|---|---|---|---|---|
| Acyclovir | 10 mg/kg of body weight i.v. tid | 90 (9) | 31.00 ± 9.00 | 3 |
| Valacyclovir | 2 g p.o. qid | 38 (14) | 1 | |
| Ganciclovir | 5 mg/kg i.v. bid | 32 (7) | 2.61 ± 1.42 | 12 |
| Valganciclovir | 900 mg p.o. bid | 24 (4) | 9 | |
| Adefovir (dipivoxil) | 10 mg p.o. od | 0.069 (15) | 18.00 ± 6.36 | 0.004 |
| Cidofovir | 5 mg/kg i.v. weekly | 72 (12) | 0.43 ± 0.27 | 167 |
| Foscarnet | 90 mg/kg i.v. bid | 766 (1) | 34.15 ± 1.87 | 22 |
i.v., intravenous; p.o., oral; od, once a day; bid, twice a day; tid, thrice a day; qid, four times a day.
IC50 results are means plus standard deviations of two experiments, and each experiment was performed in triplicate.
Therapeutic ratio is defined as the achievable peak serum level for the intravenous (i.e., acyclovir, ganciclovir, cidofovir, and foscarnet) or oral (i.e., valacyclovir, valganciclovir, and adefovir dipivoxil) formulation divided by the drug IC50 value.
In this study, we report an innovative and reproducible susceptibility assay for HHV-8 based on real-time PCR using supernatant from TPA-induced BCBL-1 cells. Cidofovir and ganciclovir had the lowest IC50 values, whereas adefovir had intermediate activity and foscarnet and acyclovir exhibited the highest values (Table 1). Noninduced controls expressed very low levels of viral replication, whereas induced controls without drug expressed peak replication levels at 6 to 7 days after TPA induction (data not shown).
Our IC50 values for HHV-8 followed the same trend—although they were not exactly identical—compared to those reported elsewhere using other methodologies (16, 20, 22). For example, although our IC50 values for ganciclovir and cidofovir were virtually identical to those reported by Kedes and Ganem (16), values for foscarnet and acyclovir were approximately half of those determined by the same authors. Such differences may be related to cell type, drug uptake and metabolism, and the methodology used to detect and quantify HHV-8. The greatest inhibitory effect on HHV-8 replication was seen with cidofovir (Table 1). This drug is also the most potent anticytomegalovirus (CMV) drug on the market based on in vitro susceptibility testing (12). Adefovir, which is another acyclic nucleoside phosphonate, had notably higher IC50 values against HHV-8. More importantly, if we consider the average peak serum level for each drug after intravenous administration (oral administration in the case of adefovir dipivoxil, which is approved for treatment of chronic hepatitis B infection) (15), the most potent inhibitor is cidofovir, with a peak serum level/IC50 ratio of 167 compared to 22, 12, 3, and 0.004 for foscarnet, ganciclovir, acyclovir, and adefovir, respectively. However, because the primary use of these drugs is likely to be for prophylaxis of KS in high-risk groups or for preemptive therapy at a time of active viral replication but before the development of HHV-8-associated diseases (8, 11, 13, 21, 26), the convenience of administration and the absence of toxicity are two key factors (in addition to IC50 values) that should be considered in the selection of an optimal antiviral agent. In that context, valganciclovir, which is a new bioavailable prodrug of ganciclovir, appears particularly interesting (2, 19). Accordingly, the protective effect of oral ganciclovir (which is 10-fold less bioavailable than valganciclovir) for the development of KS has been previously reported in a CMV study in HIV-infected subjects (18). However, larger clinical trials using KS as the primary endpoint are needed to confirm this finding.
In conclusion, we designed a new in vitro system for evaluation of HHV-8 susceptibility to antiviral drugs which is more rapid, sensitive, and objective than previous assays based on Southern blot analysis. Similar real-time PCR assays could also be used to assess drug susceptibility of other herpesviruses (24).
Acknowledgments
We thank Tomas Cihlar for careful review of the manuscript.
REFERENCES
- 1.Aweeka, F., J. Gambertoglio, J. Mills, and M. A. Jacobson. 1989. Pharmacokinetics of intermittently administered intravenous foscarnet in the treatment of acquired immunodeficiency syndrome patients with serious cytomegalovirus retinitis. Antimicrob. Agents Chemother. 33:742-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin, G., C. Gilbert, A. Gaudreau, I. Greenfield, R. Sudlow, and N. A. Roberts. 2001. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J. Infect. Dis. 184:1598-1602. [DOI] [PubMed] [Google Scholar]
- 3.Boivin, G., S. Côté, N. Cloutier, Y. Abed, M. Maguigad, and J. P. Routy. 2002. Quantification of human herpesvirus 8 by real time PCR in blood fractions of AIDS patients with Kaposi's sarcoma and multicentric Castleman's disease. J. Med. Virol. 68:399-403. [DOI] [PubMed] [Google Scholar]
- 4.Brown, F., L. Banken, K. Saywell, and I. Arum. 1999. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin. Pharmacokinet. 37:167-176. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman, E., Y. Chang, P. S. Moore, J. S. Said, and D. M. Knowles. 1995. Kaposi's sarcoma associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Crumpacker, C. S. 1996. Ganciclovir. N. Engl. J. Med. 335:721-729. [DOI] [PubMed] [Google Scholar]
- 8.Decker, L. L., P. Shankar, G. Khan, R. B. Freeman, B. J. Dezube, J. Lieberman, and D. A. Thorley-Lawson. 1996. The Kaposi sarcoma associated virus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J. Exp. Med. 184:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsky, D. I., and C. S. Crumpacker. 1987. Drugs five years later: acyclovir. Ann. Intern. Med. 107:859-874. [DOI] [PubMed] [Google Scholar]
- 10.Flore, O., and S. J. Gao. 1997. Effect of DNA synthesis inhibitors on Kaposi's sarcoma associated virus cyclin and major capsid protein gene expression. AIDS Res. Hum. Retrovir. 13:1229-1233. [DOI] [PubMed] [Google Scholar]
- 11.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock, M. J. M., H. S. Jaffe, J. C. Martin, and R. J. Stagg. 1996. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir. Chem. Chemother. 7:115-127. [Google Scholar]
- 13.Jacobson, L. P., F. J. Jenkins, G. Springer, A. Munoz, K. V. Shab, J. Phair, Z. Zhang, and H. Armenian. 2000. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J. Infect. Dis. 181:1940-1949. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, M. A. 1993. Valacyclovir (BW256U87): the l-valyl ester of acyclovir. J. Med. Virol. 1(Suppl.):150-153. [DOI] [PubMed] [Google Scholar]
- 15.Kearny, B. P., W. K. Knight, J. L. Wolf, G. M. Currie, J. M. Contreras, J. Fry, and C. L. Brosgart. 2001. Pharmacokinetics of adefovir and the effect of food on the absorption of adefovir dipivoxil 10 mg. Hepatology 34:620A.
- 16.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, M. M., S. B. Lucas, R. R. Jones, D. D. Howells, S. J. Picton, E. E. Hanks, J. O. Mcgee, and J. J. O'Leary. 1997. HHV-8 and Kaposi's sarcoma: a time cohort study. Mol. Pathol. 50:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin, D. F., B. D. Kuppermann, R. A. Wolitz, A. G. Palestine, H. Li, and C. A. Robinson. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N. Engl. J. Med. 340:1063-1070. [DOI] [PubMed] [Google Scholar]
- 19.Martin, D. F., J. Sierra-Madero, S. Walmsley, R. A. Wolitz, K. Macey, P. Georgiou, C. A. Robinson, and M. J. Stempien. 2002. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N. Engl. J. Med. 15:1119-1126. [DOI] [PubMed] [Google Scholar]
- 20.Medveczky, M. M., E. Horvath, T. Lund, and P. G. Medveczky. 1997. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 11:1327-1332. [DOI] [PubMed] [Google Scholar]
- 21.Moore, P. S., L. A. Kingsley, S. D. Holmberg, T. Spira, P. Gupta, D. R. Hoover, J. P. Parry, L. J. Conley, H. W. Jaffe, and Y. Chang. 1996. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175-180. [DOI] [PubMed] [Google Scholar]
- 22.Neyts, J., and E. De Clercq. 1997. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob. Agents Chemother. 41:2754-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 24.Stranska, R., A. M. van Loon, M. Polman, and R. Schuurman. 2002. Application of real-time PCR for determination of antiviral drug susceptibility of herpes simplex virus. Antimicrob. Agents Chemother. 46:2943-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soulier, J., L. Grollet, E. Okenhendler, P. Cacoub, D. Casals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, and L. Degos. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 26.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. Sugget, D. M. Adam, and A. S. Denton. 1995. Detection of Kaposi's sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]