Abstract
Many single-celled eukaryotes produce prostaglandin-like molecules, but these have not been absolutely verified by mass spectrometry. We have isolated, and identified by liquid chromatography-tandem mass spectrometry, authentic prostaglandin E2 from Cryptococcus neoformans. Cyclooxygenase inhibitors did not inhibit prostaglandin synthesis, and the cryptococcal genome lacks a cyclooxygenase homolog. Thus, novel enzymes must exist.
Our laboratory previously discovered that the opportunistic fungal pathogen Cryptococcus neoformans produces an oxylipin from exogenously supplied arachidonic acid (AA) that possesses a bioactivity similar to that of prostaglandin E2 (PGE2) and is immunomodulatory in vitro (11, 12). There have been reports of the production of bioactive lipids from other fungi and single-celled eukaryotes that behave like prostaglandins (5-8, 11, 13). However, the sole use of prostaglandin immunoassays, inconsistencies in effective inhibitors, or the apparent lack of enzymes believed critical for prostaglandin production has sometimes cast doubt that these putative prostaglandins are truly authentic. It is often argued that isoprostanes, which are stereoisomers of prostaglandins formed nonenzymatically by free radical oxidation (9), are actually the type of oxylipin being measured. Because of the difficulty in distinguishing such similar molecules, we have employed a highly selective liquid chromatography-tandem mass spectrometry (LC-MS-MS) analysis that can discriminate between prostaglandins and their isoprostane stereoisomers. Our objective was to conclusively identify the cryptococcal PGE2-like oxylipin.
For LC-MS-MS analysis, stationary-phase C. neoformans cells were washed and resuspended at a concentration of 2 × 107 cells/ml in phosphate-buffered saline. Cultures were incubated with and without 500 μM AA with shaking at 37°C overnight. Supernatants were then passed over PGE2 affinity columns (Cayman Chemical), and the eluates were collected and dried down under N2 gas. Samples were resuspended in 50% methanol in water, and the material was separated using a Symmetry 2.1- by 150-mm analytical column (Waters) and reverse-phase high-performance liquid chromatography (HPLC). Reverse-phase HPLC was carried out using a gradient elution starting at 75:25:0.1 (water:acetonitrile:acetic acid) for 10 min, followed by a linear shift to 0:100:0.1 over 75 min at 500 μl/min. In this system, PGE2 standards (Cayman Chemical) elute between 14.5 and 15.5 min, and so this fraction was collected for the cryptococcal samples. This fraction was then extracted into ethyl acetate, and the organic layer was dried down under N2 gas. LC-MS-MS was carried out using a ThermoFinnigan Surveyor HPLC (San Jose, CA) interfaced directly to a ThermoFinnigan LTQ linear ion-trap mass spectrometer (San Jose, CA). Samples were resuspended in a methanol:water solution (1:1 [vol/vol]) and injected onto a Phenomenex Luna 2.00- by 150-mm 3 μ phenyl-hexyl column (Phenomenex). Mobile-phase solvents were 10 mM ammonium acetate, pH 8.5, and methanol. Compounds were separated and eluted as reported by Yang et al. (17).
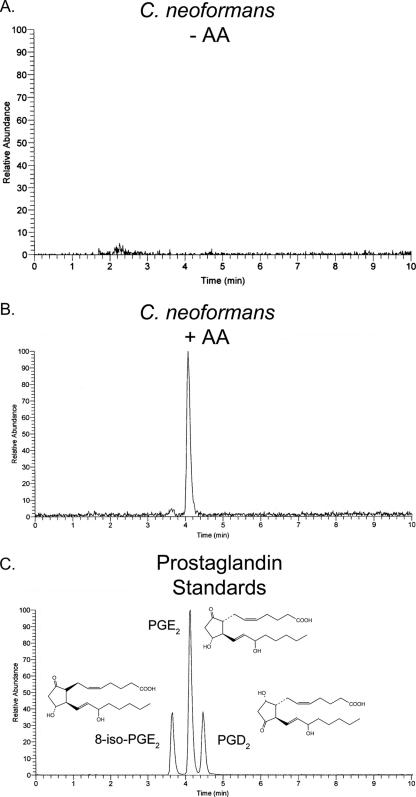
We first sought to determine whether we could identify authentic PGE2 in the supernatants from C. neoformans. Our analysis focused upon the mass range of m/z 350.5 to 351.5 for the detection of the [M-H] ion of prostaglandin E2. Focusing on a narrow m/z range rules out unrelated molecules, such as the products of β-oxidation and 3,18-diHETE, a recently discovered fungal oxylipin (2). We were unable to detect a product in the absence of exogenously supplied arachidonic acid (Fig. 1A). However, when arachidonic acid was added to the cells, a single strong peak was evident (Fig. 1B). In addition, there was a very minor peak, which corresponded to 8-iso-PGE2. This was not unexpected, because overnight incubation of AA at 37°C will generate isoprostanes. However, this minor peak also served as an internal standard for our analysis and provided additional confirmation that the main cryptococcal product was not an isoprostane. This type of analysis readily distinguishes between these three prostaglandins, despite the remarkable structural similarity between 8-iso-PGE2, PGD2, and PGE2 (structures are shown in Fig. 1C). Thus, based on the correct mass and chromatographic characteristics, C. neoformans produces PGE2.
FIG. 1.
Chromatographic analysis of the cryptococcal AA metabolite. Putative PGE2 purified from the supernatants of C. neoformans cultures was analyzed using LC-MS-MS. Peaks occur when an [M-H] ion within the mass range of m/z 350.5 to 351.5 strikes the detector. (A) Sample without AA. (B) Sample with AA. (C) Elution times of commercial prostaglandin and isoprostane standards.
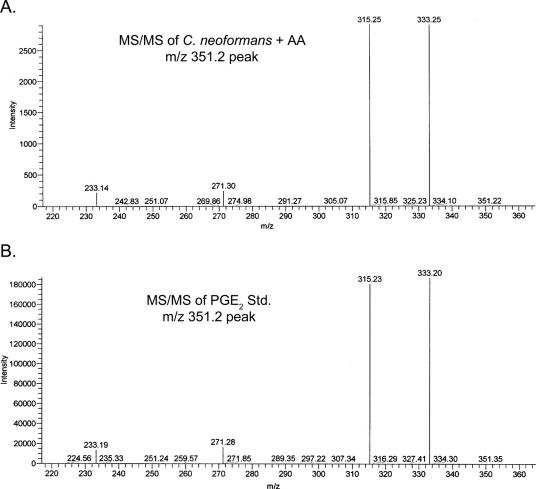
To confirm that the cryptococcal prostaglandin we identified was PGE2, MS-MS analysis was performed and the fragmentation pattern of the cryptococcal prostaglandin was compared to that of a PGE2 standard. As can be seen in Fig. 2, the fragmentation patterns of the cryptococcal prostaglandin (Fig. 2A) and the PGE2 standard (Fig. 2B) were identical. Altogether, the data in Fig. 1 and 2 unequivocally demonstrate that Cryptococcus neoformans can produce authentic PGE2.
FIG. 2.
MS-MS of the cryptococcal AA metabolite. MS-MS was performed on the 4.1-min peak found for the C. neoformans-plus-AA sample and a PGE2 standard. (A) Fragmentation pattern of the m/z 351.2 peak from C. neoformans-plus-AA samples. (B) Fragmentation pattern of the m/z 351.2 peak from the PGE2 standard (Std.).
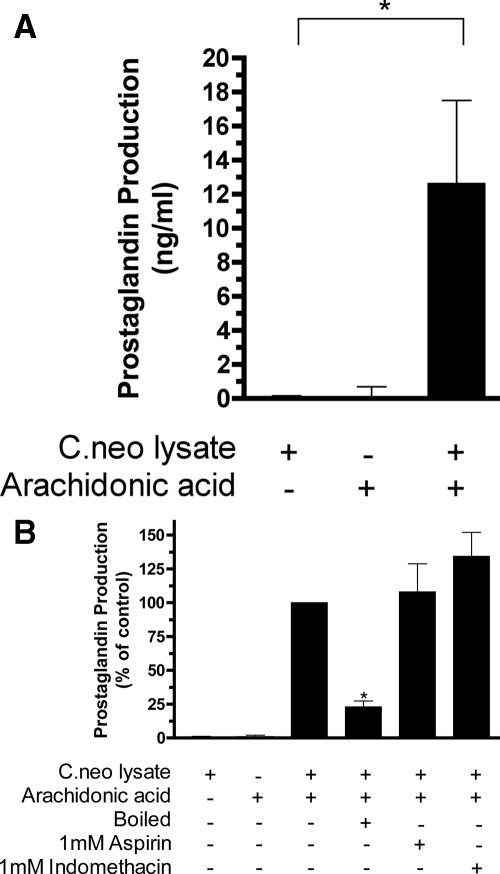
Since prostaglandins produced in mammalian cells are cyclooxygenase dependent, we next tested for the presence of a cyclooxygenase-like enzyme in cell-free lysates from C. neoformans. Lysates were generated by mechanically disrupting the cells in a pH 6.5 phosphate buffer using 0.5-μm glass beads and spinning down the debris at 15,000 × g for 45 min at 4°C. Lysates were incubated in the presence of 500 μM AA for 2 h at 37°C. Prostaglandin production was then assayed using a prostaglandin-screening enzyme immunoassay (Cayman Chemical). Background values in the absence of lysate were subtracted from each corresponding sample condition to obtain a specific measurement of prostaglandin production due to the conversion of AA. Lysates of C. neoformans that were incubated with AA produced readily detectable amounts of prostaglandins (Fig. 3A).
FIG. 3.
The effect of broad-spectrum cyclooxygenase inhibitors on cryptococcal prostaglandin production. Lysates from C. neoformans (C.neo) cells were incubated with or without AA with or without inhibitor for 2 h at 37°C. Enzymatic involvement was demonstrated using boiled lysate. Background, i.e., nonenzymatic, conversion of AA was measured for a sample with AA alone and subtracted from all values. (A) Total prostaglandin production was measured using a prostaglandin-screening enzyme immunoassay (*, P value of <0.05 by unpaired t test). (B) Inhibitory effect of cyclooxygenase inhibitors expressed as percent inhibition (where the lysate-plus-AA sample value was set at 100%) (*, P value of <0.001 by one-way analysis of variance with Bonferroni's posttest). Statistically, results for the boiled lysates are not different from those for buffer alone.
The requirement for functional enzymes in the lysate was tested by boiling a portion of the lysate for 10 min (followed by spinning down the precipitated proteins) prior to the addition of AA. The boiling of lysates resulted in a significant reduction in PGE2 production (Fig. 3B). While some residual production remains, this amount was not statistically different from that seen for the buffer control. The denaturability of this process demonstrated that the production of PGE2 by C. neoformans is dependent on a heat-denaturable enzymatic process.
We next sought to determine the effect of cyclooxygenase inhibitors. Aspirin and indomethacin (Sigma) were added to the lysates just prior to the addition of AA. As can be seen in Fig. 3B, cryptococcal prostaglandin production was not significantly inhibited by aspirin or indomethacin. This would seem to stand in contrast to previous work where cryptococcal cells treated with indomethacin decreased prostaglandin production; however, indomethacin treatment also decreased the number of viable cells in the culture (11). This suggests that the decrease seen previously was not due to a specific inhibition of the enzyme but rather to an effect on cell viability.
Recently, a family of fatty acid dioxygenase enzymes (PpoA, PpoB, and PpoC) which possess homology to the catalytic domains of mammalian cyclooxygenases were identified in Aspergillus nidulans and Aspergillus fumigatus (15). Further analysis showed that the cultures produced enzyme immunoassay-detectable prostaglandins and that ppo mutants produced less. However, these enzymes do not exist in the C. neoformans genome (16). We have carried out exhaustive BLAST analyses to search the C. neoformans genome for cyclooxygenase homologs, but we were unable to identify any significant protein or nucleotide homology. Thus, despite the fact that we identified the production of PGE2 by C. neoformans, we could not demonstrate the presence of a cyclooxygenase either by biochemical approaches or by homology searches.
Here we show that despite the lack of a cyclooxygenase, C. neoformans synthesizes PGE2 that is identical to mammalian PGE2. The absence of a cyclooxygenase goes against the current paradigm, which holds that without the cyclooxygenase the production of prostaglandins should not be possible Additionally, cryptococcus is not known to possess 20-carbon fatty acids, so they must be obtained from the environment. This fact is highlighted in the current study, where almost no prostaglandin production occurs in the absence of exogenous AA. Previous work (11) has shown greater amounts of prostaglandin produced in the absence of AA, but these studies were carried out in a rich growth medium in which free fatty acids were available. In contrast, the current work was performed in a simple phosphate buffer devoid of free fatty acids unless they were deliberately added. Within a mammalian host, cryptococcus-derived prostaglandins could be generated utilizing host AA liberated by cryptococcal phospholipase B (PLB) (1, 3, 4, 10, 14). Should cryptococcal prostaglandin synthesis prove to be important in pathogenesis, the existence of different prostaglandin-synthetic enzymes could provide novel targets for future antifungal therapies.
Acknowledgments
We thank Kathleen Noon at the University of Michigan Department of Pharmacology's Biomedical Mass Spectrometry Facility for all her help and for her mass spectrometry expertise. We also thank the Huffnagle lab and Galen Toews and Yoichi Osawa for their thought-provoking discussions.
This work was supported by funding from NIAID grant 1R01AI059201 and NHLBI grant R01HL0636.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Chen, S. C., L. C. Wright, J. C. Golding, and T. C. Sorrell. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deva, R., R. Ciccoli, T. Schewe, J. L. Kock, and S. Nigam. 2000. Arachidonic acid stimulates cell growth and forms a novel oxygenated metabolite in Candida albicans. Biochim. Biophys. Acta 1486:299-311. [DOI] [PubMed] [Google Scholar]
- 3.Djordjevic, J. T., M. Del Poeta, T. C. Sorrell, K. M. Turner, and L. C. Wright. 2005. Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor. Biochem. J. 389:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganendren, R., E. Carter, T. Sorrell, F. Widmer, and L. Wright. 2006. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 8:1006-1015. [DOI] [PubMed] [Google Scholar]
- 5.Kilunga Kubata, B., N. Eguchi, Y. Urade, K. Yamashita, T. Mitamura, K. Tai, O. Hayaishi, and T. Horii. 1998. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic, and immunosuppressive substances in humans. J. Exp. Med. 188:1197-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubata, B. K., M. Duszenko, Z. Kabututu, M. Rawer, A. Szallies, K. Fujimori, T. Inui, T. Nozaki, K. Yamashita, T. Horii, Y. Urade, and O. Hayaishi. 2000. Identification of a novel prostaglandin f(2alpha) synthase in Trypanosoma brucei. J. Exp. Med. 192:1327-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubata, B. K., Z. Kabututu, T. Nozaki, C. J. Munday, S. Fukuzumi, K. Ohkubo, M. Lazarus, T. Maruyama, S. K. Martin, M. Duszenko, and Y. Urade. 2002. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J. Exp. Med. 196:1241-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamacka, M., and J. Sajbidor. 1995. The occurrence of prostaglandins and related compounds in lower organisms. Prostaglandins Leukot. Essent. Fatty Acids 52:357-364. [DOI] [PubMed] [Google Scholar]
- 9.Morrow, J. D., and L. J. Roberts II. 1996. The isoprostanes. Current knowledge and directions for future research. Biochem. Pharmacol. 51:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noverr, M. C., G. B. Toews, and G. B. Huffnagle. 2002. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 70:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salafsky, B., and A. C. Fusco. 1985. Schistosoma mansoni: cercarial eicosanoid production and penetration response inhibited by esculetin and ibuprofen. Exp. Parasitol. 60:73-81. [DOI] [PubMed] [Google Scholar]
- 14.Santangelo, R., H. Zoellner, T. Sorrell, C. Wilson, C. Donald, J. Djordjevic, Y. Shounan, and L. Wright. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72:2229-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsitsigiannis, D. I., J. W. Bok, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 73:4548-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsitsigiannis, D. I., T. M. Kowieski, R. Zarnowski, and N. P. Keller. 2005. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology 151:1809-1821. [DOI] [PubMed] [Google Scholar]
- 17.Yang, P., E. Felix, T. Madden, S. M. Fischer, and R. A. Newman. 2002. Quantitative high-performance liquid chromatography/electrospray ionization tandem mass spectrometric analysis of 2- and 3-series prostaglandins in cultured tumor cells. Anal. Biochem. 308:168-177. [DOI] [PubMed] [Google Scholar]