Abstract
Background
Ankylosing enthesopathy (ANKENT) with progressive stiffening of ankle and tarsal joints of the hind limbs is a naturally occurring arthropathy in B10.BR mice. Some features are similar to those of the spondyloarthropathies in humans.
Objective
To study the role of sexual dimorphism and testosterone in the development of ANKENT.
Methods
The incidence of ANKENT was observed in non‐castrated, castrated, and testosterone substituted castrated male mice, and in control and testosterone treated female mice.
Results
ANKENT occurred only in males; it did not develop in males castrated at age 2–3 months but occurred in castrated males injected with testosterone. Females injected with testosterone did not develop ANKENT.
Conclusion
Testosterone can replace what castration eliminates, at least in the postpubertally castrated males, but is itself not sufficient to induce joint disease.
Keywords: spondyloarthropathy, ankylosing enthesopathy, ankylosing spondylitis, testosterone
Ankylosing enthesopathy (ANKENT) occurs in C5BL/10ScSn (B10) mice and their H‐2 congenic partners as a natural ankylosing enthesopathy with progressive stiffening of ankle and tarsal joints of the hind limbs. For a number of measures ANKENT appears analogous to the human spondyloarthropathies (SpA). ANKENT occurs only in males after sexual maturation, usually after the age of 3 months. The highest incidence of ANKENT is around age 6–8 months. It begins as an inflammation of enthesis of the hind paw ankles and tarsal joints and develops fully during a period of 2–4 weeks. It can occur up to many months later on the other hind paw. Microscopically, after a short initial stage of proliferative and destructive inflammation in para‐ossal connective tissue and synovium, with cartilage proliferation, mostly at the insertions of the ligamentous parts of the joint capsules followed by ossifications, fusions with adjacent bone, resulting in ankylosis of ankle and tarsal joints occur.1 No other disease was seen in the affected mice.
The major histocompatibility complex (MHC) haplotype, H‐2 and HLA‐B27—in transgenic mice—represents a relative risk factor(s) for the occurrence of ANKENT.2,3 B10.BR (H‐2k) mice are the most prone to the occurrence of ANKENT and the incidence of ANKENT was also increased in doubly transgenic B10 mice (transgenic for hu‐β2m and HLA‐B27). The role of the MHC haplotype as a risk factor argues for the involvement of immunology factor(s) in ANKENT pathogenesis. However, except for an increase of interleukin 6 values at the time when ANKENT becomes manifest, the occurrence of ANKENT could not be monitored by immunology strategies such as injections of interleukin 6, immunosuppression, thymectomy, or transfer of spleen, bone marrow, or lymph node cells from affected animals to healthy, irradiated animals.
Mice with ANKENT were screened for numerous bacteria and viral pathogens, bacterial load was measured by fatty acid spectra of anaerobic intestinal flora, but no association with ANKENT was found. Injection of mice with Gram negative bacteria did not induce ANKENT.2 The incidence of ANKENT varies among H‐2 congenic strains with the C57Bl/10 genetic background (5–40%).2,3 Although there was no apparent difference in the incidence of ANKENT between conventionally housed and SPF animals,2 an important role for microbial environmental factor(s) was found, because germ‐free mice did not develop ANKENT.4 ANKENT occurs, even in the most sensitive strains, exclusively in males. In humans, the SpA, and particularly ankylosing spondylitis (AS), occurs more frequently in men. The preponderance of AS in men (about 75%) is a general feature in SpA.5,6,7 Various hypotheses and theories have been proposed to explain sexual dimorphism, and particularly male hormone testosterone, for SpA in humans, but so far there is no straightforward explanation.8,9,10,11 In this work we have tested the role of male hormone testosterone on the animal model ANKENT.
Materials and methods
Mice
ANKENT prone, conventionally housed B10.BR (C57Bl/10 genetic background, H‐2k haplotype) inbred mice were used. Male mice were surgically castrated at age 2.5–3 months. The incidence of ANKENT was recorded in the following groups: control (non‐castrated) males, castrated males, testosterone treated castrated males, control females, and testosterone treated females. Mice were housed (without changes) 4–5 per cage.
Experimental protocols were approved by the Institutional Animal Care Committee of the Institute of Molecular Genetics, Prague.
Testosterone determination and treatment
For determination of total testosterone in mouse serum samples, a competitive radioimmunoassay was used (RIA kit Testosterone direct, Immunotech, catalogue No 1119), and testosterone values of individual samples were obtained from the standard curve by interpolation.
Testosterone treated females and castrated males were injected intramuscularly with testosterone (Sustanon 250, NV Organon, Netherlands) in a dose of 500 ng per mouse every eighth day during the whole experiment. The time schedule of testosterone application and the testosterone dose were designed so that a testosterone serum level was established approximately identical with testosterone levels found in control male mice.
ANKENT screening and evaluation
ANKENT is a progressive stiffening of the ankle and tarsal joints of hind paws in mice (fig 1). The stiffness of the joints was checked once a week and a score between 0 (normal mobility) and 3 (completely immobile) was assigned. Scores of 2 and 3 were interpreted as positive for ANKENT. Mice were observed until 1 year of age.
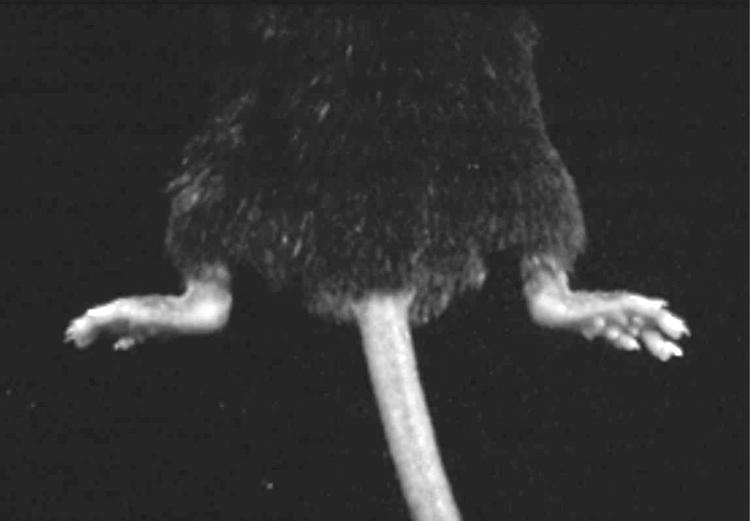
Figure 1 ANKENT in both hind limbs.
Statistical analysis
The statistical difference in the occurrence of ANKENT among individual groups was evaluated by one way analysis of variance and Newman‐Keuls tests.
Results
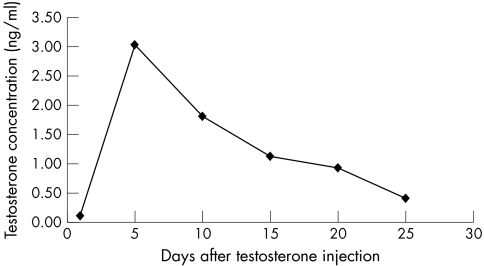
In testosterone injected castrated males the time course of testosterone serum levels was tested, and from the testosterone metabolic curve (fig 2) the testosterone injection interval was chosen. Testosterone levels were determined in all experimental groups (table 1). In control males and castrated males with testosterone substitution, the mean levels of testosterone were almost identical. Similarly, mean (very low) testosterone levels were identical in castrated males and control females.
Figure 2 Time course of testosterone metabolism in castrated males injected with 500 ng of testosterone per mouse.
Table 1 Testosterone levels in experimental groups.
| Experimental group | Testosterone treatment | Testosterone level |
|---|---|---|
| (ng/ml) | ||
| Mean (SE) | ||
| Control males | None | 3.42 (0.58) |
| Castrated males | None | 0.12 (0.02) |
| Castrated males + Te | 500 ng Te weekly | 3.49 (0.36)* |
| Control females | None | 0.09 (0.02) |
| Females + Te | 500 ng Te weekly | 2.64 (0.24)* |
*Mean Te values on day 4 after intramuscular injection of 500 ng Te. Males and females were injected with the same doses every eighth day during the whole experiment.
Te, testosterone.
Table 2 shows the incidence of ANKENT in the experimental groups. In control testosterone untreated B10.BR males, the incidence of ANKENT was 8.7%, similar to that of previously published results.2,3 In castrated males ANKENT did not occur. In castrated males injected with testosterone, the incidence of ANKENT was 8.1%, comparable to that in control males. Thus, testosterone restored the occurrence of ANKENT in castrated males. ANKENT did not occur either in control females or in females injected with testosterone. These results show that in males testosterone conditions the occurrence of ANKENT; however, testosterone itself, injected into females, does not initiate the occurrence of ANKENT.
Table 2 Incidence of ANKENT in non‐castrated and castrated B10.BR males, castrated B10.BR males with testosterone substitution, and in control and testosterone treated B10.BR females.
| No of mice | Treatment | No of ANKENT positive/total mice | Occurrence of ANKENT | % with ANKENT* |
|---|---|---|---|---|
| (months)* | ||||
| 104 Males | None | 9/104 | 4, 5.5, 5.5, 6.5, 7, 8, 9, 9, 12 | 8.7 |
| 93 Males | Castration | 0/93 | 0 | |
| 37 Males | Castration + Te | 3/37 | 6.5, 10, 11 | 8.1 |
| 30 Females | None | 0/30 | 0 | |
| 32 Females | Te | 0/32 | 0 |
*Significant difference in the incidence of ANKENT was found between the control males and castrated males (p<0.01), and between castrated males and castrated males injected with Te (p<0.01).
Te, testosterone.
Blood levels of testosterone in ANKENT+ and ANKENT– males were variable but not significantly different. In two B10.BR males tested in the initial stage of ANKENT, increased testosterone levels were found (data not shown). Such data are difficult to gather owing to daily, individual, and age dependent variations (ANKENT occurs at various ages of male mice) and mainly due to the low incidence of ANKENT.
Discussion
Interpretation of our findings includes two major possibilities. Firstly, testosterone is participating directly in the biochemical pathway(s) which induces ANKENT. Secondly, testosterone contributes to the occurrence of ANKENT by influencing the behavioural characteristics of males (but not females). These two possibilities are not mutually exclusive. So far, we have described two factors which influence the incidence of ANKENT. Firstly, ANKENT does not occur in germ‐free mice.4 Secondly, ANKENT does not occur in males caged solitarily.12
These two findings indicate that ANKENT might begin owing to some minor damage (which we have never been able to observe) induced by cohabitation of several males in one cage, and local infection by non‐pathogenic microbes present in conventional (or specific pathogen‐free) animal facilities triggers the occurrence of ANKENT. In this process, testosterone may have a role by influencing the behaviour of the males cohabitating in the cages. The role of testosterone in males caged solitarily cannot be tested, because ANKENT does not occur in such males (normal levels of testosterone).
Despite the analogies of our animal model with human joint disease development, we are aware that the situation in humans might be different and certainly is more complicated. In animal models it is possible to eliminate the variability of risk factors for development of joint disease, such as genetics, age, and environmental conditions. In our experiment we used genetically identical mice of the highly inbred mouse B10.BR strain. The animals were roughly the same age (+/− 3 weeks), and were maintained under the same conventional conditions during the whole experiment. In clinical samples such homogeneity can never be achieved. Nevertheless, various clinical data clearly show a higher incidence of AS in men than in women.5,6,7,13 Participation of sex hormones in joint disease development has been investigated repeatedly. Generally, steroid hormones are implicated in the immune response and androgens function as natural immunosuppressors. It was found that rheumatoid arthritis is associated with low serum testosterone levels.14,15 However, no significant changes in testosterone serum levels were found in men with AS.8,9,10,11
Although a direct role of testosterone or a relation between the testosterone level and the occurrence of AS was not shown, our data indicate that further research in this direction might be interesting.
In conclusion, ANKENT is similar to human AS, and our results show that male hormone testosterone itself does not induce ANKENT in mice. However, testosterone does lead to the development of joint disease in male mice.
Acknowledgements
We thank Dr Sarka Takacova for her help with the preparation of the manuscript.
This work was supported by grant 305/03/0287 from the grant agency of the Czech Republic and by research project AVOZ50520514 funded by the Academy of Sciences of the Czech Republic.
Abbreviations
ANKENT - ankylosing enthesopathy
AS - ankylosing spondylitis
MHC - major histocompatibility complex
SpA - spondyloarthropathies
References
- 1.Eulderink F, Ivanyi P, Weinreich S. Histopathology of murine ankylosing enthesopathy. Pathol Res Pract 1998194797–803. [DOI] [PubMed] [Google Scholar]
- 2.Weinreich S, Eulderink F, Capkova J, Pla M. Gaede K, Heesemann J, et al. HLA‐B27 as a relative risk factor in ankylosing enthesopathy in transgenic mice. Hum Immunol 199542101–115. [DOI] [PubMed] [Google Scholar]
- 3.Capkova J, Ivanyi P. H‐2 influence on ankylosing enthesopathy of the ankle (ANKENT). Folia Biol 199238258–262. [PubMed] [Google Scholar]
- 4.Rehakova Z, Capkova J, Stepankova R, Sinkora J, Louzecka A, Ivanyi P. Germ‐free mice do not develop ankylosing enthesopathy, a spontaneous joint disease. Hum Immunol 200061555–558. [DOI] [PubMed] [Google Scholar]
- 5.Brewerton D A. Introduction: B27‐associated diseases. Scand J Rheumatol 199087(suppl)108–110. [DOI] [PubMed] [Google Scholar]
- 6.Linssen A. B27+disease versus B27‐disease. Scand J Rheumatol 199087(suppl)111–119. [PubMed] [Google Scholar]
- 7.Dekker‐Saeys B J, Keat A C S. Follow up study of ankylosing spondylitis over a period of 12 years (1977–1989). Scand J Rheumatol 199087(suppl)120–121. [DOI] [PubMed] [Google Scholar]
- 8.Giltay E J, Popp‐Snijders C, van Schaardenburg D, Dekker‐Saeys B J, Gooren L J, Dijkmans B A. Serum testosterone levels are not elevated in patients with ankylosing spondylitis. J Rheumatol 1998252389–2394. [PubMed] [Google Scholar]
- 9.Giltay E J, van Schaardenburg D, Gooren L J, Popp‐Snijders C, Dijkmans B A. Androgens and ankylosing spondylitis: a role in the pathogenesis? Ann N Y Acad Sci 1999876340–364. [DOI] [PubMed] [Google Scholar]
- 10.Gooren L J, Giltay E J, van Schaardenburg D, Dijkmans B A. Gonadal and adrenal sex steroids in ankylosing spondylitis. Rheum Dis Clin North Am 200026969–987. [DOI] [PubMed] [Google Scholar]
- 11.Straub R H, Struharova S, Scholmerich J, Harle P. No alternations of serum levels of adrenal and gonadal hormones in patients with ankylosing spondylitis. Clin Exp Rheumatol 200228(suppl)852–859. [PubMed] [Google Scholar]
- 12.Weinreich S, Capkova J, Hoebe‐Hewryk B, Boog C, Ivanyi P. Grouped caging predisposes male mice to ankylosing enthesopathy. Ann Rheum Dis 199655645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gran J T, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle‐aged population of Tromso, northern Norway. Ann Rheum Dis 198544359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci 2002966131–142. [DOI] [PubMed] [Google Scholar]
- 15.Masi A T, Chatterton R T, Aldag J C, Malamet R L. Perspectives on the relationship of adrenal steroids to rheumatoid arthritis. Ann N Y Acad Sci 20029661–12. [DOI] [PubMed] [Google Scholar]