Abstract
Treatment of pain in rheumatoid arthritis must take into account the gastrointestinal and cardiovascular risk of individual patients. Adequate results are not yet available, and until they are, treatment recommendations must take into account, not only the more favourable gastrointestinal risk profile of selective COX‐2 inhibitors, but also the potential atherothrombotic risk of any NSAID or selective COX‐2 inhibitor treatment.
Keywords: non‐steroidal anti‐inflammatory drugs, atherothrombotic risk, COX‐2 inhibitors
Vascular atherosclerosis is an inflammatory disorder associated with characteristic lesions of the vessel wall, which induces cellular interactions that do not differ fundamentally from those of other chronic inflammatory fibroproliferative diseases.1 Inflammatory processes mediated by cyclo‐oxygenase‐2 (COX‐2) are inhibited by traditional non‐steroidal anti‐inflammatory drugs (NSAIDs) or COX‐2 selective inhibitors. This can halt the atherogenesis in its early stages.2,3
Prostacyclin (prostaglandin I2 (PGI2)) is a vasodilator that inhibits platelet function. However, inhibition of PGI2 synthesis does not lead to spontaneous thrombosis.2 In endothelial cells PGI2 synthesis is mediated by COX‐2, which in turn is haemodynamically induced or activated by oestrogen.4 PGI2 modulates platelet–vascular interactions and specifically limits the response to thromboxane A2 (TXA2). Selective COX‐2 inhibitors inhibit PGI2 but not TXA2.5 Selective COX‐2 inhibitors reduce PGI2 dependent atheroprotective processes such as platelet aggregation inhibition and vasodilatation and decrease the proliferation and contraction of smooth muscle cells. COX‐2 inhibitors promote interactions between neutrophils and platelets and the vessel wall thus contributing to atherogenesis.4,6 In premenopausal women chronic treatment of patients with selective inhibitors of COX‐2 could undermine protection from cardiovascular disease.4,5
Unlike selective COX‐2 inhibitors, NSAIDs reversibly inhibit the production of TXA2 in platelets. However, the resulting decrease in platelet aggregation does not generally persist beyond the overall dosing interval.7 Moreover, the correlation between NSAID‐induced inhibition of TXA2 production and platelet functions is not linear. The imbalance between PGI2 and TXA2, which is said to be the reason for the atherogenic potential of selective COX‐2 inhibitors, is also likely to exist in large segments of the dosing intervals during NSAID treatment.8 A possible exception is naproxen (500 mg twice daily (bid)), which—at least under study conditions—can attain stable and sufficiently high plasma concentrations to compensate for the PGI2/TXA2 imbalance.9 With most NSAIDs, therefore, an increase in the thrombogenic risk must be expected. The results of large outcome studies and of more recent intervention studies, have to be seen in this light, and treatment recommendations should be modified accordingly.
Rofecoxib
In the Vioxx Gastrointestinal Outcomes Research (VIGOR) study, the gastrointestinal (GI) superiority of rofecoxib (50 mg daily) over naproxen (500 mg bid) was demonstrated in 8076 patients with rheumatoid arthritis (RA) treated for a median period of 9 months. Rates of complicated confirmed events (perforation, obstruction, and severe upper GI bleeding) were 0.6 and 1.4 per 100 patient‐years, respectively (p = 0.005). In that study the incidence of cardiovascular (CV) thrombotic events doubled during treatment with rofecoxib. Myocardial infarction (MI) occurred more frequently with rofecoxib than with naproxen (0.4% v 0.1%; 95% confidence interval (CI) 0.1 to 0.6). There was no correlation between MI and hypertension, and CV mortality and cerebrovascular ischaemia occurred in 0.2% of patients in both groups.10 The difference in the incidence of MI was a secondary outcome in the study and may have been a chance finding. Biomedical models suggest two other seemingly contradictory yet plausible hypotheses for a possible atherogenic effect with rofecoxib and a cardioprotective effect with naproxen that is comparable to the effect of aspirin (acetylsalicylic acid (ASA)).9,10,11
A retrospective analysis found that 4% of study participants had a history of CV disorders and were included in the study contrary to the protocol. In accordance with the protocol, these patients were not treated with ASA. Thirty eight per cent of the MIs occurred in this high risk group.10 Rofecoxib may have unmasked the thrombogenic potential in these high risk patients and may even have potentiated the thrombotic potential compared with celecoxib in the CLASS study, where ASA was permitted.8 In the remaining patients, the incidence of MI was not significantly different: 0.2% with rofecoxib and 0.1% with naproxen.10
The Adenomatous Polyp Prevention on Vioxx (APPROVe) study included almost 2600 patients and started 9 months after the approval of rofecoxib in America and 1 month before the results of the VIGOR study became known.11,12 The CV risk increased, for the first time, 18 months after the start of treatment with 25 mg rofecoxib daily. After a further 18 months of treatment with rofecoxib, the difference attained significance (p = 0.008). The incidence of severe thromboembolic events was 1.92 times higher in those treated with rofecoxib than in the placebo group.12 In September 2004 the study was stopped prematurely and the manufacturer withdrew the medicine from the market.
Celecoxib
The outcome of the Celecoxib Long term Arthritis Safety Study (CLASS) was similar to VIGOR performed in 8059 patients. The annual incidence of upper GI ulcer complications combined with symptomatic ulcers for celecoxib (400 mg bid) v NSAIDs (diclofenac 75 mg bid and ibuprofen 800 mg three times a day (tid)) was 2.08% v 3.54% (p = 0.02). However, CLASS included an insufficient number of participants to achieve the primary target criterion—namely, a significant reduction in the incidence of upper GI ulcer complications between celecoxib alone versus two traditional NSAIDs combined with permitted ASA treatment. In the 6 month treatment period, the incidence of MI CV events in the celecoxib group (0.9%) did not differ from that in the NSAID group (1.0%). Among those patients not treated with ASA, CV events occurred with equal frequency (0.5% with celecoxib and 0.4% with NSAIDs).13 Any existing atherogenic potential of celecoxib may have been masked in the 21% of participants using ASA.8
“In the CLASS study the potential of celecoxib may have been masked by patients using aspirin”
The Adenoma Prevention with Celecoxib (APC) study was carried out by the National Cancer Institute in 2035 patients and was prematurely stopped by the National Institutes of Health after an average treatment period of 33 months. The study was stopped because the incidence of CV events (CV death, MI, stroke) showed a dose dependent 2.3‐fold and 3.4‐fold increase during celecoxib treatment in the 200 mg bid and 400 mg bid dose groups, respectively, compared with the placebo group.14 Additionally, in the APPROVe study, CV events also occurred dose dependently and with a similar frequency in 1–2% of patients treated with celecoxib. A second placebo controlled study (Prevention of Spontaneous Adenoma Polyps (PreSAP)) with a comparable study design did not show any increased CV risk with celecoxib at a dose of 400 mg daily after a similar mean treatment period.15,16
The placebo controlled, three arm Alzheimer's Disease Anti‐Inflammatory Prevention Trial (ADAPT), which was sponsored by the National Institute of Aging, was also stopped by the National Institutes of Health as a precautionary measure in December 2004. The study included about 2400 volunteer subjects (mean age about 70 years) who were treated with naproxen (220 mg bid) or celecoxib (200 mg bid). In this study it was the naproxen group that showed a significant increase in CV risk compared with placebo. The celecoxib group showed no abnormal findings in this respect.17
Valdecoxib
In the placebo controlled Coronary Artery Bypass Graft (CABG)‐1 study,18 patients received parecoxib (40 mg intravenously (IV) for ⩾3 days), followed by valdecoxib (40 mg bid orally for 14 days), immediately after their coronary bypass operation. This treatment regimen was used in a modified form in a second study (CABG‐2).19 A loading dose of 40 mg parecoxib by IV injection was followed by a period of at least 3 days in which 20 mg parecoxib was administered by IV injection every 12 hours, which was followed in turn by 10 days of oral treatment with valdecoxib (20 mg bid). Every phase of the study was placebo controlled. The incidence of severe CV events in the parecoxib/valdecoxib group (2.2% and 2%, respectively) was significantly higher than in the placebo/placebo group (0.0% and 0.5%, respectively). After this study, a warning about CV risk was included in the prescribing information.20
In a third study, a controlled treatment schedule was employed following general surgical interventions that was comparable to the regimen defined in the CABG‐2 protocol. No differences in the incidence of CV events between placebo/placebo and parecoxib/valdecoxib were found.21 Moreover, no sufficiently conclusive CV safety data are available for long term treatment with lower valdecoxib doses in patients with relatively minor CV risk.21,22
The lack of adequate data on the CV safety of long term use of valdecoxib, and the increased risk of adverse CV events in CABG trials, together with the increased risk of rare but serious unpredictable skin reactions associated with valdecoxib, already described in its label, seems to demonstrate a lack of any advantages for valdecoxib compared with other NSAIDs. In April 2005 the manufacturer agreed to suspend the use of valdecoxib in Europe and the United States as an interim measure pending finalisation of the assessment of COX‐2 inhibitors.23,24
Lumiracoxib
The Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) included 18 325 patients and compared lumiracoxib (400 mg daily) with naproxen (500 mg bid) and ibuprofen (800 mg tid)25,26; 24% of patients received concomitant ASA. In patients treated with lumiracoxib, the incidence of upper GI ulcer complications was two thirds lower than in the patients treated with an NSAID, this difference being significant. In the subgroup of patients treated with concomitant ASA, no significant difference was found. This is not surprising as the number of cases was too small to settle this question.26
The CV end point (non‐fatal and silent MI, stroke, or CV death27) was met by about the same number of patients in the lumiracoxib group as in the NSAID group (0.65% v 0.55%; p = 0.5074). Patients who did not receive cardioprotective treatment with ASA showed no statistically significant differences in CV events. In the naproxen substudy, a tendency towards increased MI was seen with lumiracoxib (0.21% v 0.38%; p = 0.1471) compared with the ibuprofen substudy, in which a lower incidence of MI was seen with lumiracoxib (0.16% v 0.11%; p = 0.4833).25
Naproxen and other NSAIDs
In the naproxen group of the VIGOR study, the incidence of CV side effects was significantly lower than in the rofecoxib group. The aspirin‐like cardioprotective effects of naproxen were given as a possible explanation for this.10 Retrospective analyses and follow up studies supported this view.10,28,29,30,31,32 An unequivocal confirmation of the atherothromboprotective activity of naproxen has yet to be demonstrated in clinical studies.
The outcome of the Alzheimer prevention study (ADAPT), which was announced at the end of 2004, showed a higher incidence of CV and cerebrovascular events with naproxen (220 mg bid) than with placebo over a period of 3 years,17 which is not surprising in view of the incomplete COX‐1 inhibition and complete COX‐2 inhibition. With regular ingestion, high doses of naproxen (500 mg bid) competitively and reversibly inhibit platelet COX‐1 activity and TXA2 biosynthesis beyond the 12 hour dosing interval in a manner similar (in terms of its completeness) to the irreversible COX‐1 binding achieved with low dose ASA. However, unlike ASA, the inhibitory effect rapidly subsides after the last naproxen dose.9 In practice, the cardioprotective effect cannot be ensured with low daily doses and irregular use of naproxen. In contrast with ASA, naproxen also inhibits COX‐2 dependent PGI2 synthesis9 and thus weakens any atheroprotective potential.
What has been said for naproxen probably also applies in principle to other NSAIDs. Clinical studies to assess atherogenic potential have not been carried out with traditional NSAIDs and individual safety profiles need to be established for the various NSAIDs based on clinical studies.
“With most so‐called ‘tried and tested' NSAIDs an increase in the thrombogenic risk must be expected”
These studies must take into account pharmacokinetic properties (for example, half life, bioavailability) and molecular differences of NSAIDs. Non‐enzymatic mechanisms contribute to the differences in atherogenic potential of some NSAIDs.32 For example, experiments show that in contrast with other selective COX‐2 inhibitors (celecoxib, valdecoxib, meloxicam) or non‐selective NSAIDs (ibuprofen, naproxen, diclofenac), sulfone COX‐2 inhibitors (rofecoxib, etoricoxib) exert a pro‐oxidative influence on low density lipoprotein oxidation, which promotes the pathogenesis of atherosclerosis.33 The clinical relevance of this, however, is not clear.
The need to assess the CV risk of an NSAID is not confined to the active treatment phase. The risk of a primary MI appears to be increased for several weeks after the withdrawal of NSAID treatment, especially if the treatment was long term and another systemic inflammatory disease is present at the same time. The cause is assumed to be a vascular rebound effect.34 The activation of platelet activation following the absence of COX‐1 inhibition and TXA2 synthesis, as well as the flaring up of inflammatory processes in the coronary vessel wall with subsequent plaque instability, are possible reasons for the increase in the incidence of acute MI.
Aspirin
Aspirin (acetylsalicylic acid or ASA) acetylates a single serine residue in the COX‐1 (Ser529) and the COX‐2 channel (Ser516) and thereby permanently inactivates the enzyme.35 The resulting longlasting inhibition of TXA2 synthesis in anuclear platelets is the basis for the antithrombotic cardioprotective effect of low doses of ASA.9 Daily aspirin doses of 75–325 mg are regarded as suitable for inhibiting platelet aggregation as a means of cardioprophylaxis in patients at risk (acute MI, a history of MI, a history of stroke or transient ischaemic attacks or other relevant disorders or events such as unstable angina, vascular surgery, angioplasty, atrial fibrillation, heart defects, peripheral vascular disease, etc).27 When doses higher than 100 mg/day are used, both ASA and naproxen also inhibit COX‐2 dependent PGI2 synthesis.27,36 The expected simultaneous suppression of TXA2 and PGI2 may reduce the cardioprotective effect of low dose treatment. The concomitant administration of ibuprofen, but not rofecoxib or diclofenac, antagonises the irreversible platelet inhibition induced by ASA. Treatment with ibuprofen in patients with increased CV risk may limit the cardioprotective effects of ASA.7
“The cardiovascular protective effects of aspirin may be limited by concurrent use of ibuprofen”
It is difficult to interpret the results of a recent placebo controlled study in which the use of ASA at doses of 81 mg and 325 mg was investigated over a 3 year period for the prevention of colorectal adenoma.37 In the 749 subjects treated with ASA, seven MIs and seven strokes (of which one was a haemorrhagic insult) occurred compared with only one MI among the 372 patients in the placebo group.37 When the published data are analysed according to the criteria of the Antiplatelet Trialists' Collaboration (APTC), the difference is significant (p = 0.006, 95% CI 1.3 to 78).27 Coronary revascularisation was performed equally frequently in the ASA group (eight cases in 749 patients) and in the placebo group (four cases in 372 patients). This surprising result may be a chance finding. However, it may also be suggestive of an increased CV risk in at least some of the study participants treated with ASA.
For CV secondary prophylaxis, low dose ASA is prescribed, where appropriate, in addition to selective COX‐2 inhibitors. Concomitant selective COX‐2 inhibition causes the rate of gastroduodenal ulcers to rise close to that of a dual COX‐1/COX‐2 inhibitor alone.38 In the CLASS outcomes study, reductions in ulcer complications were not significant in those taking aspirin (0.79, p = 0.4876).13 In another study, celecoxib together with ASA (325 mg/day) induced significantly more ulcers at 1 week than ASA alone (18.7% v 7.6%), but significantly fewer ulcers than the non‐selective NSAID naproxen plus ASA (18.7% v 27.3%).39 In the TARGET study, the GI advantage of lumiracoxib in the group treated with ASA was only discernible as a trend.26 The use of enteric coated rather than plain ASA does not decrease the risk of GI bleeding.40,41
Lower GI tract
Serious lower GI events occurred at a rate of 0.9% per year in patients with RA taking the non‐selective NSAID naproxen, accounting for nearly 40% of the serious GI events that developed in these patients. Serious lower GI events were 54% lower with the use of the selective COX‐2 inhibitor rofecoxib.42 A clinically meaningful decrease in haemoglobin (>20 g/l) or packed cell volume (>10%) level was seen in significantly more patients taking ibuprofen (5.4%) than in those taking placebo, ASA, or ASA plus rofecoxib (0.8%–1.6%).38 In capsule endoscopic studies, celecoxib leads to a significant reduction in lower bowel lesions compared with the combination of naproxen with a proton pump inhibitor.43
Assessment and recommendations
The seemingly contradictory results of numerous studies on the atherogenic potential of various NSAIDs and selective COX‐2 inhibitors may be, at least in part, explained by epidemiological differences in the study groups, the primary indications for treatment, differences in the length of the studies, and other factors dependent on the study design. Differences in the CV safety of NSAIDs should be studied prospectively using direct comparisons between NSAIDs, where possible, in three arm studies including placebo. These requirements are met, at least partly, by the outcomes studies performed to date with COX‐2 selective inhibitors—namely, VIGOR,10 CLASS,13 and TARGET.25,26 The results of APPROVe,12 APC,14 PreSAP,15,16 ADAPT,17 CABG‐1,18 and CABG‐219 allow indirect comparisons of the agents to be made owing to the placebo arms (see table 1). Contradictory results may arise from differing patient groups, which may also differ substantially from the licensed indications of the agents. Some non‐CV primary study results, which have yet to be published, would possibly facilitate the benefit–risk evaluation.
Table 1 Summary of findings in relevant studies discussed.
| Study acronym | Patient No | Treatment arms | Treatment duration | Primary target criterion | Outcome | CV events |
|---|---|---|---|---|---|---|
| VIGOR10 | 8076 | Rheumatoid arthritis (RA); rofecoxib 50 mg daily (twice the maximum RA doses) or naproxen 500 mg bid | Median follow up of 9.0 months | Confirmed clinical upper GI events (gastroduodenal perforation or obstruction, upper GI bleeding, and symptomatic gastroduodenal ulcers) | 2.1 confirmed GI events per 100 patient‐years occurred with rofecoxib, as compared with 4.5 per 100 patient‐years with naproxen (RR = 0.5; 95% CI 0.3 to 0.6; p<0.001) | Incidence of MI was lower among patients in the naproxen group than among those in the rofecoxib group (0.1% v 0.4%; RR = 0.2; 95% CI 0.1 to 0.7); the overall mortality rate and the rate of death from CV causes were similar in the two groups |
| CLASS13 | 8059 | Osteoarthritis (OA); RA; celecoxib 400 mg bid (2 and 4 times the maximum RA and OA doses, respectively); ibuprofen 800 mg tid; or diclofenac 75 mg bid. Aspirin use for CV prophylaxis (325 mg/day) was permitted | 57% received treatment for 6 months | Incidence of prospectively defined symptomatic upper GI ulcers and ulcer complications (bleeding, perforation, and obstruction) | For patients not taking aspirin, the annualised incidence rates of upper GI ulcer complications alone and combined with symptomatic ulcers for celecoxib v NSAIDs were 0.44% v 1.27% (p = 0.04) and 1.40% v 2.91% (p = 0.02) | No difference was noted in the incidence of CV events between celecoxib and NSAIDs, irrespective of aspirin use |
| TARGET26,25 | 18325 | OA; lumiracoxib 400 mg once a day, naproxen 500 mg bid, or ibuprofen 800 mg tid in two substudies of identical design. Randomisation was stratified for low dose aspirin use and age | 1 Year | Difference in time‐to‐event distribution of upper GI ulcer complications (bleeding, perforation, or obstruction)26Antiplatelet Trialists' Collaboration end point of non‐fatal and silent MI, stroke, or CV death25 | In patients not taking aspirin, the cumulative 1 year incidence of ulcer complications was 1.09% (95% CI 0.82 to 1.36) with NSAIDs (64 events) v 0.25% (95% CI 0.12 to 0.39) with lumiracoxib (14 events; HR = 0.21 (95% CI 0.12 to 0.37), p<0.0001) | Incidence of the primary end point was low, both with lumiracoxib (59 events (0.65%)) and the NSAIDs (50 events (0.55%); HR = 1.14 (95% CI 0.78 to 1.66), p = 0.5074) |
| APPROVe12,44 | 2586 | Rofecoxib 25 mg bid, and placebo | 36 Months | Colorectal adenoma chemoprevention in subjects with an increased risk | Reduced rate of adenoma recurrence, years 0–3 RR = 0.75 (95% CI 0.67 to 0.83), p<0.001, | 1.50 confirmed thrombotic events per 100 patient‐years in the rofecoxib group, as compared with 0.78 events per 100 patient‐years in the placebo group. Corresponding RR = 1.92 (95% CI 1.19 to 3.11; p = 0.008) |
| APC 14,15,16 | 2035 | Comparing two doses of celecoxib (200 mg or 400 mg bid) with placebo | 2.8–3.1 Years | Colorectal adenoma chemoprevention | Not reported | Potentially serious CV events were reached in 1% in the placebo group, as compared with 2.3% in the celecoxib 200 mg twice daily group (HR = 2.3; 95% CI 0.9 to 5.5) and with 3.4% in the 400 mg celecoxib twice daily group (HR = 3.4; 95% CI 1.4 to 7.8) |
| PreSAP15,16 | 1561 | A similar study to APC comparing celecoxib 400 mg once a day versus placebo | About 33 months | To prevent colon polyps | Not reported | Patients taking celecoxib 400 mg once a day versus placebo did not have increased CV risk |
| Aspirin to Prevent Colorectal Adenomas37 | 1121 | Placebo, 81 mg of aspirin, or 325 mg of aspirin daily | About 3 years | Chemoprevention against colorectal adenomas in subjects with an increased risk | Unadjusted relative risks of any adenoma (as compared with the placebo group) were 0.81 in the 81 mg aspirin group (95% CI 0.69 to 0.96) and 0.96 in the 325 mg group (95% CI 0.81 to 1.13) | Non‐fatal MI and stroke, respectively, occurred somewhat more frequently in the aspirin groups than in the placebo group. The calculated RR of combined CV end point was 10 (95% CI 1.3 to 78; p = 0.006) |
| ADAPT17 | About 2400 volunteer participants | Naproxen 220 mg bid and celecoxib 200 mg bid or placebo | Up to 3 years | Decreasing the risk of developing Alzheimer's disease in people ⩾70 years of age | Not reported | No significant increase in CV and cerebrovascular risk for celecoxib; increase in events among the participants taking naproxen in comparison with those receiving placebo |
| CABG‐118 | 462 | Intravenous parecoxib 40 mg given within 30 minutes after extubation and every 12 hours for a minimum of 3 days followed by oral valdecoxib 40 mg every 12 hours for a combined total of 14 days | 14 Days | Analgesic efficacy of the study drug in patients undergoing coronary artery bypass grafting surgery through a median sternotomy | Study drug was significantly better than control treatment | Myocardial infarction was reported in 1.6% (5/311) of P/V group patients versus 0.7% (1/151) of control patients (p = 0.669). Cerebrovascular complications occurred in 9 (2.9%) P/V group patients versus 1 (0.7%) patient in the control group (p = 0.177) |
| CABG‐219 | 1671 | Intravenous parecoxib 40 mg for at least 3 days, followed by oral valdecoxib 20 mg every 12 hours through day 10; intravenous placebo followed by oral valdecoxib 20 mg every 12 hours; or placebo for 10 days | 10 Days | Frequency of predefined adverse events, including CV events, renal failure or dysfunction, gastroduodenal ulceration, and wound healing complications | As compared with placebo alone, both parecoxib and valdecoxib and placebo and valdecoxib had a higher proportion of patients with at least one confirmed adverse event | Cardiovascular events (including MI, cardiac arrest, stroke, and pulmonary embolism) were more common among the patients given parecoxib and valdecoxib than among those given placebo (2.0% v 0.5%; RR = 3.7; 95% CI 1.0 to 13.5; p = 0.03) |
| General Surgery Safety Study 21 | 1050 | Initial dose of parecoxib 40 mg IV, then 20 mg IV Q12H for a minimum of 3 days followed by oral valdecoxib (20 mg Q12H) (n = 525) for the remainder of a 10 day treatment period, or placebo IV followed by oral placebo (n = 525) | 10 Days | Analgesic efficacy of the study drug in patients undergoing orthopaedic/general surgery | Not reported | No significant differences in the overall safety profile |
Despite the inconsistency of their conclusions on CV safety, the listed studies have, nevertheless, provided crucial data for changes in the licences for these medicines. They have had an influence on pending approval procedures, treatment recommendations, and practical treatment decisions, which in addition to GI safety issues, now increasingly have to take CV aspects into account. The results of VIGOR and APPROVe may have been only the tip of the iceberg, concealing comparable CV side effect profiles of the so‐called “tried and tested” NSAIDs. No studies are available which have been able to shed light on these questions, and none are likely to be performed because of the high costs involved. Precautionary notes have been included in the prescribing information, and CV signs such as oedema or hypertension are regularly listed as side effects in the prescribing information of traditional NSAIDs.
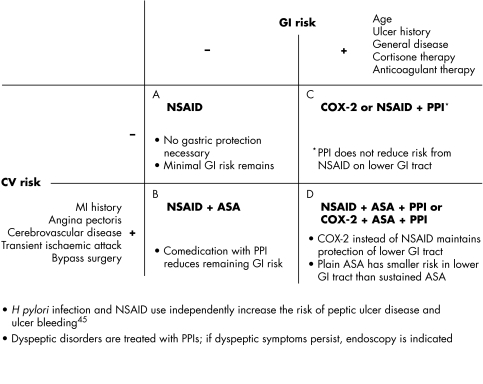
The new results from these studies influence recommendations for prescribing behaviour (see fig 1). There is a real need to avoid making hasty conclusions about the safety of traditional NSAIDs. In the course of any NSAID treatment, the individual indication must be regularly reviewed. Unnecessary courses of treatment must be avoided, and alternatives with minimal side effects and adequate efficacy must be used. Selective COX‐2 inhibitors remain a sensible choice for patients with low CV risk who have experienced severe GI events, especially during treatment with NSAIDs. Based on the data currently available, selective COX‐2 inhibitors should not, as far as possible, be used in patients with CV disorders or increased CV risks.6 For the protection of patients requiring treatment, precautionary measures should be extended to include all NSAIDs and selective COX‐2 inhibitors in what is a rapidly changing field, both scientifically and clinically. For this reason, “tried and tested” substances should be re‐examined for their atherogenic potential. However, abrupt withdrawal of NSAIDs should be avoided because of possible vascular rebound effects in patients with systemic inflammatory disorders.34
Figure 1 Treatment of RA inflammatory pain taking into account the individual GI and CV risk. (A) Without GI risk, traditional NSAIDs can be used. (B) With increased CV risk, combination of NSAID with low dose ASA is also justified. (C) If GI risks are present, then either a proton pump inhibitor (PPI) should be added or the NSAID should be replaced by a selective COX‐2 inhibitor. In the lower GI tract, PPIs do not reduce the (albeit lower) risk of NSAID lesions. (D) With increased CV risk, plain forms of ASA can be used for CV prophylaxis and the predominantly gastroduodenal lesion risk can be reduced by PPIs. At the same time, PPIs reduce the potential of NSAIDs to cause gastroduodenal lesions. The potential of NSAIDs for causing lesions in distal sections of the gut can be combated by replacing NSAIDs with a selective COX‐2 inhibitor and, where applicable, sustained‐release ASA with plain ASA. The indication for treatment (dose of drug, duration of treatment), GI risk, and CV risk are a basis for the individual risk–benefit evaluation of anti‐inflammatory treatment.
Abbreviations
APC - Adenoma Prevention with Celecoxib
APPROVe - Adenomatous Polyp Prevention on Vioxx
ASA - acetylsalicylic acid
bid - twice a day
CABG - Coronary Artery Bypass Graft
CI - confidence interval
CLASS - Celecoxib Long term Arthritis Safety Study
COX‐2 - cyclo‐oxygenase‐2
CV - cardiovascular
GI - gastrointestinal
IV - intravenous
MI - myocardial infarction
NSAIDs - non‐steroidal anti‐inflammatory drugs
PGI2 - prostaglandin I2
PPI - proton pump inhibitor
PreSAP - Prevention of Spontaneous Adenoma Polyps
RA - rheumatoid arthritis
tid - three times a day
TARGET - Therapeutic Arthritis Research and Gastrointestinal Event Trial
TXA2 - thromboxane A2
VIGOR - Vioxx Gastrointestinal Outcomes Research
Footnotes
Disclosure: Dr W W Bolten has received speaker's fees from Pfizer, MSD, Novartis and AstraZeneca for his presentations.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 2.FitzGerald G A. Cardiovascular pharmacology of nonselective nonsteroidal anti‐inflammatory drugs and coxibs: clinical considerations. Am J Cardiol 20028926–32D. [DOI] [PubMed] [Google Scholar]
- 3.Chenevard R, Hurlimann D, Bechir M, Enseleit F, Spieker L, Hermann M.et al Selective COX‐2 inhibition improves endothelial function in coronary artery disease. Circulation 2003107405–409. [DOI] [PubMed] [Google Scholar]
- 4.Egan K M, Lawson J A, Fries S, Koller B, Rader D J, Smyth E M.et al COX‐2‐derived prostacyclin confers atheroprotection on female mice. Science 20043061954–1957. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Austin S C, Rocca B, Koller B H, Coffman T M, Grosser T.et al Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 2002296539–541. [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald G A. Coxibs and cardiovascular disease. N Engl J Med 20043511709–1711. [DOI] [PubMed] [Google Scholar]
- 7.Catella‐Lawson F, Reilly M P, Kapoor S C, Cucchiara A J, DeMarco S, Tournier B.et al Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 20013451809–1817. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe M M. Rofecoxib, Merck, and the FDA. N Engl J Med 20043512877. [PubMed] [Google Scholar]
- 9.Capone M L, Tacconelli S, Sciulli M G, Grana M, Ricciotti E, Minuz P.et al Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low‐dose aspirin in healthy subjects. Circulation 20041091468–1471. [DOI] [PubMed] [Google Scholar]
- 10.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 11.Kim P S, Reicin A S, Villalba L, Witter J, Wolfe M M, Topol E J. Rofecoxib, Merck, and the FDA. N Engl J Med 20043512875–2878. [PubMed] [Google Scholar]
- 12.Bresalier R S, Sandler R S, Quan H, Bolognese J A, Oxenius B, Horgan K.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein F E, Faich G, Goldstein J L, Simon L S, Pincus T, Whelton A.et al Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. celecoxib long‐term arthritis safety study. JAMA 20002841247–1255. [DOI] [PubMed] [Google Scholar]
- 14.Solomon S D, McMurray J J V, Pfeffer M A, Wittes J, Fowler R, Finn P.et al Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 15.EMEA Statement on celecoxib. [Press release, Doc. Ref: EMEA/205831/2004] 2004 [cited 9 January 2005]. Available from: http://www.emea.eu.int/htms/hotpress/h20583104.htm, accessed 4 October 2005
- 16.FDA Statement on the halting of a clinical trial of the cox‐2 inhibitor Celebrex. 2004 [cited 9 January 2005]. Available from: http://www.fda.gov/bbs/topics/news/2004/NEW01144.html, accessed 4 October 2005
- 17.NIH Use of non‐steroidal anti‐inflammatory drugs suspended in large Alzheimer's disease prevention trial. 2004 [cited 9 January 2005]. Available from: http://www.nih.gov/news/pr/dec2004/od‐20.htm, accessed 4 October 2005
- 18.Ott E, Nussmeier N A, Duke P C, Feneck R O, Alston R P, Snabes M C.et al Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 20031251481–1492. [DOI] [PubMed] [Google Scholar]
- 19.Nussmeier N A, Whelton A A, Brown M T, Langford R M, Hoeft A, Parlow J L.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 20.FDA Bextra label updated with boxed warning concerning severe skin reactions and warning regarding cardiovascular risk. [Talk Paper T04‐56] 2004 [cited 9 January 2005]. Available from: http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01331.html, accessed 4 October 2005
- 21.Pfizer BEXTRA: valdecoxib tablets; safety studies. 2004 [cited 9 January 2005]. Available from: http://www.pfizer.com/download/uspi_bextra.pdf, accessed 4 October 2005
- 22.Ray W A, Griffin M R, Stein C M. Cardiovascular toxicity of valdecoxib. N Engl J Med 20043512767. [DOI] [PubMed] [Google Scholar]
- 23.FDA Alert for healthcare professionals—valdecoxib. 2005 [cited 24 April 2005]. Available from: http://www.fda.gov/cder/drug/InfoSheets/HCP/valdecoxibHCP.pdf, accessed 4 October 2005
- 24.EMEA European Medicines Agency statement on the suspension of use of Bextra. 2005 [cited 25 April 2005]. Available from: http://www.emea.eu.int/pdfs/human/press/pus/12163705en.pdf, accessed 4 October 2005
- 25.Farkouh M E, Kirshner H, Harrington R A, Ruland S, Verheugt F W, Schnitzer T J.et al Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004364675–684. [DOI] [PubMed] [Google Scholar]
- 26.Schnitzer T J, Burmester G R, Mysler E, Hochberg M C, Doherty M, Ehrsam E.et al Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004364665–674. [DOI] [PubMed] [Google Scholar]
- 27.Antiplatelet Trialists' Collaboration Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 199430881–106. [PMC free article] [PubMed] [Google Scholar]
- 28.Mamdani M, Rochon P, Juurlink D N, Anderson G M, Kopp A, Naglie G.et al Effect of selective cyclooxygenase 2 inhibitors and naproxen on short‐term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003163481–486. [DOI] [PubMed] [Google Scholar]
- 29.Watson D J, Rhodes T, Cai B, Guess H A. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med 20021621105–1110. [DOI] [PubMed] [Google Scholar]
- 30.Solomon D H, Glynn R J, Levin R, Avorn J. Nonsteroidal anti‐inflammatory drug use and acute myocardial infarction. Arch Intern Med 20021621099–1104. [DOI] [PubMed] [Google Scholar]
- 31.Rahme E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med 20021621111–1115. [DOI] [PubMed] [Google Scholar]
- 32.Ray W A, Stein C M, Hall K, Daugherty J R, Griffin M R. Non‐steroidal anti‐inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet 2002359118–123. [DOI] [PubMed] [Google Scholar]
- 33.Walter M F, Jacob R F, Day C A, Dahlborg R, Weng Y, Mason R P. Sulfone COX‐2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX‐2 inhibitors and NSAIDs. Atherosclerosis 2004177235–243. [DOI] [PubMed] [Google Scholar]
- 34.Fischer L M, Schlienger R G, Matter C M, Jick H, Meier C R. Discontinuation of nonsteroidal anti‐inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med 20041642472–2476. [DOI] [PubMed] [Google Scholar]
- 35.Lecomte M, Laneuville O, Ji C, Dewitt D L, Smith W L. Acetylation of human prostaglandin endoperoxide synthase‐2 (cyclooxygenase‐2) by aspirin. J Biol Chem 199426913207–13215. [PubMed] [Google Scholar]
- 36.Patrono C, Coller B, Dalen J E, FitzGerald G A, Fuster V, Gent M.et al Platelet‐active drugs: the relationships among dose, effectiveness, and side effects. Chest 2001119(suppl)39–63S. [DOI] [PubMed] [Google Scholar]
- 37.Baron J A, Cole B F, Sandler R S, Haile R W, Ahnen D, Bresalier R.et al A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003348891–899. [DOI] [PubMed] [Google Scholar]
- 38.Laine L, Maller E S, Yu C, Quan H, Simon T. Ulcer formation with low‐dose enteric‐coated aspirin and the effect of COX‐2 selective inhibition: a double‐blind trial. Gastroenterology 2004127395–402. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein J L, Gibofsky A, Fort J G. Incidence of endoscopic gastroduodenal ulcers in subjects on 325 mg qd of aspirin for cardiovascular prophylaxis with placebo, a COX‐2 specific inhibitor, or a nonspecific NSAID; results from a randomized, double‐blind, controlled trial. Arthritis Rheum 200346(suppl)LB14, poster 523 [Google Scholar]
- 40.Kelly J P, Kaufman D W, Jurgelon J M, Sheehan J, Koff R S, Shapiro S. Risk of aspirin‐associated major upper‐gastrointestinal bleeding with enteric‐coated or buffered product. Lancet 19963481413–1416. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen H T, Mellemkjaer L, Blot W J, Nielsen G L, Steffensen F H, McLaughlin J K.et al Risk of upper gastrointestinal bleeding associated with use of low‐dose aspirin. Am J Gastroenterol 2000952218–2224. [DOI] [PubMed] [Google Scholar]
- 42.Laine L, Connors L G, Reicin A, Hawkey C J, Burgos‐Vargas R, Schnitzer T J.et al Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003124288–292. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein J L, Eisen G M, Lewis B, Gralnek I M, Zlotnick S, Fort J G. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 20053133–141. [DOI] [PubMed] [Google Scholar]
- 44.Bresalier R S, Sandler R S, Quan H, Bolognese J, Oxenius B, Horgan K.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 45.Huang J Q, Sridhar S, Hunt R H. Role of Helicobacter pylori infection and non‐steroidal anti‐inflammatory drugs in peptic‐ulcer disease: a meta‐analysis. Lancet 200235914–22. [DOI] [PubMed] [Google Scholar]