Abstract
Objective
To study in parallel the outflow of the sympathetic nervous system (SNS) and the hypothalamic‐pituitary adrenal (HPA) axis tone in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Methods
32 patients with SLE, 62 with RA, and 65 healthy subjects (HS) were included. To measure the tone of the HPA axis, plasma ACTH and serum cortisol were determined. Serum neuropeptide Y (NPY) was used to evaluate the sympathetic outflow.
Results
Patients with SLE had increased NPY levels in comparison with HS, irrespective of prior prednisolone treatment (p<0.001). For patients with RA, only those with prednisolone treatment had increased NPY levels in comparison with HS (p = 0.016). Daily prednisolone dose correlated positively with serum NPY in RA (RRank = 0.356, p = 0.039). In contrast, plasma ACTH levels were generally decreased significantly in comparison with HS in SLE with prednisolone, and in RA with/without prednisolone. Similarly, serum cortisol levels were also decreased in SLE with/without prednisolone, and in RA with prednisolone. The NPY/ACTH ratio was increased in SLE and RA, irrespective of prior prednisolone treatment. The NPY/cortisol ratio was increased in SLE with/without prednisolone, and in RA with prednisolone. Twelve weeks' anti‐TNF antibody treatment with adalimumab did not decrease NPY levels in RA, irrespective of prednisolone treatment.
Conclusions
An increased outflow of the SNS was shown and a decreased tone of the HPA axis in patients with SLE and RA. Low levels of cortisol in relation to SNS neurotransmitters may be proinflammatory because cooperative anti‐inflammatory coupling of the two endogenous response axes is missing.
Keywords: adrenal hormones, neuropeptide Y, rheumatoid arthritis, sympathetic nervous system hormones, systemic lupus erythematosus
During acute inflammation in humans and animals, activation of the hypothalamic‐pituitary adrenal (HPA) axis and the sympathetic nervous system (SNS) is seen.1,2,3,4,5,6 In chronic inflammatory diseases such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) the HPA axis alters markedly : (a) secretion of adrenocorticotropic hormone (ACTH) relative to circulating cytokines is inadequate7; (b) patients have inappropriately low spontaneous and stimulated cortisol secretion in relation to inflammation7,8,9,10,11; (c) adrenal androgens decrease dramatically.11,12,13,14,15 The reasons for these changes are only partly understood, but striking changes on all levels of the HPA axis seem to have a role. For example, during repetitive administration of interleukin (IL) 6 over 3 weeks, the stimulatory capacity of IL6 on the central level is normally lost, but stimulation of the adrenal glands remains relatively stable.4,5
At this point, the question arises as to what happens with the SNS in chronic inflammatory diseases. Some studies have indicated that patients with chronic inflammatory diseases have an increased activity of the SNS.16,17,18,19,20 Such an increased sympathetic tone may be a consequence of hypothalamic changes, with an observed shift from corticotropin‐releasing hormone (CRH) to vasopressin, which has been demonstrated in experimental arthritis.21 However, none of these studies investigated the tone of the HPA axis in parallel. Thus, a possible preponderance of one system over the other was not investigated.
Why might it be important that the activity of the HPA axis and the SNS are up regulated in parallel, and what would happen if uncoupling of these axes appears? Release of cortisol is typically coupled to release of norepinephrine, which leads to stronger signalling through the β adrenoceptor; several studies have shown cooperation of cortisol and norepinephrine at a molecular level.22,23,24,25,26,27,28,29 This permissive effect of cortisol is due to β adrenoceptor up regulation and stabilisation of the cyclic AMP (cAMP)/protein kinase A/cAMP responsive element binding protein signalling pathway.30 In patients with asthma, this has led to a more effective combination treatment with local glucocorticoids and local β adrenergic agents than either substance alone.31,32 Thus, it seems that instant coupling of the two stress axes and their mediators is important for cooperative effects. Cooperation may be important in chronic inflammatory diseases to efficiently down regulate inflammation in the periphery.33
This study aimed at investigate the tone of the SNS in patients with SLE and RA by using neuropeptide Y (NPY), the relatively stable sympathetic co‐transmitter of norepinephrine. NPY is an excellent indicator of sympathetic activity,34 which is more stable and has a significantly longer half life in plasma.35 The correlation between NPY and norepinephrine release has been demonstrated in obstructive sleep apnoea syndrome,36 experimental stress,37 hypertension,38 surgery,39 hypoxia,40 and exercise.35 It is important to mention that the characteristics of norepinephrine and NPY release are not always identical: Norepinephrine is released at low exercise levels, whereas NPY is released at higher exercise levels.34 This reflects the differential release of norepinephrine and NPY from nerve terminals because norepinephrine is released at low stimulation frequencies, whereas norepinephrine and NPY are released together at higher stimulation frequencies.41 Furthermore, because NPY is produced in the sympathetic neurone in paravertebral ganglia it needs to be transported to the peripheral nerve ending. Thus, the availability of NPY, in contrast with locally produced norepinephrine, depends on production in the neuronal soma and the transport rate.42 However, when NPY is increased its most important source is the sympathetic nerve terminal.
To our knowledge, NPY has never been investigated in the serum or plasma of patients with RA and SLE. In parallel with the SNS, we studied the HPA axis tone focusing on ACTH and cortisol. Because administration of prednisolone to healthy subjects increases the SNS tone,20 we analysed separately patients with and without prior prednisolone treatment. Furthermore, in patients with RA, we investigated the effect of 12 weeks of anti‐tumour necrosis factor (TNF) treatment with adalimumab on NPY serum levels.
Patients and methods
Patients, anti‐TNF treatment, and healthy subjects
We enrolled 32 white patients with SLE according to the criteria of the American College of Rheumatology (ACR).43 Clinical activity in these patients was assessed by the SLE Disease Activity Index (SLEDAI). To study patients with another chronic inflammatory disease simultaneously, we included 62 white patients with diagnosed RA fulfilling the ACR criteria.44 Clinical variables of disease activity included the number of swollen and tender joints and erythrocyte sedimentation rate. Table 1 shows the basic characteristics of both disease groups, including their treatment. None of the patients without prednisolone received glucocorticoids during a period of 6 months before study entry, whereas patients with prednisolone had stable treatment over several weeks before study entry.
Table 1 Basic characteristics of healthy subjects and patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
| Characteristics | SLE | HS | RA | HS |
|---|---|---|---|---|
| (match SLE) | (match RA) | |||
| Number | 32 | 42 | 62 | 23 |
| Age (years) | 38.1 (2.1) | 37.1 (1.5) | 57.7 (1.7) | 51.5 (0.8) |
| Sex, female/male (%) | 24/8 (75/25) | 25/17 (60/40) | 52/10 (84/16) | 12/11 (52/48) |
| Disease duration (years) | 8.0 (1.5) | NA | 9.7 (1.0) | NA |
| SLEDAI | 10.9 (1.5) | NA | NA | NA |
| Tender joints | NA | NA | 9.0 (0.6) | NA |
| Swollen joints | NA | NA | 7.5 (0.6) | NA |
| ESR (mm/1st h) | 25.0 (3.3) | NM | 27.7 (2.4) | NM |
| Drug | ||||
| Prednisolone, No (%) | 20 (63) | NA | 34 (55) | NA |
| Prednisolone/day (mg) | 9.4 (3.4) | NA | 4.3 (0.9) | NA |
| NSAID, No (%) | 13 (41) | NA | 38 (62) | NA |
| Methotrexate, No (%) | 2 (6) | NA | 43 (69) | NA |
| Azathioprine, No (%) | 12 (38) | NA | 0 (0) | NA |
| Anti‐TNF treatment, No (%) | 0 (0) | NA | 38 (61) | NA |
| Leflunomide, No (%) | 0 (0) | NA | 8 (13) | NA |
| Cyclophosphamide, No (%) | 2 (6) | NA | 0 (0) | NA |
| Hydroxychloroquine, No (%) | 3 (9) | NA | 4 (6) | NA |
| Sulfasalazine, No (%) | 0 (0) | NA | 2 (3) | NA |
Data are given as means (SEM)unless stated otherwise.
ESR, erythrocyte sedimentation rate; NA, not applicable; NM, not measured; SLEDAI, SLE Disease Activity Index.
Some patients with RA (16 with and 16 without parallel prednisolone) were treated with adalimumab (Abbott SpA, Campoverde di Aprilia, Italy) according to the inclusion criteria of the adalimumab Research in Active RA study (ReAct). These patients with RA received additional methotrexate (stable throughout this study) but no other immunosuppressive drugs. Patients were assigned to receive single self injections of adalimumab 40 mg subcutaneously every other week. Efficacy assessments demonstrated excellent response according to ACR and EULAR response criteria (data not shown; see Atzeni et al45). A baseline, blood sample was taken 1–2 weeks before the start of adalimumab treatment. Anti‐TNF antibodies were infused on weeks 0, 2, 4, 6, 8, 10, and 12. These patients were clinically investigated and blood was drawn at baseline, and at weeks 2, 6, and 12.
For comparison, 65 white healthy subjects (HS) were recruited, and their health status verified by a 33 item questionnaire, as previously described.46 Fertile women (HS and patients) were not taking contraceptives and were in the early to mid‐follicular phase of the menstrual cycle. Owing to the different ages and sex distribution in the disease groups, subgroup analyses were carried out in order to compare the different groups of patients with HS. The subgroups were matched according to age and sex (table 1). Because serum levels of adrenal hormones are largely independent of sex, male and female subjects were not further separated into subgroups.
The study was approved by the ethics committee of the University Hospital of Regensburg, Germany, and for the adalimumab study, approval was obtained from the ethics committee of L Sacco University Hospital, Italy.
Laboratory variables
In all subjects, blood was drawn between 08:00 and 10:00 in the morning when the patients visited the outpatient clinic. The blood was immediately centrifuged and serum or plasma was stored at −80°C. We used radioimmunometric assays for the quantitative determination of serum levels of NPY (Euro‐Diagnostica AB, Malmö, Sweden, via IBL, Hamburg, Germany; detection limit: 6 pmol/l). Although the behaviour of plasma ACTH and serum cortisol is known in patients with SLE and RA, we measured these hormones to calculate ratios of serum NPY/plasma ACTH and serum NPY/serum cortisol. These ratios should give an impression of the interrelation of the two hormones included. We used a radioimmunometric assay for the quantitative determination of serum levels of cortisol (Coulter Immunotech, Marseilles, France, via IBL; detection limit: 10 nmol/l) and an enzyme immunoassay to detect plasma ACTH (Sangui BioTech, Inc, California, USA, via IBL; detection limit: 0.1 pmol/l). For all assays, intra‐assay and interassay coefficients of variation were below 10%.
Presentation of data and statistical analysis
The data are given as box plots with the 5th, 10th, 50th (median), 90th, and 95th centiles. Group medians were compared by the non‐parametric Mann‐Whitney test, correlations were calculated by Spearman rank correlation analysis (SPSS/PC, version 11.5, SPSS Inc, Chicago, USA). A decrease or increase of a variable over time (during adalimumab treatment) was tested by the non‐parametric Friedman test (SPSS). A value of p<0.05 was the level of significance.
Results
NPY serum levels in patients with SLE, RA, and in HS
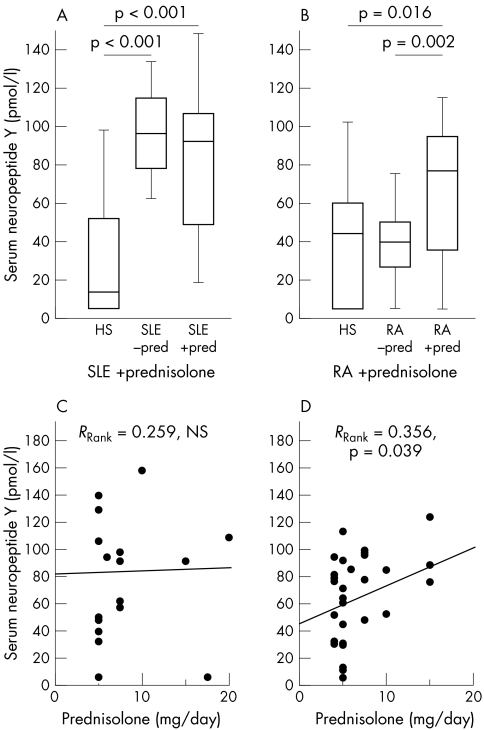
Figure 1 demonstrates higher NPY serum levels in patients with SLE than in HS, irrespective of prednisolone treatment (fig 1A). In RA, only patients with prior prednisolone had increased NPY serum levels in comparison with healthy subjects (fig 1B). Because age matched healthy subjects had relatively high NPY serum levels, no difference was noted in comparison with patients with RA without prednisolone treatment (fig 1B).
Figure 1 Serum NPY in HS, patients with SLE and RA. (A) Comparison of HS and patients with SLE. (B) Comparison of HS and patients with RA. For panels (A) and (B) data are given as box plots with the 5th, 10th, 50th (median), 90th, and 95th centiles. (C) and D) Interrelation of daily prednisolone dose and serum NPY levels in prednisolone treated patients with SLE (C) and RA (D). The linear regression line, the rank correlation coefficient and its p value are given.
The results of increased NPY levels in patients with RA treated with prednisolone prompted us to study the interrelation of the daily prednisolone dose and NPY serum levels. Clearly, the prednisolone dose correlated with NPY serum levels in patients with RA but not in patients with SLE (figs 1C and D). However, plasma NPY levels did not correlate with typical markers of disease activity such as tender joint score in RA (without prednisolone: RRank = 0.111, NS; with prednisolone: RRank = 0.227, NS), swollen joint score in RA (without prednisolone: RRank = −0.010, NS; with prednisolone: RRank = 0.356, p = 0.088), and SLEDAI in SLE (without prednisolone: RRank = 0.023, NS; with prednisolone: RRank = 0.044, NS).
Plasma NPY levels did not differ between male and female patients with or without prednisolone (data not shown). Therapeutic agents such as non‐steroidal anti‐inflammatory drugs, methotrexate, azathioprine, and leflunomide did not influence serum NPY levels in patients with RA or SLE (data not shown).
Relation of NPY serum levels and HPA axis hormones
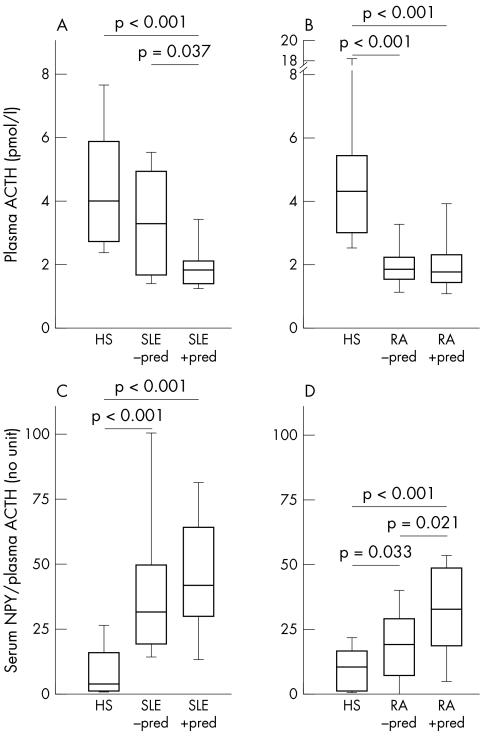
To study the relation between NPY and HPA axis hormones, molar ratios of NPY/ACTH and NPY/cortisol were calculated. These ratios express a possible preponderance of the SNS over the HPA axis or vice versa. As expected, patients with SLE and RA with prior prednisolone demonstrated decreased ACTH levels (figs 2A and B). In addition, patients with RA without prior prednisolone treatment also had decreased ACTH levels in comparison with healthy subjects (fig 2B). The ratio of NPY/ACTH was significantly higher in patients with SLE and RA than in healthy controls, irrespective of prior prednisolone treatment (figs 2C and D). In patients with RA, prednisolone treatment increased this particular ratio (fig 2D).
Figure 2 Relation of NPY and plasma ACTH in HS, patients with SLE, and RA. (A) and (B) Plasma levels of ACTH. (C) and (D) Ratio of serum NPY and plasma ACTH. All data are given as box plots with the 5th, 10th, 50th (median), 90th, and 95th centiles.
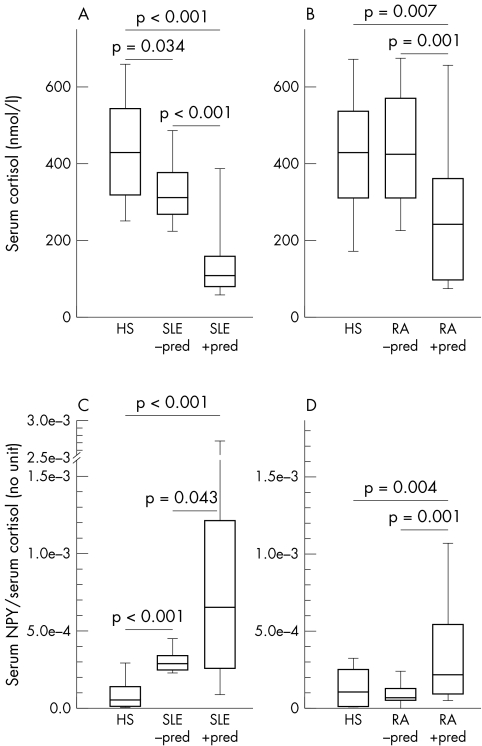
For cortisol, patients with SLE demonstrated decreased serum levels, irrespective of prior prednisolone treatment (fig 3A). In RA, only those patients with prior prednisolone treatment had decreased cortisol serum levels (fig 3B). The ratio of NPY/cortisol was increased in both SLE patient groups, irrespective of prednisolone treatment (fig 3C). Additionally, patients with SLE treated with prednisolone had a higher increased ratio of NPY/cortisol than untreated patients (fig 3C). In RA, only patients with prior prednisolone treatment had an increased ratio of NPY/cortisol in comparison with HS (fig 3D).
Figure 3 Relation of NPY and serum cortisol in HS, patients with SLE and RA. (A) and (B) Serum levels of cortisol. (C) and (D) Ratio of serum NPY and serum cortisol. All data are given as box plots with the 5th, 10th, 50th (median), 90th, and 95th centiles.
The above‐mentioned ratios did not differ between male and female patients with or without prednisolone (data not shown). Therapeutic agents such as non‐steroidal anti‐inflammatory drugs, methotrexate, azathioprine, and leflunomide did not influence serum NPY levels in patients with RA or SLE (data not shown).
Influence of anti‐TNF treatment in RA on NPY serum levels
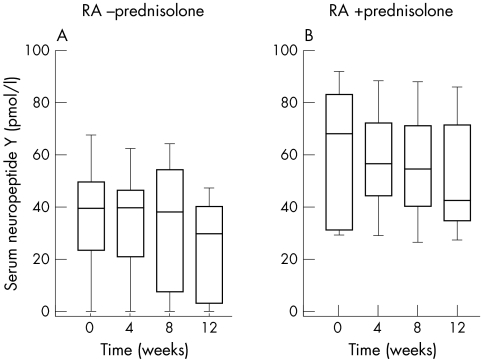
Using the Friedman test statistics, 12 weeks of anti‐TNF treatment did not change NPY serum levels, irrespective of prednisolone treatment (fig 4). Again it is obvious that patients with RA with prednisolone had increased serum NPY levels in comparison with untreated patients with RA (fig 4). In addition, neither the ratio of serum NPY/plasma ACTH nor the ratio of serum NPY/serum cortisol changed during 12 weeks of anti‐TNF treatment (data not shown).
Figure 4 Influence of 12 weeks' anti‐TNF treatment with adalimumab on serum NPY in patients with RA. All data are given as box plots with the 5th, 10th, 50th (median), 90th, and 95th centiles.
Discussion
Using NPY as a reliable read‐out measure of the SNS activity, we were able to demonstrate an increased SNS outflow in relation to the HPA axis tone in all patients with SLE and in patients with RA treated with prednisolone. For the ratio of serum NPY/plasma ACTH, also patients with RA without prednisolone demonstrated a preponderance of the SNS over the HPA axis.
Several studies have demonstrated an increased sympathetic tone in patients with chronic inflammatory diseases.16,17,18,19,20 However, none of these studies investigated the tone of the HPA axis in parallel. Thus, the preponderance of one system over the other was not investigated. In a recent study in patients with Crohn's disease and ulcerative colitis, we observed a very similar phenomenon of a preponderance of the SNS over the HPA axis47: serum NPY levels were increased, whereas serum cortisol levels were normal or decreased. We called this phenomenon uncoupling of the SNS and HPA axis in order to emphasise the loss of cooperative activities of these two endogenous response systems.47 This present study supports uncoupling of the two main response axes in patients with SLE and RA. Uncoupling is enhanced in prednisolone treated patients because prednisolone stimulates the SNS and inhibits the HPA axis even in healthy subjects.20 Interestingly, 12 weeks of anti‐TNF treatment in patients with RA slightly reduced raised NPY serum levels and SNS dominance. Thus, it seems that uncoupling is enduringly imprinted, and it is obvious that TNF is not the sole and main factor responsible for this phenomenon.
For patients without prednisolone treatment, it is interesting that the uncoupling phenomenon is obvious in our young patients with SLE but not similarly in the older patients with RA. Similarly, our recent studies in young patients with inflammatory bowel disease demonstrated the uncoupling phenomenon in patients without prednisolone.47 It may well be that aging has an influence on the uncoupling phenomenon, which has been demonstrated in HS focusing on serum cortisol and plasma norepinephrine.48 In this latter study, however, plasma NPY levels did not similarly increase during aging, which was shown for plasma norepinephrine, confirming an earlier study.49 Thus, it seems that in older subjects NPY is not produced to a similar extent as norepinephrine.48 From this point of view, it may well be that older patients with RA without prednisolone do not demonstrate high levels of NPY, though norepinephrine might have been increased. In older patients, NPY may not be the ideal measure when SNS activity is only increased to a small extent. In our study we did not measure norepinephrine because we expected a strong bias due to a prolonged storage period (norepinephrine is much more labile than NPY).
Coupling of the SNS and HPA axis is important because it supports the β adrenergic and glucocorticoid receptor pathways,22,23,24,25,26,27,28,29 which would lead to stronger cooperative effects than using one system alone. Cooperative activity of both axes is observed in asthmatic patients who use local glucocorticoids and local β2 adrenergic agents.31,32 In these patients, cooperation increases the bronchodilatory effect of each substance alone. A similar cooperation can be observed in patients with septic shock50: combined treatment with norepinephrine and cortisol leads to improved circulation and blood pressure. Similarly, a cooperative effect of cortisol and norepinephrine is also found in patients with RA (see below). In patients with chronic inflammatory diseases, a relative loss of HPA axis hormones in relation to proinflammatory cytokines may lead to deficient vasopressive activity of SNS neurotransmitters, which may consequently lead to up regulation of the SNS tone. This may counterbalance the loss of cortisol in the presence of increased circulating vasodilators such as nitric oxide, TNF, and others.
These SNS changes may be supported by the observed hypothalamic shift from initially high CRH expression to chronically increased vasopressin expression, which has been demonstrated during experimental arthritis.21 This shift to increased vasopressin production can also be viewed as a sign of an increased sympathetic tone in relation to the HPA axis (CRH) because increased vasopressin levels would support the SNS in stabilising blood pressure. Apart from effects on bronchodilation and circulation, cooperation may also lead to stronger anti‐inflammatory effects. In addition, disease related factors such as depression, chronic pain, weight gain, and others may add to the uncoupling phenomenon.
In patients with RA, we recently demonstrated anti‐inflammatory cooperation of norepinephrine and cortisol33: combined administration of norepinephrine and cortisol to cultured mixed synovial cells led to a stronger reduction of TNF, IL8, and IL6 secretion than the use of each substance alone. Furthermore, patients with RA with prednisolone treatment and presence of synovial sympathetic nerve fibres had decreased histological markers of synovial inflammation in comparison with patients without prednisolone treatment or without sympathetic innervation.33 Thus, high levels of mediators of the SNS together with cortisol at the local site of inflammation may be favourable factors which dampen inflammation.
However, it has been demonstrated that sympathetic innervation is decreased in inflammatory processes such as in the spleen of lupus lpr/lpr mice,51 in the synovium of patients with RA,52 and in inflamed islets of diabetic rats.53 Thus, an increased systemic tone of the SNS probably would not lead to increased local sympathetic neurotransmitters because sympathetic nerve fibres are lost. In such a situation, local concentrations of sympathetic neurotransmitters are low, which would support the proinflammatory process via α adrenoceptors (reviewed by Straub et al54). Loss of sympathetic nerve fibres and low levels of cortisol and androgens would lead to a proinflammatory microenvironment in inflamed tissue. In support of this notion, it has been repeatedly demonstrated that a higher SNS tone increases circulating leucocytes, such as monocytes, NK cells, and neutrophils.55,56 Probably, this has been evolutionarily conserved in order to support the immune system in the very early phase of a systemic inflammatory response (help for the innate immune system). However, in patients with chronic inflammatory diseases such a stimulation of leucocyte migration and redistribution is probably unfavourable.
In conclusion, an increased SNS tone in the presence of a defective HPA axis probably supports the continuing inflammatory process. In addition, an increased SNS tone would support atherosclerosis in patients with chronic inflammatory diseases. These observations may stimulate rheumatologists to treat patients with centrally acting drugs in order to inhibit enhanced SNS outflow.
Acknowledgements
We thank Angelika Gräber and Melanie Grünbeck for excellent technical assistance.
Abbreviations
ACR - American College of Rheumatology
ACTH - adrenocorticotropic hormone
CRH - corticotropin‐releasing hormone
HPA - hypothalamic‐pituitary‐adrenal
HS - healthy subjects
IL - interleukin
NPY - neuropeptide Y
RA - rheumatoid arthritis
SLE - systemic lupus erythematosus
SLEDAI - SLE Disease Activity Index
SNS - sympathetic nervous system
TNF - tumour necrosis factor
Footnotes
These authors contributed equally.
References
- 1.Besedovsky H O, del Rey A E, Sorkin E. Immune‐neuroendocrine interactions. J Immunol 1985135750–4s. [PubMed] [Google Scholar]
- 2.Saigusa T. Participation of interleukin‐1 and tumor necrosis factor in the responses of the sympathetic nervous system during lipopolysaccharide‐induced fever. Pflugers Arch 1990416225–229. [DOI] [PubMed] [Google Scholar]
- 3.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin‐1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst 199136183–192. [DOI] [PubMed] [Google Scholar]
- 4.Mastorakos G, Chrousos G P, Weber J S. Recombinant interleukin‐6 activates the hypothalamic‐pituitary‐adrenal axis in humans. J Clin Endocrinol Metab 1993771690–1694. [DOI] [PubMed] [Google Scholar]
- 5.Späth‐Schwalbe E, Born J, Schrezenmeier H, Bornstein S R, Stromeyer P, Drechsler S.et al Interleukin‐6 stimulates the hypothalamus‐pituitary‐adrenocortical axis in man. J Clin Endocrinol Metab 1994791212–1214. [DOI] [PubMed] [Google Scholar]
- 6.Terao A, Oikawa M, Saito M. Tissue‐specific increase in norepinephrine turnover by central interleukin‐1, but not by interleukin‐6, in rats. Am J Physiol 1994266R400–R404. [DOI] [PubMed] [Google Scholar]
- 7.Straub R H, Paimela L, Peltomaa R, Schölmerich J, Leirisalo‐Repo M. Inadequately low serum levels of steroid hormones in relation to IL‐6 and TNF in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum 200246654–662. [DOI] [PubMed] [Google Scholar]
- 8.Gudbjornsson B, Skogseid B, Oberg K, Wide L, Hallgren R. Intact adrenocorticotropic hormone secretion but impaired cortisol response in patients with active rheumatoid arthritis. Effect of glucocorticoids. J Rheumatol 199623596–602. [PubMed] [Google Scholar]
- 9.Crofford L J, Kalogeras K T, Mastorakos G, Magiakou M A, Wells J, Kanik K S.et al Circadian relationships between interleukin (IL)‐6 and hypothalamic‐ pituitary‐adrenal axis hormones: failure of IL‐6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab 1997821279–1283. [DOI] [PubMed] [Google Scholar]
- 10.Cutolo M, Foppiani L, Prete C, Ballarino P, Sulli A, Villaggio B.et al Hypothalamic‐pituitary‐adrenocortical axis function in premenopausal women with rheumatoid arthritis not treated with glucocorticoids. J Rheumatol 199926282–288. [PubMed] [Google Scholar]
- 11.Zietz B, Reber T, Oertel M, Glück T, Schölmerich J, Straub R H. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol 200027911–918. [PubMed] [Google Scholar]
- 12.Masi A T, Josipovic D B, Jefferson W E. Low adrenal androgenic‐anabolic steroids in women with rheumatoid arthritis (RA): gas‐liquid chromatographic studies of RA patients and matched normal control women indicating decreased 11‐deoxy‐17‐ketosteroid excretion. Semin Arthritis Rheum 1984141–23. [DOI] [PubMed] [Google Scholar]
- 13.Cutolo M, Balleari E, Giusti M, Monachesi M, Accardo S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonadotropin stimulation. Arthritis Rheum 1988311314–1317. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook P N, Eisman J A, Champion G D, Pocock N A. Sex hormone status and osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum 198831973–978. [DOI] [PubMed] [Google Scholar]
- 15.van Vollenhoven R F, Engleman E G, McGuire J L. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum 1994371305–1310. [DOI] [PubMed] [Google Scholar]
- 16.Leden I, Eriksson A, Lilja B, Sturfelt G, Sundkvist G. Autonomic nerve function in rheumatoid arthritis of varying severity. Scand J Rheumatol 198312166–170. [DOI] [PubMed] [Google Scholar]
- 17.Kuis W, de Jong‐de Vos van Steenwijk C, Sinnema G, Kavelaars A, Prakken B, Helders P M.et al The autonomic nervous system and the immune system in juvenile rheumatoid arthritis. Brain Behav Immun 199610387–398. [DOI] [PubMed] [Google Scholar]
- 18.Perry F, Heller P H, Kamiya J, Levine J D. Altered autonomic function in patients with arthritis or with chronic myofascial pain. Pain 19893977–84. [DOI] [PubMed] [Google Scholar]
- 19.Dekkers J C. Psychophysiological responsiveness in recently diagnosed patients with rheumatoid arthritis. Dordrecht: Drukkerij Dekkers, 2000, (Thesis. )
- 20.Glück T, Oertel M, Reber T, Zietz B, Schölmerich J, Straub R H. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. I. The hypothalamus‐autonomic nervous system axis. J Rheumatol 200027903–910. [PubMed] [Google Scholar]
- 21.Harbuz M S, Rees R G, Eckland D, Jessop D S, Brewerton D, Lightman S L. Paradoxical responses of hypothalamic corticotropin‐releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF‐41 peptide and adenohypophysial propiomelanocortin mRNA during chronic inflammatory stress. Endocrinology 19921301394–1400. [DOI] [PubMed] [Google Scholar]
- 22.Oikarinen J, Hamalainen L, Oikarinen A. Modulation of glucocorticoid receptor activity by cyclic nucleotides and its implications on the regulation of human skin fibroblast growth and protein synthesis. Biochim Biophys Acta 1984799158–165. [DOI] [PubMed] [Google Scholar]
- 23.Gruol D J, Campbell N F, Bourgeois S. Cyclic AMP‐dependent protein kinase promotes glucocorticoid receptor function. J Biol Chem 19862614909–4914. [PubMed] [Google Scholar]
- 24.Nakada M T, Stadel J M, Poksay K S, Crooke S T. Glucocorticoid regulation of beta‐adrenergic receptors in 3T3‐L1 preadipocytes. Mol Pharmacol 198731377–384. [PubMed] [Google Scholar]
- 25.Dong Y, Aronsson M, Gustafsson J A, Okret S. The mechanism of cAMP‐induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem 198926413679–13683. [PubMed] [Google Scholar]
- 26.DiBattista J A, Martel‐Pelletier J, Cloutier J M, Pelletier J P. Modulation of glucocorticoid receptor expression in human articular chondrocytes by cAMP and prostaglandins. J Rheumatol Suppl 199127102–105. [PubMed] [Google Scholar]
- 27.Korn S H, Wouters E F, Wesseling G, Arends J W, Thunnissen F B. Interaction between glucocorticoids and beta2‐agonists: alpha and beta glucocorticoid‐receptor mRNA expression in human bronchial epithelial cells. Biochem Pharmacol 1998561561–1569. [DOI] [PubMed] [Google Scholar]
- 28.Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M.et al Ligand‐independent activation of the glucocorticoid receptor by beta2‐adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem 19992741005–1010. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt P, Holsboer F, Spengler D. beta(2)‐Adrenergic receptors potentiate glucocorticoid receptor transactivation via g protein betagamma‐subunits and the phosphoinositide 3‐kinase pathway. Mol Endocrinol 200115553–564. [DOI] [PubMed] [Google Scholar]
- 30.Schramm C M. beta‐Adrenergic relaxation of rabbit tracheal smooth muscle: a receptor deficit that improves with corticosteroid administration. J Pharmacol Exp Ther 2000292280–287. [PubMed] [Google Scholar]
- 31.Motulsky H J, Insel P A. Adrenergic receptors in man: direct identification, physiologic regulation, and clinical alterations. N Engl J Med 198230718–29. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels R A, Lofdahl C G, Postma D S, Tattersfield A E, O'Byrne P, Barnes P J.et al Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med 19973371405–1411. [DOI] [PubMed] [Google Scholar]
- 33.Straub R H, Günzler C, Miller L E, Cutolo M, Schölmerich J, Schill S. Anti‐inflammatory cooperativity of corticosteroids and norepinephrine in rheumatoid arthritis synovial tissue in vivo and in vitro. FASEB J 200216993–1000. [DOI] [PubMed] [Google Scholar]
- 34.Morris M J, Russell A E, Kapoor V, Cain M D, Elliott J M, West M J.et al Increases in plasma neuropeptide Y concentrations during sympathetic activation in man. J Auton Nerv Syst 198617143–149. [DOI] [PubMed] [Google Scholar]
- 35.Pernow J, Lundberg J M, Kaijser L, Hjemdahl P, Theodorsson‐Norheim E, Martinsson A.et al Plasma neuropeptide Y‐like immunoreactivity and catecholamines during various degrees of sympathetic activation in man. Clin Physiol 19866561–578. [DOI] [PubMed] [Google Scholar]
- 36.Barcelo A, Barbe F, Llompart E, de la Pena M, Duran‐Cantolla J, Ladaria A.et al Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med 2005171183–187. [DOI] [PubMed] [Google Scholar]
- 37.Tidgren B, Theodorsson E, Hjemdahl P. Renal and systemic plasma immunoreactive neuropeptide Y and calcitonin gene‐related peptide responses to mental stress and adrenaline in humans. Clin Physiol 1991119–19. [DOI] [PubMed] [Google Scholar]
- 38.Edvinsson L, Ekman R, Thulin T. Increased plasma levels of neuropeptide Y‐like immunoreactivity and catecholamines in severe hypertension remain after treatment to normotension in man. Regul Pept 199132279–287. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg J M, Torssell L, Sollevi A, Pernow J, Theodorsson N E, Anggard A.et al Neuropeptide Y and sympathetic vascular control in man. Regul Pept 19851341–52. [DOI] [PubMed] [Google Scholar]
- 40.Kaijser L, Pernow J, Berglund B, Grubbstrom J, Lundberg J M. Neuropeptide Y release from human heart is enhanced during prolonged exercise in hypoxia. J Appl Physiol 1994761346–1349. [DOI] [PubMed] [Google Scholar]
- 41.Lundberg J M, Rudehill A, Sollevi A, Fried G, Wallin G. Co‐release of neuropeptide Y and noradrenaline from pig spleen in vivo: importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience 198928475–486. [DOI] [PubMed] [Google Scholar]
- 42.Fried G, Lundberg J M, Theodorsson‐Norheim E. Subcellular storage and axonal transport of neuropeptide Y (NPY) in relation to catecholamines in the cat. Acta Physiol Scand 1985125145–154. [DOI] [PubMed] [Google Scholar]
- 43.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 44.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 45.Atzeni F, Sarzi‐Puttini P, Capsoni F, Dell'Acqua D, Boccassini L, Santalena G.et al Autoantibodies profile of rheumatoid arthritis patients during treatment with adalimumab [abstract]. Ann Rheum Dis 200463(suppl.I)298 [Google Scholar]
- 46.Straub R H, Konecna L, Hrach S, Rothe G, Kreutz M, Schölmerich J.et al Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin‐6 (IL‐6), and DHEA inhibits IL‐6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab 1998832012–2017. [DOI] [PubMed] [Google Scholar]
- 47.Straub R H, Herfarth H, Falk W, Andus T, Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic‐pituitary‐adrenal axis in inflammatory bowel disease? J Neuroimmunol 2002126116–125. [DOI] [PubMed] [Google Scholar]
- 48.Straub R H, Cutolo M, Zietz B, Schölmerich J. The process of aging changes the interplay of the immune, endocrine and nervous systems. Mech Ageing Dev 20011221591–1611. [DOI] [PubMed] [Google Scholar]
- 49.Hetland M L, Eldrup E, Bratholm P, Christensen N J. The relationship between age and venous plasma concentrations of noradrenaline, catecholamine metabolites, DOPA and neuropeptide Y‐like immunoreactivity in normal human subjects. Scand J Clin Lab Invest 199151219–224. [DOI] [PubMed] [Google Scholar]
- 50.Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G.et al Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double‐blind, single‐center study. Crit Care Med 199927723–732. [DOI] [PubMed] [Google Scholar]
- 51.Del Rey A, Kabiersch A, Petzoldt S, Besedovsky H O. Sympathetic abnormalities during autoimmune processes: potential relevance of noradrenaline‐induced apoptosis. Ann N Y Acad Sci 2003992158–167. [DOI] [PubMed] [Google Scholar]
- 52.Miller L E, Jüsten H P, Schölmerich J, Straub R H. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J 2000142097–2107. [DOI] [PubMed] [Google Scholar]
- 53.Mei Q, Mundinger T O, Lernmark A, Taborsky G J., Jr Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 2002512997–3002. [DOI] [PubMed] [Google Scholar]
- 54.Straub R H, Dhabhar F S, Bijlsma J W, Cutolo M. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum 20055216–26. [DOI] [PubMed] [Google Scholar]
- 55.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol 19951545511–5527. [PubMed] [Google Scholar]
- 56.Schedlowski M, Hosch W, Oberbeck R, Benschop R J, Jacobs R, Raab H R.et al Catecholamines modulate human NK cell circulation and function via spleen‐independent beta 2‐adrenergic mechanisms. J Immunol 199615693–99. [PubMed] [Google Scholar]