Abstract
Background
Cardiac involvement in idiopathic inflammatory myopathy has been recognised as an important prognostic factor, but treatment remains empirical.
Objective
To investigate the effects of corticosteroids and immunosuppressors on myocarditis in patients with inflammatory myopathies.
Methods
Patients with inflammatory myositis of recent onset who had not received treatment were evaluated for associated myocarditis by magnetic resonance imaging (MRI) and reinvestigated after treatment with high dose corticosteroids and immunosuppressors.
Results
Four patients with histologically proven myositis were included. Two patients with polymyositis had cardiac clinical symptoms. Two other patients with dermatomyositis and diffuse cutaneous systemic sclerosis‐polymyositis overlap syndrome were asymptomatic. In three cases the usual conventional screening tests were normal. For all patients an area of contrast enhancement and hypokinesia detected by cardiac MRI was markedly reduced after treatment with corticosteroids and immunosuppressors for 6 months.
Conclusion
Treatment with intravenous methylprednisolone followed by prednisone and immunosuppressive therapy seems to be effective for treating myocardial involvement in patients with idiopathic inflammatory myopathies, either alone or presenting as overlap syndromes. Cardiovascular MRI is a non‐invasive technique that may be a powerful tool for diagnosis and monitoring of myocardial inflammation in this setting.
Keywords: polymyositis, dermatomyositis, myocarditis, heart, magnetic resonance imaging
Cardiac disease in idiopathic inflammatory myopathies, including heart failure, arrhythmias, cardiac arrest, and myocardial infarction, has been recognised as the main prognostic factor for death.1 About 15% of these patients have a cardiac clinical abnormality at their initial visit, but some cardiac involvement is diagnosed by sensitive non‐invasive methods in up to 70% of patients during the course of their disease.2 Cardiac involvement may manifest itself as isolated electrocardiographic changes or clinical rhythm abnormalities and, less frequently, as valve disease, coronary vasculitis, myocardial ischaemia, congestive heart failure, or myocarditis.1,2
Myocarditis has been shown to occur in up to 30% of patients at necropsy.2 In clinical practice, it is generally diagnosed by electrocardiography (ECG), laboratory investigations, and Doppler echocardiography, but its prevalence has not been defined in large series. Using myocardial scintigraphic techniques, some authors reported 57% of abnormal tracer uptake in an uncontrolled study.3 After recent technical improvements, cardiovascular magnetic resonance imaging (MRI) has already been recognised as a valuable tool for the diagnosis of viral myocarditis4 and in systemic diseases—for example, for cardiac sarcoidosis5 or microcirculation impairment evaluation in systemic sclerosis.6
As idiopathic inflammatory myopathies are relatively rare disorders, a treatment strategy has not been fully evaluated in randomised controlled trials. Corticosteroids and immunosuppressive therapy are commonly used without clear evidence from trials. Our objective was to investigate the effects of corticosteroids and immunosuppressors on myocarditis evaluated by cardiac MRI in patients with inflammatory myopathies.
Methods
Patients referred to the department of rheumatology with recent onset and untreated inflammatory myopathies during a 12 month period were investigated for associated myocarditis by cardiac MRI (Echospeed 1.5 T, GEMS, Milwaukee, USA), and early and delayed enhancement evaluated on the gadolinium enhanced acquisition (segmented inversion‐recovery gradient echo sequence). When established, myocardial involvement was treated with high dose of corticosteroids and immunosuppressors and was reinvestigated by cardiac MRI after 6 months of treatment. Antinuclear antibodies were detected by indirect immunofluorescence on Hep‐2 cells and anti‐Jo‐1 antibodies by counterimmunoelectrophoresis.
Results
Four patients responding to the classification criteria of Tanimoto et al7 were included; table 1 gives details of their characteristics. All patients had myalgia, inflammatory arthralgia and two patients (cases 2 and 4) had significant symmetric proximal muscle weakness. Two patients with polymyositis (cases 1 and 2) had cardiac clinical symptoms. Two other patients with dermatomyositis and diffuse cutaneous systemic sclerosis‐polymyositis overlap syndrome were asymptomatic, with the exception of unspecific grade II dyspnoea (NYHA). In three cases, the usual conventional cardiac screening tests, including laboratory tests, ECG, echocardiography, were normal. None had pulmonary disease on computed tomography.
Table 1 Patients characteristics.
| Patient No | Age/sex/symptoms' duration (years) | Myopathy subtype | Cardiac symptoms | Cardiac investigations | Cardiac biological tests | ESR/CRP* | CK/LDH | Autoantibodies |
|---|---|---|---|---|---|---|---|---|
| 1 | 24/F/1 | PM | Chest pain Dyspnoea | Normal ECG, ECHO, radionuclide ventriculography | TI = 0.08 Nt‐pro‐BNP = 33.6 | 70/48 | 1200/880 | ANA (1/320), anti‐Jo‐1 |
| 2 | 62/M/2 | PM | Constrictive chest pain Dyspnoea | ECG: inferior ST elevation ECHO: mild septal hypokinesia Coronarography without significant narrowing | TI = 2.6 Nt‐pro‐BNP = ND | 60/38 | 680/840 | ANA (1/160) |
| 3 | 53/F/1 | DM | Dyspnoea | normal ECG, ECHO : mild mitral insufficiency | TI = 0.04 Nt‐pro‐BNP = 39 | 30/14 | 271/680 | ANA (1/10 000) |
| 4 | 37/F/1 | PM SSc | Dyspnoea | Normal ECG, ECHO | TI<0.04 Nt‐pro‐BNP = 108 | 14/5.4 | 831/710 | ANA (1/160, nucleolar) |
PM, polymyositis; DM, dermatomyositis; SSc, systemic sclerosis; ECHO, echocardiography; TI (normal <0.15 ng/ml), troponin I; NT‐pro‐BNP (normal <155 pg/ml), N‐terminal pro‐brain natriuretic peptide; CK (normal <200 IU/l), creatine kinase; LDH (normal <450 IU/l), lactic dehydrogenase; ANA, antinuclear antibodies.
*ESR in mm/1st h; CRP in mg/l.
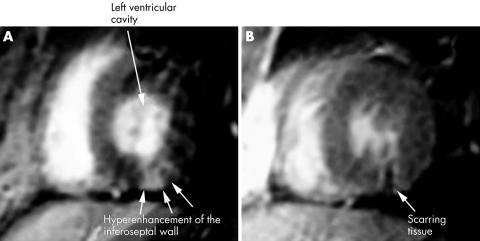
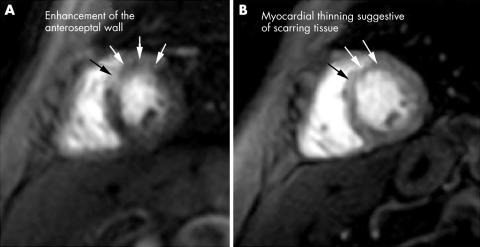
Cardiac MRI showed an area of early and delayed enhancement consistent with the diagnosis of myocarditis in all four patients: in the septal subepicardial area in case 1, inferoseptal left ventricular myocardium in case 2 (fig 1A), anteroseptal subepicardial area in case 3, and in a large area of the anteroseptal wall in case 4 (fig 2A), with hypokinesia on cine‐MRI in all cases (steady state free precession gradient echo sequence).
Figure 1 Gadolinium‐diethylenetriamine‐pentaacetic acid (Gd‐DTPA) enhanced inversion recovery prepared gradient echo acquisitions in short axis view. (A) Before treatment: transmural hyperenhancement of the inferoseptal wall suggesting acute necrosis. (B) After treatment: regression of contrast enhancement after steroid and cyclophosphamide treatment with myocardial thinning suggestive of scarring tissue.
Figure 2 Gd‐DTPA first pass perfusion MRI in short axis view. (A) Before treatment: early increased enhancement of the anteroseptal wall due to acute myocardial inflammation. (B) After treatment: regression of abnormal anteroseptal enhancement with myocardial thinning suggestive of scarring tissue.
Patients 1, 2, and 4 were treated with daily infusions of 1 g methylprednisolone for 3 days, followed by 1 mg/kg prednisone (tapered to about 0.5 mg/kg at 6 months) and monthly infusions of cyclophosphamide (0.7 g/m2) for 6 months, followed by azathioprine (150 mg/day). Patient 3 was treated with daily infusions of methylprednisolone (1 g) for 3 days, followed by prednisone (20 mg/day), hydroxychloroquine (400 mg/day), and azathioprine (150 mg/day). After 2 months of follow up, all clinical cardiovascular signs for the two symptomatic patients had disappeared and dyspnoea improved in all cases. After 6 months of treatment, cardiac MRI showed that the kinetic abnormalities had normalised and that contrast enhancement, with scarring tissue was clearly reduced (figs 1B and 2B). No clinical recurrence had occurred after 1 year's follow up.
Discussion
We report herein four cases of patients with idiopathic inflammatory myopathy related myocarditis, in which treatment with methylprednisolone followed by immunosuppressive therapy led to a clinical improvement, with striking reduction in myocardial contrast enhancement on cardiac MRI.
Cardiac involvement in idiopathic inflammatory myopathies is diagnosed by non‐invasive methods in up to 70% of patients during the course of their disease, and myocarditis has been shown to occur in up to 30% of patients at necropsy.2 The overall prognosis of idiopathic inflammatory myopathy has substantially improved in recent years, but cardiac disease has been identified as the main prognostic factor for death.1
Autoantibodies against nuclear or cytoplasmic antigens are found in about 20% of patients; antibodies against SRP were initially described in cases of severe cardiac disease.8 Patient 1 had anti‐Jo‐1 antibodies, which are usually associated with interstitial lung disease, but she had no pulmonary involvement on computed tomography scan at this stage.
The onset of myocarditis is difficult to recognise clinically, and diagnosis is generally based on laboratory investigations, ECG, echocardiography, gallium citrate or indium labelled antimyosin antibody detection, and scintigraphic techniques.9 However, these techniques are limited by their poor sensitivity and specificity. Endomyocardial biopsy has long been considered the “gold standard” for the diagnosis of myocarditis.4 Laboratory assay may be helpful in the detection of myocardial involvement, and cardiac troponin I is the best test currently available,10 as emphasised by the case of patient 2, but the value of brain natriuretic peptides will have to be examined in the future.
In 1998, Friedrich et al showed that cardiac MRI could be used to diagnose myocarditis of viral origin.11 Mahrholdt et al showed that contrast enhancement, was a common finding in patients with suspected viral myocarditis and was associated with active myocarditis defined histologically.4 The value of cardiac MRI has also been demonstrated in the early diagnosis and follow up of cardiac sarcoidosis5 and for the assessment of vasodilator effects on microcirculation impairment in systemic sclerosis.6 Patient 2 had been admitted to an intensive care unit for suspected myocardial infarction, but coronarography ruled out coronary artery stenosis; MRI distinguished clearly between inflammatory and ischaemic myocardium involvement because the subendocardial layer was shown not to be affected in enhanced delayed sequences in our case.
The cases reported here demonstrate the existence of myocardial inflammatory areas in patients with inflammatory myopathies consistent with myocarditis, which could not have been diagnosed with the usual techniques but were clearly identified by MRI. Only these four patients were referred with recent onset and untreated inflammatory myopathies during a 12 month period, and only two of the four had cardiac symptoms. Thus, MRI may be of considerable potential value in this field, although its sensitivity and specificity remain to be evaluated on a large scale. To our knowledge, only one previous case of polymyositis with myocarditis diagnosed by endomyocardial biopsy and suggested by gadolinium enhanced MRI has been published.12
As idiopathic inflammatory myopathies are relatively rare disorders, a treatment strategy has not been fully evaluated in randomised controlled trials.8 There is a consensus that oral corticosteroids should be used as the standard treatment,8 but intravenous pulse infusion of methylprednisolone has also been advocated.13 Immunosuppressive agents are often used in patients with refractory disease, but their role in the treatment of myocarditis is unclear.14
We believed that patients 1 and 2 required “aggressive” treatment because of their clinical presentation and MRI features. Given the lack of controlled data available and the potential lethality of myocarditis, we chose to treat patients 1 and 2 with an intensive regimen, including pulses of 1 g methylprednisolone, followed by a tapering regimen of oral corticosteroids in association with monthly infusions of 0.7 g/m2 cyclophosphamide for 6 months. This therapeutic combination has been used with some success in lupus related myocarditis.15 Patient number 3 was treated with three intravenous pulses of 1 g methylprednisolone, followed by prednisone (20 mg/day), hydroxychloroquine, and azathioprine because of apparently less severe disease. Patient number 4 was treated with the same regimen as patients 1 and 2 because of the intensity of muscular involvement associated with myocarditis. In all four cases, these regimens led to markedly reduced contrast enhancement on MRI. Although we did not perform endomyocardial biopsy for our patients, we assume that the presence of early and untreated disease in patients with active inflammatory myositis, together with the finding of pronounced myocardial contrast enhancement and its reduction after treatment, is highly suggestive of myocarditis.
In conclusion, a therapeutic regimen of methylprednisolone followed by immunosuppressors improved the cardiac clinical symptoms and markedly reduced myocardial MRI contrast enhancement in patients with inflammatory myopathies. Cardiac MRI is a recently developed non‐invasive technique that has been efficiently used here to determine the localisation, extent, and regression of myocardial inflammation in four patients undergoing this therapeutic regimen. This powerful tool could be used for the diagnosis and monitoring of myocarditis related to acute idiopathic inflammatory myopathy. Its evaluation on a large scale is warranted.
Footnotes
Conflict of interest: None.
References
- 1.Danko K, Ponyi A, Constantin T, Borgulya G, Szegedi G. Long‐term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 20048335–42. [DOI] [PubMed] [Google Scholar]
- 2.Lie J T. Cardiac manifestations in polymyositis/dermatomyositis: how to get to the heart of the matter. J Rheumatol 199522809–811. [PubMed] [Google Scholar]
- 3.Buchpiguel C A, Roizemblatt S, Pastor E H, Hironaka F H, Cossermelli W. Cardiac and skeletal muscle scintigraphy in dermato‐ and polymyositis: clinical implications. Eur J Nucl Med 199623199–203. [DOI] [PubMed] [Google Scholar]
- 4.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H.et al Multimedia article. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology, Circulation 20041091250–1258. [DOI] [PubMed] [Google Scholar]
- 5.Vignaux O, Dhote R, Duboc D, Blanche P, Dusser D, Weber S.et al Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1‐year follow‐up study. Chest 20021221895–1901. [DOI] [PubMed] [Google Scholar]
- 6.Vignaux O, Allanore Y, Meune C, Pascal O, Duboc D, Weber S.et al Evaluation of nifedipine efficacy on myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Ann Rheum Dis 2005641268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanimoto K, Nakano K, Kano S, Mori S, Ueki H, Nishitani H.et al Classification criteria for polymyositis and dermatomyositis. J Rheumatol 199522668–674. [PubMed] [Google Scholar]
- 8.Dalakas M C, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003362971–982. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl U, Lauer B, Souvatzoglu M, Vosberg H, Schultheiss H P. Antimyosin scintigraphy and immunohistologic analysis of endomyocardial biopsy in patients with clinically suspected myocarditis: evidence of myocardial cell damage and inflammation in the absence of histologic signs of myocarditis. J Am Coll Cardiol 1998321371–1376. [DOI] [PubMed] [Google Scholar]
- 10.Kiely P D, Bruckner F E, Nisbet J A, Daghir A. Serum skeletal troponin I in inflammatory muscle disease: relation to creatine kinase, CKMB and cardiac troponin I. Ann Rheum Dis 200059750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich M G, Strohm O, Schulz‐Menger J, Marciniak H, Luft F C, Dietz R. Contrast media‐enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998971802–1809. [DOI] [PubMed] [Google Scholar]
- 12.Ohata S, Shimada T, Shimizu H, Murakami Y, Matsuno Y. Myocarditis associated with polymyositis diagnosed by gadolinium‐DTPA enhanced magnetic resonance imaging. J Rheumatol 200229861–862. [PubMed] [Google Scholar]
- 13.Matsubara S, Hirai S, Sawa Y. Pulsed intravenous methylprednisolone therapy for inflammatory myopathies: evaluation of the effect by comparing two consecutive biopsies from the same muscle. J Neuroimmunol 19977675–80. [DOI] [PubMed] [Google Scholar]
- 14.Mason J W, O'Connell J B, Herskowitz A, Rose N R, McManus B M, Billingham M E.et al A clinical trial of immunosuppressive therapy for myocarditis: the Myocarditis Treatment Trial Investigators. N Engl J Med 1995333269–275. [DOI] [PubMed] [Google Scholar]
- 15.Chan Y K, Li E K, Tam L S, Chow L T, Ng H K. Intravenous cyclophosphamide improves cardiac dysfunction in lupus myocarditis. Scand J Rheumatol 200332306–308. [DOI] [PubMed] [Google Scholar]