Abstract
Objective
To estimate the incremental cost‐utility of etanercept and infliximab compared with usual care in active ankylosing spondylitis.
Methods
A Markov model over five years with cycle times of three months was computed. Patients included all had active disease, defined as Bath ankylosing spondylitis disease activity index (BASDAI) ⩾4 and could reach low disease activity, defined as BASDAI <4. Non‐response to tumour necrosis factor α (TNFα) inhibitors was always followed by cessation of treatment. Response to TNFα inhibitors could be followed at any time by either relapse to BASDAI ⩾4, leading to cessation of treatment, or toxicity, leading to cessation of treatment if major. Probabilities for efficacy, relapse, and toxicity were derived from two European randomised controlled trials. Utilities and costs assigned to the BASDAI disease states were derived from a two year observational Dutch cohort. In sensitivity analyses probabilities of effectiveness, toxicity, costs, and utilities were varied.
Results
Over five years the total quality adjusted life years varied from 2.57 to 2.89 for usual care, compared with 3.13 to 3.42 and 3.07 to 3.35 for etanercept or infliximab. Cumulative costs were between €49 555 to 69 982 for usual care compared with €59 574 to 91 183 or €28 3330 to 106 775 for etanercept and infliximab. This resulted in incremental cost‐utility ratios varying between €42 914 and 123 761 per QALY for etanercept compared with usual care and €67 207 to 237 010 for infliximab. The model was sensitive to drug prices.
Conclusion
Etanercept and infliximab have large clinical effects in ankylosing spondylitis. The present model suggests the high drug costs restricts efficient use in all patients who have a BASDAI >4. The validity of the model is limited by insufficient insight in the natural course of the disease and long term effectiveness and toxicity of TNFα inhibitors.
Keywords: ankylosing spondylitis, etanercept, infliximab, TNFα inhibitors, cost‐effectiveness
Until 2002 the treatment of ankylosing spondylitis was limited to non‐steroidal anti‐inflammatory drugs (NSAIDs) and physical therapy. In several open studies followed by two short term randomised placebo controlled trials in 2002, the tumour necrosis factor α (TNFα) inhibitors etanercept and infliximab showed important improvement in disease activity as well as in physical function.1,2,3,4,5,6 As the long term effectiveness of the drugs, the capability to inhibit radiographic damage, and the severity of side effects are still insufficiently clear, and as the TNFα inhibitors are expensive, knowledge of incremental cost‐utility might help in identifying the best way to use these drugs.7,8,9 The cost‐utility of infliximab over two years in patients with ankylosing spondylitis was reported recently.10 Data from 70 patients in a randomised trial were used in an individualised model to assess effects of treatment on combined Bath ankylosing spondylitis disease activity index (BASDAI) and Bath ankylosing spondylitis functional index (BASFI) during the first 54 weeks. In the reference case patients were treated for 54 weeks and returned to the baseline clinical state over 12 weeks. The incremental cost per quality adjusted life year (QALY) gained in the reference case (direct costs) was to £73 3000 (€112 882). Our analysis is a full Markov model over five years and concerns infliximab as well as etanercept. We also discuss the methodological limitations when modelling the cost‐utilities of treatment strategies in ankylosing spondylitis.
Methods
A Markov model was chosen as it takes into account changes over time by redistributing the patient cohort after each cycle over the health states distinguished. The time horizon of the model was five years and cycle times of three months were chosen. The point of view was the societal perspective. In the reference case direct as well as productivity costs were included.
Model
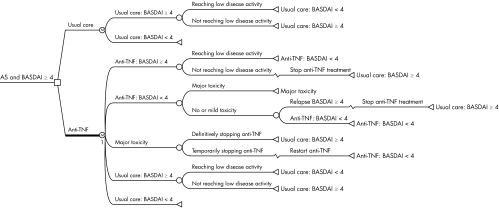
Patients entering the model had active ankylosing spondylitis defined as BASDAI ⩾4.They could be treated either with infliximab, 5 mg/kg every six weeks after the usual loading dose at weeks 0, 2, and 6; or (2) with etanercept, 25 mg twice weekly; or (3) with usual care, comprising NSAIDs or physiotherapy or both. In each treatment option, two disease states were distinguished, contrasting patients with active disease (BASDAI ⩾4) with patients with low disease activity (BASDAI <4). Response to treatment was defined as achieving a state of low disease activity (BASDAI <4). Response could be followed at any time during treatment by a relapse to BASDAI ⩾4 or by major toxicity. Non‐response or relapse was followed by stopping TNFα inhibitors and continuing usual care. Major toxicity was followed by treating the toxicity and a temporary (three months) cessation of the TNFα inhibitors while continuing the beneficial clinical effect of TNFα inhibition. After treating toxicity, patients could either continue with TNFα inhibitors or discontinue them, followed by relapsing to high disease activity and continuing usual care. All patients entering the model were screened for tuberculosis and were treated (prophylactically) if applicable. It was assumed that screening would prevent occurrence of active tuberculosis during treatment. Figure 1 presents the Markov model and health states. Mid‐cycle time corrections were applied because response, toxicity, and relapse can occur at any time during the three month cycle. The yearly transition probabilities were converted to quarterly probabilities, as required for the three month cycle time, by applying the appropriate correction formula.11 Costs and effects including utilities were discounted at 4% following Dutch guidelines.12 Modelling was undertaken in Data 3.5 software.
Figure 1 Markov model computed over a period of five years with cycle times of three months and comparing treatment with either etanercept or infliximab with usual care. AS, ankylosing spondylitis; BASDAI, Bath ankylosing spondylitis disease activity index; TNF, tumour necrosis factor.
Efficacy and toxicity data
The data on the efficacy and toxicity of etanercept and infliximab were derived from two randomised controlled trials (RCTs) that had included patients with active disease, defined as BASDAI ⩾4, and which were available when the model was computed.6,13,14 The proportion of patients reaching a BASDAI score of <4 and the proportions relapsing during follow up were obtained after additional analyses of the original RCTs. As the double blind phase of the etanercept study was six weeks, this response was carried forward to 12 weeks. The probabilities for relapse and toxicity beyond the duration of observational trial were estimated after discussion with rheumatologists experienced in the treatment of patients with ankylosing spondylitis using TNFα inhibitors, and they assumed no difference between etanercept and infliximab. When estimating the probability of toxicity, published open studies on TNFα inhibitors were considered.1,5,13,15,16,17,18 Two of these studies considered toxicity beyond six months (both studies related to infliximab).13,17
The probability of reaching a low disease activity state (BASDAI <4) when receiving usual care in the first cycle in the reference case equalled the response in the placebo groups used to adjust for the placebo effect. The probability of reaching a low disease activity state beyond the duration of the trial was determined from the four year period of observation of the Dutch cohort, which also provided data on the costs and utilities assigned to the disease states (see below). Table 1 presents the probabilities for changes between disease states of the model for each treatment option.
Table 1 Estimates of the probabilities for response, relapse, and toxicity and for costs and utilities used in the reference case, and the sensitivity analyses.
| Usual care | Etanercept | Infliximab | |
|---|---|---|---|
| Probabilities for response | |||
| First three months | 0.16 (0.06 to 0.26) | 0.71 (0.45 to 0.88)* | 0.62 (0.45 to 0.76)* |
| First year after first cycle | 0.02 (0.01 to 0.04) | NA | NA |
| Second to fifth year after first cycle | 0.01 (0.005 to 0.02) | NA | NA |
| Probabilities for relapse to BASDAI ⩾4 | |||
| First year after first cycle | NA | 0.26 (0.08 to 0.44)* | 0.16 (0.02 to 0.31)* |
| Second year | NA | 0.08 (0.005 to 0.15) | 0.08 (0.005 to 0.15)* |
| Third to fifth year after first cycle | NA | 0.02 (0.01 to 0.04) | 0.02 (0.01 to 0.04) |
| Probability of major toxicity | |||
| First three months | NA | 0* | 0.12 (0.01 to 0.23)* |
| First year after first cycle | NA | 0.08 (0.03 to 0.18)* | 0.13 (0.05 to 0.26)* |
| Second to fifth year after first cycle | NA | 0.04 (0.015 to 0.09) | 0.04 (0.015 to 0.09) |
| Response after relapse or toxicity related withdrawal | |||
| First cycle in that state | 0.05 (0.025 to 0.1) | 0.05 (0.025 to 0.1) | |
| First year after first cycle | 0.05 (0.025 to 0.1) | 0.05 (0.025 to 0.1) | |
| Second to fifth year after first cycle | 0 | 0 | |
| Discontinuation after major toxicity | 0.5 (0 to 1.0) | 0.5 (0 to 1.0) |
*Estimates based on randomised controlled trials.
BASDAI, Bath ankylosing spondylitis disease activity index; NA, not applicable; UC, usual care.
Costs and utility
Data on costs of usual care and utilities for each disease state (BASDAI <4 versus BASDAI ⩾4) were obtained from the initial two years' observation period in a longitudinal study of 130 Dutch patients with ankylosing spondylitis (OASIS, the Outcome Assessments in Ankylosing Spondylitis International Study). Detailed data on methods and results can be found in the original publications.19,20,21 Patients completed the BASDAI every two months (scores are from 0 to 10, higher values representing greater disease activity).22 A patient was considered to have low disease activity if time‐averaged BASDAI during the study period was less than 4, and high disease activity if the time‐averaged BASDAI was 4 or more.
For the costs and utilities assigned to the health states, the time‐averaged values over the two years' observation were used to assure robust estimates. The EuroQol 5 dimensions (EQ‐5D) was completed every six months.23 In case of toxicity, utility was reduced by 50%. The costs were assessed by two monthly cost questionnaires, and average ankylosing spondylitis related cost of illness per patient per year and the bootstrapped 95% confidence intervals (CI) were calculated. The indirect costs were based on the friction cost method, productivity costs to costs of sick leave in the friction period.24 All costs were adapted to 2002 prices using consumer price indices. In the reference case analysis total (direct and indirect) mean costs and the bootstrapped 95% CI per three months were used.25,26
The costs of screening for tuberculosis comprised a PPD skin test, a chest x ray, and further diagnosis and treatment if applicable. In the Netherlands, in individuals born between 1945 and 1965 a positive PPD is present in 3.5% and there is a lifetime incidence of active tuberculosis of 10%.
The drug cost of etanercept was the price of two self administered subcutaneous injections of 25 mg/week. The drug cost of infliximab was considered to be a dose of 5 mg/kg at weeks 0, 2, and 6, and every six weeks thereafter; it included a four hour infusion at a day care centre supervised by a qualified nurse (€45 per infusion).
The average weight of a patient was estimated at 70 kg (no waste). No further drug monitoring costs were considered. To estimate the costs of treating major toxicity, the type of toxicity reported in the original trials was taken into account.
Table 2 summarises the utilities and costs for the different disease states in the model.
Table 2 Costs and utilities used in the reference case and sensitivity analyses.
| Point estimate and 95% CI | |
|---|---|
| Total costs (FC) of AS if BASDAI ⩾4* | 4996 (3830 to 6737) |
| Total costs (FC) of AS if BASDAI <4* | 2456 (1825 to 3275) |
| Direct costs of AS if BASDAI ⩾4 | 4686 |
| Direct costs of AS if BASDAI <4 | 1671 |
| Total costs (HC) of AS if BASDAI ⩾4 | 15 950 |
| Total costs (HC) of AS if BASDAI <4 | 10 453 |
| Treatment costs for etanercept | 13 759 |
| Treatment costs for infliximab first cycle (€/patient) | 7385 |
| Treatment costs for infliximab | 21 335 |
| Treatment costs for low dose infliximab first cycle (€/patient) | 3974 |
| Treatment costs for low dose infliximab | 8612 |
| Screening and prophylaxis TB (€) | 82 |
| Treatment toxicity (€) | 2007 |
| EQ‐5Dtime averaged if BASDAI ⩾4 | 0.59 (0.55 to 0.63) |
| EQ‐5Dtime averaged if BASDAI <4 | 0.76 (0.74 to 0.79) |
| EQ‐5D if BASDAI <4 and toxicity present | 0.5 (0 to 1.0)* utility BASDAI <4 |
Values are €/patient/year unless stated otherwise.
*CI based on the 95th centile method of bootstrapped costs.
AS, ankylosing spondylitis; BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; CI, confidence interval; EQ‐5D, EuroQol 5 dimensions; FC, friction costs method; HC, human capital approach; TB, tuberculosis.
Sensitivity analyses
Several one way sensitivity analyses were undertaken. First, the costs of illness were varied by including direct costs only, and then by using the human capital approach instead of the friction method to estimate the productivity costs. Second, the dose of infliximab was reduced to 3 mg/kg every eight weeks after loading, while assuming the same efficacy. Third, the time horizon was limited to two years. Fourth, the placebo response in patients receiving usual care was neglected, assuming no change in health status. Fifth, several best case analyses were carried out. The first best case analysis imputed probabilities of response, toxicity, and relapse which favoured treatment with TNFα inhibitors. This probability was either the lowest or the highest value (whichever was more favourable) in the range of the 95% confidence interval (or between 0.5 and 2.0 in the absence of empirical data) surrounding the point estimate. In addition, all patients experiencing major toxicity were assumed to resume treatment. The second best case analysis used the costs and utilities associated with the disease states (BASDAI ⩾4 and BASDAI <4) that favoured TNFα inhibitors. These were again defined as either the lowest or the highest value (whichever was more favourable) in the range of the 95% confidence interval. In addition, the disutility associated with toxicity was discarded. The fourth best case sensitivity analysis combined the most favourable probabilities (response, toxicity, and relapse) as well as the most favourable costs and utilities. In addition to the use of the most favourable probabilities, costs, and utilities, the full best case also assumed there would be no change in health status with usual care. Finally, a threshold analysis was undertaken to estimate the drug acquisition cost for which the cost‐utility would be acceptable for Dutch society (€18 000 per QALY).
Results
Characteristics of patients
A comparison of the characteristics of the patients in the RCTs with the patients from our observational cohort is shown in table 3.
Table 3 Comparison of the clinical characteristics of the patients from the cohort study, providing the data on the costs and utilities of the distinguished disease states, and of the patients participating in each of the randomised controlled trials, providing data on the probabilities of response, relapse, and toxicity from treatment.
| Longitudinal cohort (n = 130) | RCTs as source for effectiveness | |||
|---|---|---|---|---|
| BASDAI <4 (n = 59) | BASDAI ⩾4 (n = 71) | Etanercept study (n = 40) | Infliximab study (n = 69) | |
| Male (%) | 63 | 77 | 73 | 65 |
| Age (y) | 47 (12) | 45 (12) | 36 (8.5) | 40 (7.9) |
| Disease duration (y) | 12 (9) | 12 (9) | 13 (8.8) | 16 (8.6) |
| BASDAI | 2.1 (1.1) | 5.6 (1.2) | 6.6 (1.4) | 6.4 (1.3) |
| BASFI | 2.8 (2.1) | 5.4 (1.8) | 5.9 (2.1) | 5.3 (2.0) |
| BASMI | 3.7 (1.7) | 4.1 (1.6) | 3.9 (1.4) | 3.7 (2.1) |
Values are mean (SD) unless stated otherwise.
BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; BASMI, Bath ankylosing spondylitis metrology index; RCT, randomised controlled trial; y, years.
Twenty two per cent of patients with BASDAI ⩾4 at the start of the observational cohort reached a BASDAI <4 after four months. The change in BASDAI over four years in patients from the cohort with BADAI ⩾4 was −0.012 (95% CI, −0.03 to 0.02) points per year. In the cohort the Spearman correlations between BASDAI and costs or utilities were moderate (r = 0.38) and good (r = −0.67), respectively.
Incremental cost‐utility ratios
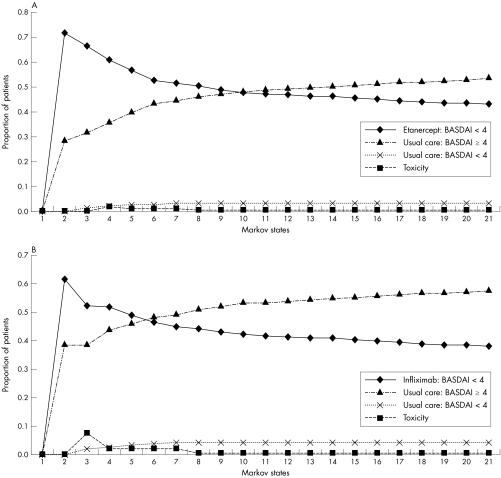
Figure 2A and 2B present the distribution of the patients over the Markov states over time for etanercept and infliximab, respectively. Over five years, the total time (maximum 60 months) in low disease activity (BASDAI <4) for the reference case was 11 months for patients receiving usual care, compared with 31 months and 28 months for patients receiving etanercept and infliximab, respectively. The total QALYs (maximum five) were 2.89, 3.16, and 3.11 for usual care, etanercept, and infliximab, respectively. The costs associated with ankylosing spondylitis and its treatment were €21 261, €52 137 and €62 047 for usual care, etanercept, and infliximab, respectively. This resulted in an incremental cost‐utility for the reference case analysis of €118 022/QALY for etanercept and €189 564/QALY for infliximab.
Figure 2 Probability plots of the distribution of the cohort over the identified Markov states over five years (20 cycles) of treatment from starting (A) etanercept and (B) infliximab onward. BASDAI, Bath ankylosing spondylitis disease activity index.
Sensitivity analyses
Table 4 shows the results of the different sensitivity analyses.
Table 4 Results of the Markov model over the five year time horizon for the reference case and for the five sensitivity analyses.
| Usual care | Etanercept | Infliximab | |
|---|---|---|---|
| Reference case | |||
| Months in BASDAI <4 over five years | 10.7 | 31.4 | 28.4 |
| Total QALY over five years | 2.89 | 3.16 | 3.11 |
| Total costs over five years (€) | 21 261 | 52 137 | 62 047 |
| Incremental cost‐utility ratio (€/QALY) | 118 022 | 189 564 | |
| Incremental cost (€) per extra month low disease activity | 1492 | 2307 | |
| Reference case with direct costs only | |||
| Total costs over five years | 19 425 | 49 555 | 59 574 |
| Incremental cost‐utility ratio (€/QALY) | 115 169 | 187 522 | |
| Reference case with human capital approach | |||
| Total costs over five years | 69 982 | 91 183 | 106 775 |
| Incremental cost‐utility ratio (€/QALY) | 81 038 | 171 848 | |
| Reference case with low dose infliximab | |||
| Total costs over five years (€) | 39 788 | ||
| Incremental cost‐utility ratio (€/QALY) | 86 533 | ||
| Reference case with two year time horizon | |||
| Months in BASDAI <4 over two years | 3.8 | 13.7 | 12.3 |
| Total QALY over two years | 1.26 | 1.39 | 1.37 |
| Total costs over two years (€) | 9462 | 25 675 | 31 972 |
| Incremental cost‐utility ratio (€/QALY) | 123 761 | 237 010 | |
| No response in health status in patients receiving usual care | |||
| Months in BASDAI <4 over five years | 0 | 29.7 | 26.3 |
| Total QALY over five years | 2.76 | 3.14 | 3.08 |
| Total costs over five years (€) | 23 307 | 52 458 | 62 448 |
| Incremental cost‐utility ratio (€/QALY) | 77 088 | 120 369 | |
| Best case analysis with probabilities (effectiveness, toxicity, and relapse) favouring TNFα inhibitors | |||
| Months in BASDAI <4 over five years | 4.4 | 48.2 | 44.3 |
| Total QALY over five years | 2.81 | 3.37 | 3.32 |
| Total costs over five years (€) | 22 473 | 67 418 | 82 601 |
| Incremental cost‐utility ratio (€/QALY) | 81 173 | 117 855 | |
| Best case analysis with costs and utilities favouring TNFα inhibitors | |||
| Total QALY over five years | 2.76 | 3.13 | 3.07 |
| Total costs over five years (€) | 27 478 | 54 595 | 65 052 |
| Incremental cost‐utility ratio (€/QALY) | 73 368 | 122 780 | |
| Best case analysis with probabilities, costs, and utilities favouring TNFα inhibitors | |||
| Total QALY over 5 years | 2.64 | 3.41 | 3.35 |
| Total costs over 5 years (€) | 29 811 | 66 891 | 82 787 |
| Incremental cost‐utility ratio (€/QALY) | 48 443 | 76 622 | |
| Best case with probabilities, costs, and utilities but no response in health status with usual care | |||
| Months in BASDAI <4 over five years | 0 | 47.6 | 43.2 |
| Total QALY over five years | 2.57 | 3.40 | 3.33 |
| Total costs over five years (€) | 31 426 | 67 139 | 83 173 |
| Incremental cost‐utility ratio (€/QALY) | 42 914 | 67 207 | |
| Reducing drug acquisition costs | 1/4 of the price | 1/5 of the price | |
| Total costs over five years (€) | 26 480 | 28 330 | |
| Incremental cost‐utility ratio (€/QALY) | 18 950 | 21 750 |
BASDAI: Bath ankylosing spondylitis disease activity index; QALY: quality adjusted life year.
In the best case analyses the incremental cost‐utility ratios (ICUR) were reduced to one third of the initial value but were still high for the viewpoint of Dutch society. Only the price of the drugs had substantial impact. The acceptable value of €18 000 per QALY was achieved when reducing the price of etanercept to one quarter and infliximab to one fifth. As even the cost‐utility of the best case analysis exceeded the Dutch acceptability threshold, we refrained from probabilistic analyses that would allow assessment of the 95% confidence interval for the incremental cost‐utility of the reference case.
Discussion
This study shows that the ICUR of etanercept or infliximab varies between €42 443 and €189 564 per QALY when compared with usual care. Whether these ratios are acceptable depends on what society feels one QALY is worth. There are large differences between countries in the threshold for considering ratios as acceptable. No country applies explicit thresholds, but the implicit thresholds cited vary between US$50 000 per QALY gained for the USA, £30 000 for the United Kingdom, and €18 000 for the Netherlands.27 Even in the best case analyses the ratios were above what would be considered favourable in most countries. From the point of view of the clinician, however, it is difficult to accept that an effective treatment should be denied because of cost implications only. It is noteworthy that our model proved especially sensitive to the price of the drugs. With increasing evolution towards targeted treatments it is to be expected that most future potential disease controlling drugs in ankylosing spondylitis will be expensive. One can discuss whether the thresholds that societies apply for decision making should be revised. However, budget restrictions cannot simply be denied, and they force the medical community to make choices in the management of disease. It should be borne in mind that the incremental cost‐utility ratios are limited in their ability to help health care systems allocate available resources. The number of patients eligible for the intervention and the available budget of the specific payer play an additional and essential role in decision making.
The resulting ICUR in this study should be seen in the light of methodological limitations in modelling the cost‐effectiveness in ankylosing spondylitis. These considerations are summarised in table 5 and are discussed below.
Table 5 Key issues that limit modelling cost‐utility in ankylosing spondylitis and recommendations for future research.
| Model issue | Present study | Limitation | Proposal | |||
|---|---|---|---|---|---|---|
| Definition of disease states that are clinically and economically relevant | BASDAI | • Limited in relating clinically and economically relevant disease states | • Combined (BADAI‐BASFI) outcome measures. Application in models would require data from large RCTs and cohorts | |||
| • Patient perspective without external (objective) criterion | • New measure including external criterion | |||||
| Number of disease states that are distinguished | BASDAI <4 opposed to ⩾4 | Limited number of disease states hampers identification of groups for which treatment is more or less cost‐effective | Determination of more disease states that are clinically (and economically) relevant. Application in models would require data from large RCTs | |||
| Natural course of the disease | • 5 year time horizon | Reduced ability to show additional long term beneficial effects of TNFα inhibition. | Gain insight into progression of the disease and measures to capture the progression | |||
| • After initial placebo response no change in BASDAI over 5 years | ||||||
| Utilities | • Utilities measured in cohort | • Patients in the cohort did not experience the beneficial influence of the TNFα inhibitors | • Utilities derived directly from observational studies | |||
| • EQ‐5D as only utility measure | • Different utilities can give different results | • Consensus of the recommended utility | ||||
| Long term toxicity | From RCTs and open studies with low sample sizes and short observations | • Initial RCTs showed more toxicity than later RCTs | Long term observational studies | |||
| • Limited long term observational data |
BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; EQ‐5D, EuroQol 5 dimensions utility index; RCT, randomised controlled trial.
The results are based on modelling data in a Markov analysis. This type of model allows a long term perspective and can take into account changes over time by redistributing the patient cohort after each cycle over the health states. The time horizon of five years was chosen as modelling beyond that time would not be realistic in the absence of empirical data. Patients are redistributed every three months over the distinguished health states.
Patients with active ankylosing spondylitis defined as BASDAI ⩾4 were considered for treatment. This inclusion criterion was chosen because it was identical to the inclusion of patients in the effectiveness studies used for the model. The choice was later supported by a consensus publication from the Assessment in Ankylosing Spondylitis (ASAS) working group on the use of TNFα inhibitors.7 A drawback of the model is that it is likely that patients from the cohort that had a BASDAI ⩾4 or <4 were not similar in disease characterises to the patients with a BASDAI ⩾4 or <4 included in the intervention trials. This explains why the average disease activity score in the patients from the cohort with a BASDAI ⩾4 was lower than the average BASDAI of the patients in the intervention trials (table 3). An additional survey in patients from the observational cohort confirmed that only 48% of those with a BASDAI ⩾4 were eligible for treatment with a biological agent according to the treating rheumatologists.28 As the costs and utilities of patients from the observational cohort who had a BASDAI ⩾4 were attributed to the patients receiving anti‐TNF treatment, the true initial costs and utilities of the cohort in the model might have been underestimated. However, when applying higher costs and worse utilities to these patients in the sensitivity analyses, the ICURs remained high.
A relevant limitation of the BASDAI defined disease states was the inability to discriminate productivity costs, which explains the small difference between the ICURs with direct costs only as opposed to the total costs including the productivity costs. Despite the substantial work disability (42%) in the cohort providing the data on productivity costs, the BASDAI was similar among the groups with or without work disability. Disease states including a measure of physical functioning might better discriminate between patients with less severe and more severe disease. Further, only two disease states of BASDAI (less than 4 versus 4 or more) were distinguished, reducing the possibility of identifying groups of patients for whom the treatment might be economically more favourable. The limited number of patients included in the source intervention trials hampered modelling in subgroups. In future analyses, alternative definitions of disease states and a larger number of disease states could help identifying patients for whom the treatment might have a more favourable cost‐effectiveness ratio.
The probabilities of transitions between the disease states of the model were obtained from two RCTs.6,13,14 Two additional RCTs with etanercept could not be used because the BASDAI was not included5 or because inclusion criteria were different from the model.15 Our model used as a criterion for response the achievement of an absolute state of low disease activity defined as BASDAI <4. When comparing the response in the model with rates based on response criteria reported in the source trials and the two RCTs that were not included, the response in the model was in the ranges reported in other trials, arguing for the validity and generalisability of this definition of initial response.1,5,6,13,14,15,29
The incremental gain in utility over five years of 0.27 and 0.22 for etanercept and infliximab, respectively, was surprisingly small compared with the large effects on patient reported disease activity and function. The gain in utility could be underestimated because utilities were derived from the patients in the observational cohort who had less severe disease and who had not experienced the beneficial effects of TNFα inhibitors. For example, TNFα inhibitors have an important effect on fatigue, and such effects are not directly captured by the EQ‐5D. As yet, utilities have not been measured directly in trials with biological agents in ankylosing spondylitis. When considering alternative outcomes and calculating an incremental cost‐effectiveness ratio (ICER) with the additional months in BASDAI <4 as an effectiveness measure, the ICER would be €1491 and €2307 per additional month for etanercept and infliximab, respectively, in the reference case, and €751 and €1197 in the best case analysis. If a BASDAI of <4 were experienced by patients as minimal clinical disease activity and if continuing low disease activity could prevent long term structural damage and functional disability, these costs seem very acceptable.
In the model, patients who relapsed to a BASDAI ⩾4 at any point beyond 12 weeks discontinued treatment with the TNFα inhibitor. This was the major reason for the high withdrawal rate of about 50% after the first year. When assuming no discontinuation, the further administration of the expensive drug would result in less favourable ratios. Based on expert opinion, it was assumed that patients experiencing toxicity would maintain their clinical response during the three months of toxicity. This was confirmed by a recent communication reporting that 63% of patients who stopped infliximab had a relapse after a mean of 13 weeks, but all responded to readministration of the treatment.30
A major consideration is that the placebo response was applied to patients receiving usual care. Despite the fact that in the observational cohort a small (non‐significant) improvement in disease activity over time was confirmed in patients with initial active disease, we undertook sensitivity analyses assuming no change in health status when receiving usual care. This approach had a large impact on the cost‐utility ratio. Assuming no placebo response in short term studies would overestimate the true effectiveness of the intervention, but we cannot exclude the possibility that in long term models this would overestimate withdrawals due to inefficacy. Better methods are needed to adjust for the short term placebo response in long term models.
It should be emphasised that it was not the intention of the present study to compare etanercept with infliximab. No head to head drug comparisons were made, and incremental cost‐utility comparisons of one drug with the other would not be justified at this time. Also, the differential effects of both drugs on the extra‐articular manifestations of the disease such as uveitis, psoriasis, and inflammatory bowel disease should also be taken into account, requiring additional data not available at present. Nevertheless, it cannot be denied that infliximab resulted in higher ICURs, the most important reason being the higher drug price. In the sensitivity analysis, infliximab at 3 mg/kg every eight weeks (assuming similar efficacy) provided a comparable ICUR to etanercept. The dose of infliximab should receive careful consideration as the drug costs proved to be the bottleneck of the economic evaluation.
Only two other ICU analyses have been reported in ankylosing spondylitis. One compared a three weeks spa intervention treatment with usual care in a group of Dutch patients31; effects, costs, and EuroQol‐5D utilities were measured directly alongside a one year RCT. The ICUR for a spa‐exercise treatment in Austria was €7465 per QALY and for spa‐exercise treatment in the Netherlands (the home country) it was €18 575 per QALY. One other study10 reported on the cost‐utility in patients with active ankylosing spondylitis but considered treatment with infliximab only. The reference case with direct costs reported £73 000 per QALY (€112 882), which was still lower than our €187 500. This model used individual patient data for the first 54 weeks of treatment and therefore patients started with a worse disease state than our cohort simulation. In addition, the model was based on disease states assessed by BASDAI and BASFI, and so identified better future savings in productivity costs. As a result, the ICUR fell to £35 000 (€53 900) when considering both direct and indirect costs. In addition, the utility gain in this study cohort was greater than in our cohort. The extent to which these differences can be attributed to cultural or methodological differences needs to be explored.
Conclusions
Despite the large clinical effects of etanercept and infliximab in patients with ankylosing spondylitis who have a BASDAI ⩾4, the extra costs are substantial, especially the high drug acquisition costs. The model is hampered by limited insight into the natural progression of the disease and long term effects of the drugs. This analysis helps to define future research to improve the economic model and identify patients for who the treatment might not only be effective but also cost‐effective.
Acknowledgements
We wish to acknowledge J Listing for carrying out the additional analyses from the source trials.
Abbreviations
BASDAI - Bath ankylosing spondylitis disease activity index
BASFI - Bath ankylosing spondylitis functional index
ICER - incremental cost‐effectiveness ratio
ICUR - incremental cost‐utility ratio
NSAID - non‐steroidal anti‐inflammatory drug
QALY - quality adjusted life year
RCT - randomised controlled trial
References
- 1.Stone M, Salonen D, Lax M, Payne U, Lapp V, Inman R. Clinical and imaging correlates of response to treatment with infliximab in patients with ankylosing spondylitis. J Rheumatol 2001281605–1614. [PubMed] [Google Scholar]
- 2.Brandt J, Haibel H, Sieper J, Reddig J, Braun J. Infliximab treatment of severe ankylosing spondylitis: one‐year followup. Arthritis Rheum 2001442936–2937. [DOI] [PubMed] [Google Scholar]
- 3.Breban M A, Vignon E, Claudepierre P, Saraux A, Wendling D, Lespessailles E.et al Efficacy of infliximab in severe refractory ankylosing spondylitis (AS). Results of an open label study. Ann Rheum Dis 200160(suppl 1)59. [DOI] [PubMed] [Google Scholar]
- 4.Van den Bosch F, Baeten D, Kruithof E, De Keyser F, Mielants H, Veys E M. Treatment of active spondyloarthropathy with infliximab, the chimeric monoclonal antibody to tumour necrosis factor alpha. Ann Rheum Dis 200160(suppl 3)iii33–iii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman J D, Sack K E, Davis J C. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 20023461349–1356. [DOI] [PubMed] [Google Scholar]
- 6.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W.et al Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 20023591187–1193. [DOI] [PubMed] [Google Scholar]
- 7.Braun J, Pham T, Sieper J, Davis J, van der Linden S, Dougados M.et al International ASAS consensus statement for the use of anti‐tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis 200362817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maksymowych W P, Inman R D, Gladman D, Thomson G, Stone M, Karsh J.et al Canadian Rheumatology Association Consensus on the use of anti‐tumor necrosis factor‐alpha directed therapies in the treatment of spondyloarthritis. J Rheumatol 2003301356–1363. [PubMed] [Google Scholar]
- 9.Pham T, van der Heijde D, Calin A, Khan M A, van der Linden S, Bellamy N.et al Initiation of biological agents in patients with ankylosing spondylitis: results of a Delphi study by the ASAS Group. Ann Rheum Dis 200362812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobelt G, Andlin‐Sobocki P, Brophy S, Jonsson L, Calin A, Braun J. The burden of ankylosing spondylitis and the cost‐effectiveness of treatment with infliximab (Remicade®). Rheumatology (Oxford) 2004431158–1166. [DOI] [PubMed] [Google Scholar]
- 11.Hunink M, Glasziou P, Siegel J, Weeks J, Pliskin J, Elsien A.et alDecision making in health and medicine. Cambridge: Cambridge University Press, 2001
- 12.Oostenbrink J, Koopmanschap M, Rutten F. Handleiding voor kostenonderzoek. Methoden en richtlijnprijzen voor economische evaluaties in de gezondheidszorg; [Guideline for cost‐evaluations. Methods and cost‐estimates for economic evaluations in healthcare]. Amstelveen: College voor zorgverzekeraars, 2000
- 13.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G.et al Long‐term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three‐month, randomized, placebo‐controlled trial. Arthritis Rheum 2003482224–2233. [DOI] [PubMed] [Google Scholar]
- 14.Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Grassnickel L.et al Six‐month results of a double‐blind, placebo‐controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003481667–1675. [DOI] [PubMed] [Google Scholar]
- 15.Davis J C, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg D O.et al Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003483230–3236. [DOI] [PubMed] [Google Scholar]
- 16.Braun J, Xiang J, Brandt J, Maetzel H, Haibel H, Wu P.et al Treatment of spondyloarthropathies with antibodies against tumour necrosis factor alpha: first clinical and laboratory experiences. Ann Rheum Dis 200059(suppl 1)i85–i89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeten D, Kruithof E, Van den Bosch F, Van den Bossche N, Herssens A, Mielants H.et al Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 200362829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maksymowych W P, Jhangri G S, Lambert R G, Mallon C, Buenviaje H, Pedrycz E.et al Infliximab in ankylosing spondylitis: a prospective observational inception cohort analysis of efficacy and safety. J Rheumatol 200229959–965. [PubMed] [Google Scholar]
- 19.Boonen A, van der Heijde D, Landewe R, Guillemin F, Rutten‐van Molken M, Dougados M.et al Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis 200362732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonen A, van der Heijde D, Landewe R, Guillemin F, Spoorenberg A, Schouten H.et al Costs of ankylosing spondylitis in three European countries: the patient's perspective. Ann Rheum Dis 200362741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boonen A, van der Heijde D, Landewe R, Spoorenberg A, Schouten H, Rutten van Molken M.et al Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 200261429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calin A, Nakache J P, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath Ankylosing Spondylitis Disease Activity Index) an appropriate instrument? Rheumatology (Oxford) 199938878–882. [DOI] [PubMed] [Google Scholar]
- 23.The EuroQol Group The EroQol‐a new facility for the measurement of health‐related quality of life. Health Policy 199016199–208. [DOI] [PubMed] [Google Scholar]
- 24.Koopmanschap M A, Rutten F F H. A practical guide for calculating the indirect costs of disease. Pharmacoeconomics 199610460–466. [DOI] [PubMed] [Google Scholar]
- 25.Severens J L, De Boo T M, Konst E M. Uncertainty of incremental cost‐effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 199915608–614. [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986154–77. [Google Scholar]
- 27.Eichler H G, Kong S X, Gerth W C, Mavros P, Jonsson B. Use of cost‐effectiveness analysis in health‐care resource allocation decision‐making: how are cost‐effectiveness thresholds expected to emerge? Value Health 20047518–528. [DOI] [PubMed] [Google Scholar]
- 28.Landewe R, Rump B, van der Heijde D, van der Linden S. Which patients with ankylosing spondylitis should be treated with tumour necrosis factor inhibiting therapy? A survey among Dutch rheumatologists. Ann Rheum Dis 200463530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H.et al Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum 200246755–765. [DOI] [PubMed] [Google Scholar]
- 30.Baraliakos X, Brandt J, Listing J, Rudwaleit M, Sieper J, Braun J. Clinical response to withdrawal of anti‐TNF therapy in patients with ankylosing spondylitis after three years pf continuous treatment with infliximab [abstract]. Arthritis Rheum 200551(suppl 9)a452 [Google Scholar]
- 31.Van Tubergen A, Boonen A, Landewe R, Rutten‐Van Molken M, Van Der Heijde D, Hidding A.et al Cost effectiveness of combined spa‐exercise therapy in ankylosing spondylitis: a randomized controlled trial. Arthritis Rheum 200247459–467. [DOI] [PubMed] [Google Scholar]