Abstract
Objective
To study the expression of laminin and type IV collagen as biomarkers of the organisation of the basal lamina of acini and ducts in labial salivary glands from patients with Sjögren's syndrome, and to relate this organisation to inflammatory cell invasion of acini and ducts.
Methods
Immunohistochemistry for laminin and type IV collagen was undertaken on sections of labial salivary glands from 30 patients with Sjögren's syndrome, 10 control subjects, and 24 controls with chronic sialoadenitis. Immunohistochemistry reaction, alterations to cell morphology, and the presence of inflammatory cells in acini and ducts were evaluated and scored using a semiquantitative method.
Results
Changes in the expression of laminin and type IV collagen in the basal lamina of acini and ducts of labial salivary glands from patients with Sjögren's syndrome were more pronounced than in labial salivary glands from control groups. A remarkable characteristic was the disorganisation of the basal lamina in the labial salivary glands in Sjögren's syndrome. The pattern of immunoreactivity of the basal lamina of other structures (for example, blood vessels) did not change. In Sjögren's syndrome, invasion of cytotoxic T lymphocytes was only observed in acini and ducts which had a disorganised basal lamina.
Conclusions
The high state of disorganisation of the basal lamina of acini and ducts could allow invasion of cytotoxic T lymphocytes in Sjögren's syndrome, contributing to cell death and ductal hyperplasia.
Keywords: basal lamina, salivary gland, Sjögren's syndrome
The basal lamina participate in the inside‐outside signalling that contributes to the maintenance of the architecture, differentiation, proliferation, growth, migration, and death of cells, as well as providing a protecting barrier against cellular invasion.1 The remodelling of the basal lamina in physiological and pathological processes is regulated by matrix metalloproteinases (MMPs) and their tissue inhibitors.2,3,4
Sjögren's syndrome is an autoimmune disease characterised by lymphocytic infiltration of salivary and lacrimal glands, with disruption of acini and ducts and important changes in the expression and activity of MMP‐3 and 9.4 Whole saliva from patients with Sjögren's syndrome has been reported to possess increased levels of MMP‐9.5,6 Acinar and ductal cell lines from human salivary glands transfected with SV‐40 virus and stimulated with cytokines show a high level of mRNA, protein expression, and gelatinolytic activity for MMP‐2.7 Additionally, extracts of labial salivary glands from patients with Sjögren's syndrome show a high degree of proteolytic activity toward laminin, type IV collagen, and stromal proteins.8
The mechanisms of damage to the parenchyma of labial salivary glands that have been described in Sjögren's syndrome involve the participation of inflammatory cells, including the perforin‐granzyme and Fas‐FasL pathways.9,10 NOD and NOD.scid mice—an experimental model of Sjögren syndrome—have high activity of MMPs in labial salivary gland tissue and saliva compared with the control strain (BALB/c)11,12; however, experiments in NOD.scid mice show that a loss of the labial salivary gland parenchyma may occur even in the absence of inflammatory cells.13 Thus labial salivary gland parenchymal damage in individuals with Sjögren's syndrome occurs in the presence or absence of inflammatory cells.
In Sjögren's syndrome, changes in basal lamina–epithelial cell interactions and their consequences for the architecture and function of labial salivary glands have been little studied, although they could be among the important events involved in labial salivary gland damage. Studies focused on these features may make an important contribution to our understanding of the molecular processes that take place before acinar and ductal cells can interact with inflammatory cells.8 It should be emphasised that only an action of the cytokines and chemokines, together with the structural disorganisation of the basal lamina, would permit the migration and invasion of inflammatory cells, and their direct interaction with acinar and ductal cells. These events would precede the cytotoxic mechanisms involved in the gland damage.
In this study, our aim was to examine the organisation of the basal lamina, evaluating in situ the expression of laminin and type IV collagen, two proteins that are fundamental to the organisation of the three dimensional network of the basal lamina. We also sought to correlate changes in the basal lamina organisation with mononuclear cell infiltration within the acini and ducts.
Methods
Patients with Sjögren's syndrome and controls
For this study, we selected 30 patients with primary Sjögren's syndrome (mean age 48.9 years, range 34 to 63 years) diagnosed according to the American‐European Consensus Group criteria.14 Patients were evaluated for keratoconjunctivitis sicca, xerostomia, and the presence of anti‐La and anti‐Ro autoantibodies.
The control groups consisted of 10 age matched healthy individuals (mean age 42.1 years, range 32 to 55 years, p = 0.1) and 24 patients with non‐specific chronic sialoadenitis (mean age 48.7, range 23 to 64 years, p = 0.9). Neither group had clinical or instrumental evidence of Sjögren's syndrome.
Biopsies
Labial salivary glands were obtained as previously described.8 Tissues were fixed for six hours at 4°C in 1% paraformaldehyde in 0.01M phosphate buffered saline, pH 7.4, and embedded in paraffin. Biopsies were obtained after receiving the informed consent of all study subjects. The study protocol was approved by the ethics committee of the Faculty of Medicine, University of Chile.
Immunohistochemistry
Two components with widespread distribution in the basal lamina were identified—laminin and type IV collagen. In some slides, mononuclear cells were identified before the detection of laminin or type IV collagen. After incubation with specific primary antibodies, the detection was completed using the avidin‐biotin complex method. Nuclear staining was done with Mayer's haematoxylin.
Primary antibodies
Antibodies directed against type IV collagen (mouse anti‐human type IV collagen; Dako, Glostrup, Denmark) or laminin (rabbit anti‐laminin; Sigma, St Louis, Missouri, USA) were used. Mononuclear cells were detected with anti‐CD4 and anti‐CD8 (Novocastra, Newcastle‐upon‐Tyne, UK). All primary antibodies were incubated overnight at 4°C. As negative controls, pre‐immune serum or immune serum adsorbed with purified protein (mouse serum (Dako) and rabbit serum (Sigma)) were used instead of primary antibodies.
Quantification of the immunohistochemical reaction of laminin and type IV collagen
The immunohistochemical reaction (IHR) was evaluated subjectively in a blinded manner, based on the reactivity, continuity, and disorganisation of the basal lamina staining. The features were scored semiquantitatively, using a visual scale (no change; light to moderate change; severe change). Three different slides containing ⩾2 labial salivary gland sections were examined for each study subject. Thirty random fields were examined in each section and for each molecule of interest.
Statistical analysis
The final analysis of the observations was carried out by comparing each field separately. Between‐group comparisons were evaluated using the χ2 test. Probability (p) values less than 0.05 were considered significant.
Results
Pattern of immunohistochemical reactions in the basal lamina of acini and ducts
Laminin and type IV collagen were analysed by immunohistochemical assays to study their modifications in Sjögren's syndrome compared with controls. We also investigated morphological alterations and the presence of mononuclear cell infiltrates. The results observed for both proteins were similar; therefore only the results for laminin are described.
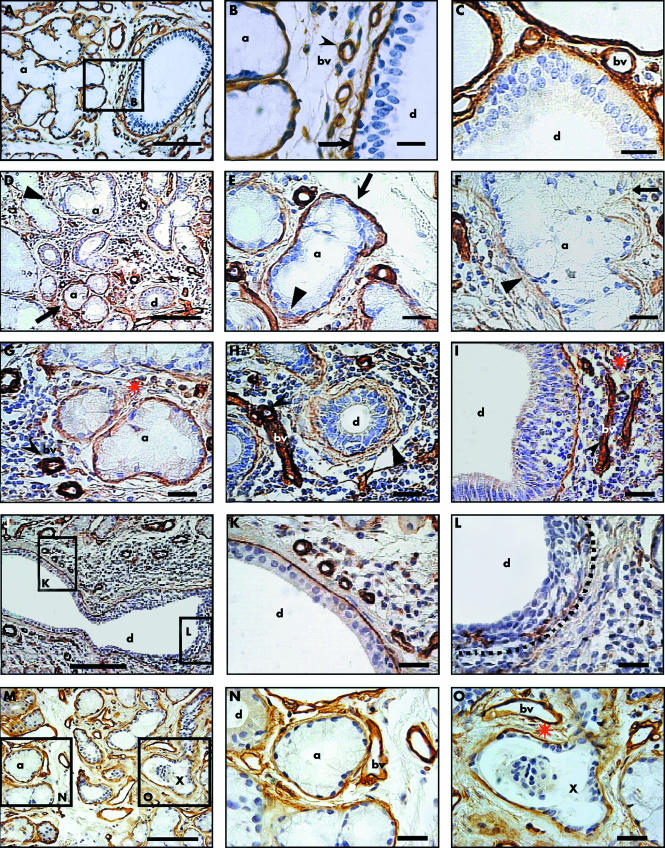
In control labial salivary glands, there was a well defined, continuous, and intense IHR in the basal lamina around acini, ducts, and blood vessels (fig 1, panels A to C). In labial salivary glands from Sjögren's syndrome, the IHR of the acinar and ductal basal lamina was heterogeneous and showed some zones with very low reactivity that lay near zones of high reactivity (fig 1, panel D). These changes were found in areas surrounded by inflammatory cells (fig 1D) as well as in areas without inflammatory cells (fig 1, panels M to O). These differences were also found in an acinus, showing at one end an area of IHR similar to that observed in controls, whereas at its opposite end, the basal lamina was disorganised (fig 1, panel E). In some areas, very poor IHR, and the occasional absence of IHR in acinar and ductal basal lamina was noted (fig 1, panels F and L). Ductal cell hyperplasia was observed (fig 1, panels I and L). It is important to note that some nuclei observed in ducts with hyperplasia were morphologically different from the nuclei of normal ductal cells but were similar to the nuclei of inflammatory cells (fig 1, panels I and L).
Figure 1 Detection of laminin in labial salivary glands from control subjects (panels A to C) and patients with Sjögren's syndrome (panels D to O). (A) Panoramic view of an labial salivary gland section from a control subject. (B) Higher magnification view of boxed area in (A), showing intense and continued reactivity around a group of acini (a). Arrow indicates duct basal lamina, cleaved arrowhead a blood vessel. (C) Closer view of a duct (d) and blood vessels (bv). (D) Section from a patient with Sjögren's syndrome showing a zone of intense inflammatory cell infiltration, where some acini maintain their reactivity (arrow), whereas neighbouring areas have lost it (arrowhead). (E) Acinus showing at one end an area of immunoreactivity similar to that observed in controls (arrow), whereas at the opposite end, the basal lamina is disorganised (arrowhead). (F) Acinus showing decreased immunoreactivity and a strongly disorganised basal lamina (arrowhead). (G) In an area of lymphocytic infiltration, there is a decrease in reactivity of the acini but no loss of reactivity of the blood vessels (cleaved arrowhead). (H) and (I) Duct showing weak reactivity (arrowhead in (H)), whereas reactivity is strongly maintained in the blood vessels (cleaved arrowhead). The duct in (I) shows hyperplasia. (J) Panoramic view of a duct with an inflammatory cell focus at one of its extremes (boxed area L) and a region without an infiltrate (boxed area K). (K) Higher magnification view of boxed area K in (J), showing an organised basal lamina and ductal cells forming a monolayer. (L) Higher magnification view of boxed area L in (J), showing a disorganised, practically absent basal lamina (dotted line) surrounded by an inflammatory cell focus. There is hyperplasia and disorganisation of the ductal cells similar to that in (I). (M) Labial salivary gland section without inflammatory cells, showing acini with normal basal lamina immunoreactivity (boxed area N) lying near others with decreased immunoreactivity (boxed area O); X indicates an apparent duct structure. (N) Higher magnification view of boxed area N in (M). (O) Higher magnification view of boxed area O in (M), showing an apparent duct structure (X), the basal lamina of which has low reactivity and shows disorganisation, and its lumen contains a group of epithelial cells. However, the blood vessel reactivity in both (N) and (O) is maintained. The red asterisk near the top of (G), (I), and (O) indicates the presence of immunoreactive fragments. Bars = 80 μm in panels A, D, J, and M; 18 μm in panels B, C, E, F, G, H, I, K, L, N, and O.
A very frequent and relevant characteristic of the IHR in the basal lamina of acini and ducts from patients with Sjögren's syndrome was its disorganisation, which had a diffuse and ill‐defined pattern and a cotton‐like appearance. The disorganisation was more evident in zones with abundant inflammatory cells (fig 1, panels F, G, H, J, and L), but this also occurred in zones without inflammatory cells (fig 1, panel O). Within the same duct, there were areas with intense inflammatory cell infiltration associated with a disorganised basal lamina (inset L in fig 1J), as well as areas free of inflammatory cells in which the basal lamina had a normal appearance (inset K in fig 1J). This disorganised basal lamina was found in acini and ducts with typical morphology, which were present in zones containing inflammatory cells (figs 1, panels F and H). However, this was also associated with structures having clear morphological changes (inset O in fig 1M, and also in fig 1, panel O), but no inflammatory cells. Very often, fragments of immunoreactive material were observed in the gland stroma (red asterisk in fig 1, panels I, G, and O).
It is important to emphasise that the variations in IHR were observed only in the gland parenchyma and not in the basal lamina of the blood vessels (fig 1, panels B, G, H, and I, cleaved arrowhead). Similarly, the basal lamina of the adipose, muscle, and nervous tissues did not show any alterations (data not shown).
Semiquantitative evaluation of immunohistochemical reactions for laminin
In order to evaluate these changes objectively, we designed a method that took into account the different characteristics of the basal lamina IHRs. These characteristics were the loss of reactivity, lack of continuity, and disorganisation of the basal lamina. The results were scored semiquantitatively on a visual scale and they were similar for laminin and type IV collagen. Thus only the results obtained for laminin reactivity are described here.
Reduction of reactivity was more often observed in labial salivary glands from Sjögren's syndrome than from controls (p = 0.014); nevertheless in some zones in the Sjögren salivary glands the immunoreaction was very intense. There were similar differences between Sjögren's syndrome and chronic sialoadenitis (p<0.001). Intense loss of reactivity was observed as a discontinuous reaction and was more often found in Sjögren's syndrome than in the normal controls or the controls with chronic sialoadenitis (p = 0.05 and 0.007, respectively). Disorganisation was defined as a diffuse pattern with a cotton‐like appearance, and it extended without clear limits toward the gland stroma. This was an interesting and characteristic finding in Sjögren's syndrome and was less often observed in the normal controls and the patients with chronic sialoadenitis (p<0.001).
Infiltration of acini and ducts by mononuclear cells
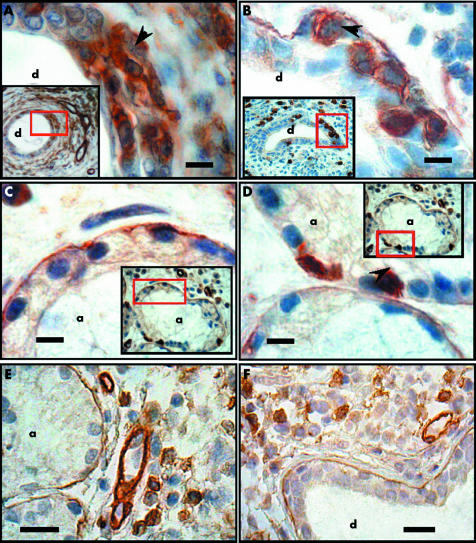
The presence of mononuclear cells within acini and ducts was used as a marker of the disarrangement of the basal lamina. Thus the localisation of CD8+ and CD4+ T lymphocytes was evaluated simultaneously to the detection of laminin or type IV collagen. In Sjögren's syndrome, CD4+ T lymphocytes were located in the periductal area as dense lymphocytic foci, but some of them were also found infiltrating ducts with disrupted basal lamina (fig 2A). CD8+ T lymphocytes were located within acini and ducts, the basal lamina of which was disrupted and occasionally absent (fig 2, panels B to D). In sections of labial salivary gland from patients with chronic sialoadenitis, inflammatory cells did not invade acini (fig 2E) or ducts (fig 2F) in areas with disrupted basal lamina.
Figure 2 Detection of CD4+ and CD8+ T lymphocytes in acini and ducts of labial salivary glands from patients with Sjögren's syndrome and chronic sialoadenitis. The panels show high magnification views of the red boxed areas in the insets (panoramic views) of each panel. (A) Duct (d) in which there is invasion of CD4+ T lymphocytes in the ductal epithelium (arrowhead) and loss of immunoreactivity of the basal lamina. (B) An identical phenomenon to that in (A), but identifying CD8+ T cells (arrowhead). (C) Acinus (a) in which the immunoreactivity of the basal lamina is normal and no CD8+ T lymphocytes are identified. (D) Acinus in which there is a loss of immunoreactivity of the basal lamina and infiltration of CD8+ T lymphocytes. CD4+ T lymphocytes were not detected in acini (data not shown). CD8+T lymphocytes in patients with chronic sialoadenitis were not detected in acini (E) or ducts (F). Bars in A to D = 5 μm; E and F 20 μm.
Discussion
In labial salivary glands from patients with Sjögren's syndrome, we have previously described significant proteolytic activity of MMPs toward purified proteins of the basal lamina.8 In these glands, high expression of MMP‐3 and MMP‐9 was found exclusively in acinar and ductal cells.4 In the present, we identified two proteins that are widely distributed in the basal lamina—laminin and type IV collagen—which are substrates of MMP‐3 and MMP‐9.15 In situ detection of laminin and type IV collagen was used as a biomarker of the molecular integrity of the basal lamina, allowing the simultaneous evaluation of the basal lamina of different structures in labial salivary glands. The expression of laminin and type IV collagen was similar in both acini and ducts both in regions with a large number of inflammatory cells and in regions where they were absent. In both regions, it was possible to observe some acini with strong IHR and others with very weak IHR. These observations indicated that the remodelling processes in the acini and ducts of the basal lamina occur apparently independently of the presence of inflammatory cells. Furthermore, in the same acinus we observed zones with intact basal lamina lying near zones with degraded basal lamina. This feature might be explained by asynchronous secretion of MMPs by acinar cells. Preliminary studies at both the messenger RNA (mRNA) and protein levels of laminin 1, laminin 5, and nidogen from Sjögren salivary glands showed that these molecules are upregulated. This suggests that the basal lamina of acini and ducts was undergoing remodelling (Kwon Y‐J, González MJ, unpublished results). This process of exchange could explain the high IHR for laminin and type IV collagen observed in some acini from labial salivary glands in patients with Sjögren's syndrome. A similar compensatory synthesis has been described previously in mammary glands.16 Additionally, McArthur and coworkers reported high levels of laminin mRNA in labial salivary glands in Sjögren's syndrome,17 which occurred before the inflammatory cells infiltrated the glands.18 Altogether, all these observations are very relevant because they support a widely held view that this pathological condition has an epithelial origin.19,20,21
The expression of laminin and type IV collagen in the basal lamina of blood vessels and other structures did not show any changes. This interesting finding supports the local proteolytic action of the acinar and ductal cells on their own basal lamina. This is a significant finding because, in some areas (figs 2, panels G, H, and I), the blood vessels were immersed in the inflammatory cell infiltrate. There is abundant information indicating that inflammatory cells produce MMPs22; however, previous observations show that in Sjögren's syndrome the main source of MMP‐2, MMP‐3, and MMP‐9 are the epithelial cells of the labial salivary glands.4 In addition, our study shows that the MMPs secreted by acinar and ductal cells are present in areas of limited activity, because, if they had spread into the extracellular matrix, proteolytic activity of the MMPs on the basal lamina of other structures would have been expected.
The disorganisation of the basal lamina of acini and ducts of labial salivary glands from patients with Sjögren's syndrome was the most frequent alteration we observed. This disorganisation showed a positive correlation with the number of inflammatory cells in the gland, regardless of whether the distribution was diffuse or focal. Both inflammatory cells and acinar and ductal cells in labial salivary glands from patients with Sjögren's syndrome synthesise cytokines,23,24 and some of them induce the synthesis of MMPs.25,26 Thus an increase in cytokine levels in the labial salivary glands would result in the maintenance of a degree of permanent synthesis of MMPs, which would trigger a greater level of remodelling activity in the basal lamina.
Nidogen and proteoglycans act as links between laminin and type IV collagen27 Preliminary studies have shown that there is increased proteolysis of nidogen in labial salivary glands in Sjögren's syndrome; this could afford an explanation for the basal lamina disorganisation described in this study. Future investigation will address this issue further.
The structural disorganisation of the basal lamina of acini and ducts is a factor that triggers its vulnerability. The basal lamina disorganisation would facilitate the direct contact between inflammatory cells and epithelial cells, favouring cytotoxic processes. Hyperplasia of the ductal epithelium is a common finding in salivary glands from Sjögren's syndrome patients. However, close observation of hyperplastic regions shows that some cells have a morphological appearance that is similar to that of inflammatory cells rather than epithelial cells. These changes coincide with areas of weak IHR for laminin (fig 2, panels I, J, and L) or type IV collagen (data not shown). In attempting to evaluate this, we used as markers of invasion the presence of CD4+ and CD8+ T lymphocytes. Results presented here indicate that the CD4+ and CD8+ cellular frequency was higher in ducts than in acini. This is the first demonstration of basal lamina structural changes in labial salivary glands in Sjögren's syndrome that could explain how inflammatory cells can infiltrate acini and ducts. These findings support related findings reported previously, where cells morphologically similar to inflammatory cells were shown in ducts but were not further characterised.28 Furthermore, in lacrimal glands from patients with Sjögren's syndrome, Fujihara and co‐workers have found that CD8+ but not CD4+ T lymphocytes were located around the acinar epithelial cells.29 Both the perforin/granzyme B and Fas/Fas ligand pathways were implicated in the process of programmed cell death in lacrimal glands.29 Disruption of basal lamina associated with further infiltration of CD4+ and CD8+ cells has also been proposed as a pathogenic mechanism of another immune response—the non‐auto‐immune disease oral lichen planus.30
In conclusion, we have described in detail the immunocytochemical and morphological changes that occur in the basal lamina of labial salivary glands from patients with Sjögren's syndrome and offer an explanation for these changes. We propose that the basal lamina becomes disorganised as result of degradation of at least two components, laminin and type IV collagen, by overexpression of MMP‐3 and MMP‐9 present in acinar and ductal cells.4 This disorganisation by itself may be the cause of the detachment of the acinar and ductal cells, provoking cellular death by anoikis.8 This detachment was observed with moderate frequency. The anoikis occurs in sites with and without inflammatory foci. However, there are other studies suggesting different mechanisms of cell death where the destruction of the gland occurs by cytotoxic mechanisms.9,10 The basal lamina permits the flux of water, ions, molecules, and macromolecules, but this structure also acts as a barrier to cells. Therefore, since contact between acinar or ductal cells and inflammatory cells requires direct cell–cell interaction, basal lamina disruption is a likely mechanism for this to occur. Moreover, patients with non‐specific chronic sialoadenitis rarely showed any T cells, despite the fact that the basal lamina could be disorganised or even absent, as in Sjögren's syndrome. The latter indicates that simple basal lamina disruption is not enough to account for infiltration of leucocytes in the acini and ducts in Sjögren's syndrome; the presence of cytokines or chemokines and active antigen presentation by acinar or ductal cells could provide an explanation for this difference.
The detection and quantification of the basal lamina disorganisation would improve our understanding of the different steps in the pathway that leads to apoptosis of acinar and ductal cells, and of the activation of cell survival mechanisms in Sjögren's syndrome.
Acknowledgements
We thank Drs Remigio López and Andrew Quest (Universidad de Chile, Facultad de Medicina, ICBM, Santiago, Chile) for their support, discussions, and critical review of the manuscript. This work was supported by a grant from the Fondecyt‐Chile (grants 1020755 and 1050192 to MJG), by a doctoral fellowship from Conicyt and by a grant from Mecesup‐Postgraduate University of Chile (grant 99‐03) to PP. LL is supported by Fogarty‐NIH grant No 1‐R03‐TW06024‐01, FONDECYT grant No 1040390; and FONDAP grant No 15010006.
Abbreviations
IHR - immunohistochemical reaction
MMP - matrix metalloproteinase
References
- 1.Giancotti F G, Ruoslahti E. Integrin signaling. Science 19992851028–1032. [DOI] [PubMed] [Google Scholar]
- 2.Bissell M J, Weaver V M, Lelievre S A, Wang F, Petersen O W, Schmeichel K L. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59: 1757–63s; discussion 1763–4s, (7 suppl) [PubMed]
- 3.Vincenti M P, Brinckerhoff C E. Transcriptional regulation of collagenase (MMP‐1, MMP‐13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene‐specific transcription factors. Arthritis Res 20024157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez P, Goicovich E, Alliende C, Aguilera S, Leyton C, Molina C.et al Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjogren's syndrome. Arthritis Rheum 2000432807–2817. [DOI] [PubMed] [Google Scholar]
- 5.Hanemaaijer R, Visser H, Konttinen Y T, Koolwijk P, Verheijen J H. A novel and simple immunocapture assay for determination of gelatinase‐B (MMP‐9) activities in biological fluids: saliva from patients with Sjogren's syndrome contain increased latent and active gelatinase‐B levels. Matrix Biol 199817657–665. [DOI] [PubMed] [Google Scholar]
- 6.Konttinen Y T, Halinen S, Hanemaaijer R, Sorsa T, Hietanen J, Ceponis A.et al Matrix metalloproteinase (MMP)‐9 type IV collagenase/gelatinase implicated in the pathogenesis of Sjogren's syndrome. Matrix Biol 199817335–347. [DOI] [PubMed] [Google Scholar]
- 7.Azuma M, Motegi K, Aota K, Hayashi Y, Sato M. Role of cytokines in the destruction of acinar structure in Sjogren's syndrome salivary glands. Lab Invest 199777269–280. [PubMed] [Google Scholar]
- 8.Goicovich E, Molina C, Perez P, Aguilera S, Fernandez J, Olea N.et al Enhanced degradation of proteins of the basal lamina and stroma by matrix metalloproteinases from the salivary glands of Sjogren's syndrome patients: correlation with reduced structural integrity of acini and ducts. Arthritis Rheum 2003482573–2584. [DOI] [PubMed] [Google Scholar]
- 9.Alpert S, Kang H I, Weissman I, Fox R I. Expression of granzyme A in salivary gland biopsies from patients with primary Sjogren's syndrome. Arthritis Rheum 1994371046–1054. [DOI] [PubMed] [Google Scholar]
- 10.Bolstad A I, Eiken H G, Rosenlund B, Alarcon‐Riquelme M E, Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte‐associated antigen 4, and programmed cell death 1 in primary Sjogren's syndrome. Arthritis Rheum 200348174–185. [DOI] [PubMed] [Google Scholar]
- 11.Robinson C P, Yamachika S, Alford C E, Cooper C, Pichardo E L, Shah N.et al Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjogren syndrome. Proc Natl Acad Sci USA 1997945767–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamachika S, Nanni J M, Nguyen K H, Garces L, Lowry J M, Robinson C P.et al Excessive synthesis of matrix metalloproteinases in exocrine tissues of NOD mouse models for Sjogren's syndrome. J Rheumatol 1998252371–2380. [PubMed] [Google Scholar]
- 13.Robinson C P, Yamamoto H, Peck A B, Humphreys‐Beher M G. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)‐SCID mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clin Immunol Immunopathol 19967950–59. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et al Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem 1997378151–160. [PubMed] [Google Scholar]
- 16.Streuli C H, Bissell M J. Expression of extracellular matrix components is regulated by substratum. J Cell Biol 19901101405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArthur C P, Fox N W, Kragel P. Monoclonal antibody detection of laminin in minor salivary glands of patients with Sjogren's syndrome. J Autoimmun 19936649–661. [DOI] [PubMed] [Google Scholar]
- 18.McArthur C P, Daniels P J, Kragel P, Howard P F, Julian L. Sjogren's syndrome salivary gland immunopathology: increased laminin expression precedes lymphocytic infiltration. J Autoimmun 19971059–65. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys‐Beher M G, Peck A B. New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model. Arch Oral Biol 199944(suppl 1)S21–S25. [DOI] [PubMed] [Google Scholar]
- 20.Anaya J M, Talal N. Sjogren's syndrome comes of age. Semin Arthritis Rheum 199928355–359. [DOI] [PubMed] [Google Scholar]
- 21.Moutsopoulos H M. Sjogren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol 199472162–165. [DOI] [PubMed] [Google Scholar]
- 22.Seitz M, Dayer J M. Enhanced production of tissue inhibitor of metalloproteinases by peripheral blood mononuclear cells of rheumatoid arthritis patients responding to methotrexate treatment. Rheumatology (Oxford) 200039637–645. [DOI] [PubMed] [Google Scholar]
- 23.Fox R I, Kang H I, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol 19941525532–5539. [PubMed] [Google Scholar]
- 24.Sun D, Emmert‐Buck M R, Fox P C. Differential cytokine mRNA expression in human labial minor salivary glands in primary Sjogren's syndrome. Autoimmunity 199828125–137. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B.et al Selective interaction of JNK protein kinase isoforms with transcription factors. Embo J 1996152760–2770. [PMC free article] [PubMed] [Google Scholar]
- 26.Baeuerle P A. IkappaB‐NF‐kappaB structures: at the interface of inflammation control. Cell 199895729–731. [DOI] [PubMed] [Google Scholar]
- 27.Ries A, Gohring W, Fox J W, Timpl R, Sasaki T. Recombinant domains of mouse nidogen‐1 and their binding to basement membrane proteins and monoclonal antibodies. Eur J Biochem 20012685119–5128. [DOI] [PubMed] [Google Scholar]
- 28.Fox R I, Kang H I. Pathogenesis of Sjogren's syndrome. Rheum Dis Clin North Am 199218517–538. [PubMed] [Google Scholar]
- 29.Fujihara T, Fujita H, Tsubota K, Saito K, Tsuzaka K, Abe T.et al Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren's syndrome. J Immunol 19991632226–2235. [PubMed] [Google Scholar]
- 30.Sugerman P B, Savage N W, Walsh L J, Zhao Z Z, Zhou X J, Khan A.et al The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med 200213350–365. [DOI] [PubMed] [Google Scholar]