Abstract
Background
An imbalance in cytokine homoeostasis is thought to have a key role in the neuropsychiatric syndromes of systemic lupus erythematosus (NPSLE), and recently, a role for chemokines has been noted.
Objective
To compare concentrations of monocyte chemotactic protein‐1 (MCP‐1)/CCL2 in cerebral spinal fluid (CSF) of patients with SLE, and with and without neuropsychiatric symptoms.
Methods
CSF was obtained from 185 patients with SLE: 96 with NPSLE and 89 patients with SLE without neuropsychiatric symptoms (non‐NPSLE patients). MCP‐1/CCL2 concentrations were measured with an ELISA.
Results
The average concentration of CSF MCP‐1/CCL2 in patients with NPSLE was 1959 pg/ml, and in non‐NPSLE patients 712 pg/ml. The average MCP‐1/CCL2 concentration was significantly higher in the NPSLE group than in the non‐NPSLE group (p<0.001). In one representative patient with NPSLE, MCP‐1/CCL2 levels in the CSF decreased in parallel with a decline in neuropsychiatric symptoms.
Conclusions
CSF MCP‐1/CCL2 levels are higher in patients with NPSLE than in non‐NPSLE patients. MCP‐1/CCL2 may have an important role in the expression of NPSLE. These results indicate that CSF MCP‐1/CCL2 reflects an inflammatory activity in the brain, suggesting that it might be used as a diagnostic tool and a monitor for therapeutic responses in patients with NPSLE.
Keywords: lupus, neuropsychiatric lupus, central nervous system, monocyte chemotactic protein‐1
Systemic lupus erythematosus (SLE) is an autoimmune disease characterised by widespread immunological abnormalities and multiorgan involvement, including the skin, joints, kidney, serosal membranes, and peripheral and central nervous system (CNS). Neuropsychiatric syndromes of systemic lupus erythematosus (NPSLE) may occur at any time during the course of the disease, and symptoms are extremely diverse, ranging from depression, psychosis, and seizures to stroke.1 It is difficult to know the origin of minor clinical symptoms, such as headaches and mood swings, and patients with SLE may have other conditions capable of causing neuropsychiatric symptoms, such as infections, severe hypertension, metabolic derangement, steroid psychosis, and other drug toxicities.2 Without proper treatment, neuropsychiatric disease in SLE is known to increase morbidity and mortality, and because beneficial treatment is available, it needs to be recognised early. An effective method of monitoring disease activity and response to treatment and a more specific diagnostic tool are crucial in the management of NPSLE.
Currently, tests for diagnosing NPSLE include brain magnetic resonance imaging (MRI), electroencephalography (EEG), a neuropsychological test, and lumbar puncture. These findings are found to be abnormal in some but not all patients, and therefore, none of the findings are specific for NPSLE. The large discrepancy in reported incidence of neuropsychiatric involvement in patients with SLE (14–75%) further proves the need for a single confirmatory diagnostic tool.3,4 Proinflammatory cytokines have been known to be increased in cerebral spinal fluids (CSF) of patients with NPSLE, and some reports have shown that cytokines such as interleukin (IL) 6, IL1, IL8, IL10, and tumour necrosis factor (TNF) α, interferon (IFN) γ are increased intrathecally, allowing these cytokines to be used as diagnostic tools.5,6
Recently, a role for chemokines has been reported. The chemokine monocyte chemotactic protein 1 (MCP‐1)/CCL2 is mainly expressed by activated monocyte/macrophages, T cells, and natural killer cells, and it attracts leucocytes and other mediators into sites of inflammation. Few studies showing an association between NPSLE and MCP‐1/CCL2 have been reported. MCP‐1/CCL2 is a high affinity ligand for the CCR2 chemokine receptor, which is mainly expressed on helper T1 (Th1) cells. The relative predominance of Th1 versus Th2 cells in patients with NPSLE remains unknown. As far as we know, MCP‐1/CCL2 levels in the CSF of NPSLE have never been reported. To examine the role of MCP‐1/CCL2 in NPSLE, we measured concentrations of MCP‐1/CCL2 in CSF obtained from patients with SLE with and without neuropsychiatric involvement.
Patients and methods
Patients
A total of 185 patients fulfilling the criteria of the American College of Rheumatology for the classification of SLE were selected. The patients (174 female and 11 male, aged 16–62 years; average age 33.6) were admitted to either the Institute of Rheumatology, Tokyo Women's Medical University or the Division of Rheumatology and Clinical Immunology, Jichi Medical School, from 1992 to 2002. NPSLE was diagnosed according to the American College of Rheumatology criteria for neuropsychiatric lupus syndromes.1 Examinations of neuropsychiatric symptoms were carried out by experienced clinicians, and specific tests for the diagnosis of NPSLE included neuropsychological tests, lumbar puncture, brain MRI, and EEG. Patients were divided into two groups: 96 with SLE with neuropsychiatric symptoms, and 89 with SLE without neuropsychiatric symptoms. CSF was collected from these patients. Of the 96 patients with NPSLE, 35 samples were collected before NPSLE treatment, while 61 samples were collected after or during glucocorticoid treatment, specifically for the neuropsychiatric symptoms.
Methods
Levels of MCP‐1/CCL2 in CSF were determined by an enzyme linked immunosorbent assay (ELISA; Quantikine human MCP‐1 immunoassay, R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Statistical comparisons were carried out using the non‐parametric Mann‐Whitney U test. For correlation analysis, we used Spearman's correlation coefficient.
Results
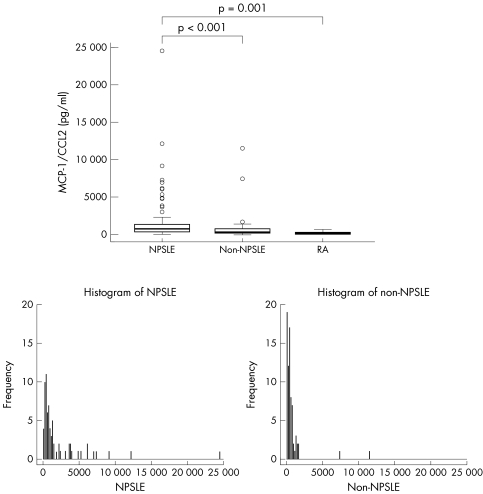
The mean (SD) concentration of MCP‐1/CCL2 in patients with NPSLE was 1959 (3199) pg/ml, and in non‐NPSLE patients 712 (1432) pg/ml. As the data were not symmetrically distributed, we present the results as a box plot and histogram. The minimum values for NPSLE, non‐NPSLE, and rheumatoid arthritis (RA) were 7, 0, and 5 pg/ml, respectively. The maximum values for NPSLE, non‐NPSLE, and RA were 24 590, 11 540, and 602 pg/ml, respectively. The median of the values for NPSLE, non‐NPSLE, and RA were 737, 400, and 120 pg/ml, respectively. The first quartile of the values for NPSLE, non‐NPSLE and RA were 380, 239, and 9 pg/ml, respectively.
Statistical analyses showed that the MCP‐1/CCL2 concentration was significantly higher in the NPSLE group than in the non‐NPSLE group (p = 0.0004) and than in the patients with RA (p = 0.001) (fig 1).
Figure 1 CSF levels of MCP‐1/CCL2 in patients with NPSLE, non‐NPSLE patients, and patients with RA.
We next studied the relationship between MCP‐1/CCL2 levels and IL6, IL8, IFNα, MDC or IP‐10/CXCL10. There were no correlations between the levels of MCP‐1/CCL2 and IL6 (p = 0.46, rs = −0.12), IL8 (p = 0.35, rs = −0.16), IFNα (p = 0.68, rs = −0.08) or MDC (p = 0.95, rs = 0.01). However, there was a close correlation between MCP‐1/CCL2 levels and IP‐10/CXCL10 levels (p<0.001, rs = 0.64). We concluded that CSF MCP‐1/CCL2 levels are higher in patients with NPSLE than in non‐NPSLE patients, indicating possible involvement of this chemokine in the pathogenesis of NPSLE. Furthermore, we compared the levels of MCP‐1/CCL2 with various neuropsychiatric symptoms. Table 1 shows the statistical data of each group and each case was categorised according to his/her dominant neuropsychiatric symptom. As some groups had very few cases, we were unable to conclude which type of symptom was associated with the increase of MCP‐1/CCL2 in CSF from the data presented.
Table 1 CSF levels of MCP‐1/CCL2 among groups of various neuropsychiatric symptoms.
| Number | Average | SD | Minimum values | Maximum values | Median values | First quartile | |
|---|---|---|---|---|---|---|---|
| Aseptic meningitis | 11 | 3144 | 3359 | 151 | 9157 | 480 | 403 |
| Cerebrovascular disease | 16 | 1416 | 1809 | 180 | 7000 | 603 | 409 |
| Demyelinating syndrome | 2 | 6109 | 1680 | 4921 | 7297 | 3109 | 5515 |
| Movement disorder | 6 | 865 | 389 | 497 | 1525 | 803 | 576 |
| Seizures | 20 | 1185 | 1770 | 31 | 6042 | 418 | 240 |
| Acute confusion state | 3 | 349 | 141 | 186 | 430 | 430 | 308 |
| Anxiety disorder | 5 | 464 | 395 | 92 | 1130 | 400 | 273 |
| Cognitive dysfunction | 4 | 153 | 98 | 98 | 300 | 107 | 103 |
| Mood disorders | 9 | 575 | 1198 | 25 | 3745 | 220 | 45 |
| Psychosis | 17 | 2010 | 5832 | 10 | 24590 | 630 | 338 |
| Neuropathy, cranial | 3 | 449 | 398 | 211 | 909 | 227 | 219 |
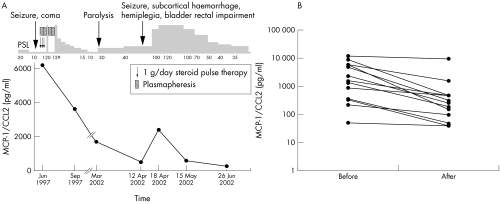
Figure 2 illustrates the changes of MCP‐1/CCL2 levels in CSF during the course of the disease of a representative patient with NPSLE symptoms. A 23 year old woman with episodes of high fever, cervical lymph node swelling, joint pain, and morning stiffness was diagnosed with SLE by the findings of arthritis, positive antinuclear antibody (speckled pattern), anti‐Sm antibody, and lymphocytopenia in July 1994. Treatment was started with 20 mg/day of oral prednisolone in January 1995, and her symptoms subsided. Her drug dosage was reduced gradually to 10 mg/day. However, in June, 1997, she became symptomatic again with a fever of 38–39°C, leucocytopenia, thrombocytopenia, and proteinuria. In June the patient had seizures and went into a coma. After CSF was collected, the patient was given methylprednisolone pulse therapy and plasmapheresis. Her daily prednisolone dose was increased to 120 mg. The patient's fever and neurological symptoms gradually subsided, and her prednisolone dosage was gradually reduced from 120 mg/day to 10 mg/day. During her first neurological symptoms, the patient's CSF MCP‐1 level was increased at 6041 pg/ml, but after treatment the level decreased to 3709 pg/ml.
Figure 2 (A) Clinical course and CSF levels of MCP‐1/CCL2 in patient 1. A 23 year old woman was diagnosed with SLE by the findings of arthritis, positive antinuclear antibody (speckled pattern), anti‐Sm antibody, lymphocytopenia. As her neuropsychiatric symptoms improved, her CSF MCP‐1/CCL2 level decreased. (B) Temporal correlation of clinical symptoms and MCP‐1 levels in 13 other cases. We show the serial data of MCP‐1 levels before and after the improvement of the symptoms.
However, in February 2002, after a brief period of stable disease activity, the patient had episodes of fever (37–38°C) and sporadic periods of paralysis. Corticosteroid pulse therapy was given and her daily oral dose was increased to 40 mg/day. On 12 April the patient had seizures, impairment of the bladder and rectum, loss of consciousness, and hemiplegia; head computed tomography showed subcortical haemorrhage. Steroid pulse therapy was immediately started and her daily dose was increased to 100 mg. Even after this treatment, her symptoms progressed and her CSF MCP‐1 was raised at 2361 pg/ml, and therefore, her daily drug dose was increased to 120 mg. Gradually, her symptoms improved along with a decrease in CSF MCP‐1 level to 594 pg/ml within 1 month, and her daily oral prednisolone was gradually reduced. Figure 2B shows temporal correlation of clinical symptoms and MCP‐1 levels in other patients. Serial data of MCP‐1 levels from 13 patients are presented.
These results indicate that the MCP‐1/CCL2 level in CSF reflects disease activity of NPSLE and it may be used as an indicator of response to treatment.
Discussion
Because patients with SLE may have a wide array of neuropsychiatric symptoms, and because this condition may lead to mortality and morbidity, early diagnosis and appropriate treatment is essential for the management of the disease. Multiple pathogenetic mechanisms may cause NPSLE, but there is no single confirmatory diagnostic test available. Tests for diagnosing NPSLE include brain MRI, EEG, neuropsychological test, and lumbar puncture. Although MRI may be useful in detecting lesions caused by NPSLE, there are no specific MRI findings and although patients may be symptomatic, not all show an MRI abnormality.7 CSF has been used for diagnostic purposes because it is directly in contact with CNS tissue, and the concentration of astroglial and neuronal degradation products reflects the extent of brain cell injury, making it suitable for studies of pathological processes in the CNS. Studies have shown raised IL6, IL1, IL8, IL10, TNFα, and IFNγ in the CSF of patients with SLE with neuropsychiatric involvement.5,6 and decreased IL6 after treatment for neuropsychiatric disease.5
Chemokines, which are chemotactic cytokines, are a family of small molecular weight proteins that function in leucocyte recruitment and cellular activation. Chemokine expression in the CNS has an important role in the inflammatory response in several diseases. The role of chemokines in the CSF of patients with NPSLE has been little reported. Although a Th1 chemokine interferon‐inducible protein (IP) 10/CXCL10 was found to be increased in the CSF of patients with NPSLE,8 to our knowledge, no studies of MCP‐1/CCL2 expression in CSF of patients with SLE have ever been conducted.
In our study, we investigated the level of MCP‐1/CCL2 in CSF and found it to be significantly increased in patients with NPSLE. Levels of MCP‐1/CCL2 decreased after treatment, and a significant difference was seen in the levels of CSF MCP‐1/CCL2 before and after treatment. Although, table 1 shows the statistical data of each group of various neuropsychiatric symptoms, we were unable to conclude which type of symptom was associated with the increase of MCP‐1/CCL2 in CSF and further study is needed to determine this.
In conclusion, the evaluation of MCP‐1/CCL2 levels in CSF reflects an inflammatory activity in the brain, making its useful as a diagnostic tool as well as a monitoring gauge of response to treatment in patients with NPSLE. Further studies may show the importance of MCP‐1/CCL2 as a possible measure of neuropsychiatric involvement in patients with SLE.
Acknowledgements
We gratefully acknowledge the expert technical help of Mika Kasahara.
Abbreviations
CNS - central nervous system
CSF - cerebrospinal fluid
EEG - electroencephalography
IFN - interferon
IL - interleukin
MCP - monocyte chemotactic protein
MRI - magnetic resonance imaging
NPSLE - neuropsychiatric syndromes of systemic lupus erythematosus
RA - rheumatoid arthritis
SLE - systemic lupus erythematosus
Th - T helper
TNF - tumour necrosis factor
References
- 1.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. Arthritis Rheum 199942599–608. [DOI] [PubMed] [Google Scholar]
- 2.Bresnihan B. CNS lupus. Clin Rheum Dis 19828183–195. [PubMed] [Google Scholar]
- 3.Brey R L, Holliday S L, Saklad A R, Navarrete M G, Hermosillo‐Romo D, Stallworth C L.et al Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002581214–1220. [DOI] [PubMed] [Google Scholar]
- 4.Ainiala H, Loukkola J, Peltola J, Korpela M, Heitaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 200157496–500. [DOI] [PubMed] [Google Scholar]
- 5.Trysberg E, Calrsten H, Tarkowski A. Intrathecal cytokines in systemic lupus erythematosus with central nervous system involvement. Lupus 20009498–503. [DOI] [PubMed] [Google Scholar]
- 6.Svenungsson E, Andersson M, Brundin L, van Vollenhoven R, Khademi M, Tarkowski A.et al Increased levels of proinflammatory cytokines and nitric oxide metabolites in neuropsychiatric lupus erythematosus. Ann Rheum Dis 200160372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCune W J, MacGuire A, Aisen A, Gebarski S. Identification of brain lesions in neuropsychiatric systemic lupus erythematosus by magnetic resonance scanning. Arthritis Rheum 198831159–166. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto H, Katsumata Y, Nishimura K, Kamatani N. Interferon‐inducible protein 10/CXCL10 is increased in the cerebrospinal fluid of patients with central nervous system lupus. Arthritis Rheum 2004503731–3732. [DOI] [PubMed] [Google Scholar]