In the generation of Sjögren's syndrome (SS), CD4 positive αβ T cells have a crucial role. Previous studies have provided evidence about the T cell receptor (TCR) Vβ and Vα genes on these T cells, and sequence analysis of the CDR3 region indicates the presence of some conserved amino acid motifs, supporting the notion that infiltrating T cells recognise relatively few epitopes on autoantigens.1
Candidate autoantigens recognised by T cells that infiltrate the labial salivary glands of patients with SS have been analysed, and Ro/SSA 52 kDa,2 α‐amylase, heat shock protein, and TCR BV6 have been identified, although Ro/SSA 52 kDa reactive T cells were not increased in peripheral blood.3 Gordon et al indicated that anti‐M3R autoantibodies occurred in SS and were associated with the sicca symptoms.4 Recently, we provided evidence for the presence of autoantibodies against the second extracellular domain of muscarinic acetylcholine receptor (M3R) in a subgroup of patients with SS.5 The M3R is an interesting molecule, because this portion has an important role in intracellular signalling,5 although the function of anti‐M3R autoantibodies remains unknown.
The mechanism through which a peptide is recognised by a TCR is flexible. If the amino acid residue of the peptide ligands for TCR is substituted by a different amino acid and can still bind to major histocompatibility complex molecules (altered peptide ligand), such an altered peptide ligand could regulate the activation of T cells. Several studies have shown that an altered peptide ligand could induce differential cytokine secretion, anergy, and antagonism of the response to the wild‐type antigens.6,7 The altered peptide ligand has the potential of being used therapeutically against T cell mediated diseases such as autoimmune diseases and allergic disorders.
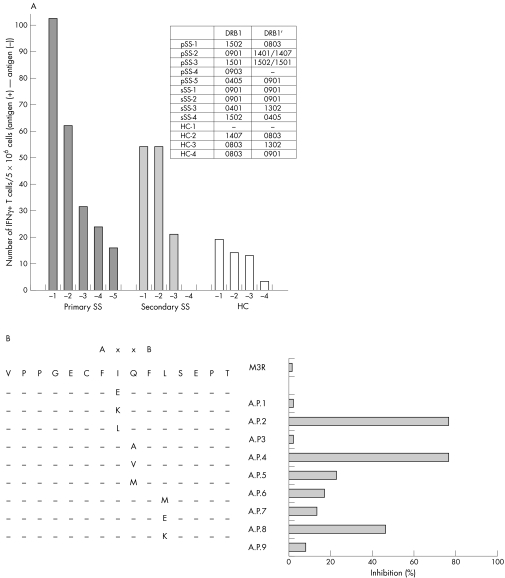
As an extension to our previous study,5 we focused in the present study on M3R reactive T cells and analysed T cell epitopes and their altered peptide ligands with the aim of regulating T cell proliferation and autoantibody production. The 25mer synthetic amino acids encoding the second extracellular domain of M3R (KRTVPPGECFIQFLSEPTITFGTAI, AA213‐237) were used as the antigen for T cells, and the number of interferon (IFN) γ producing T cells was counted by flow cytometry using a magnetic activated cell sorting (MACS) secretion assay. The proportion of IFNγ‐producing T cells among peripheral blood mononuclear cells (PBMCs) was high in two of five patients with primary SS (pSS) and two of four patients with secondary SS (sSS), compared with the level in four healthy control subjects (HC) (fig 1A). Three patients with SS and M3R reactive T cells (pSS‐2, and sSS‐1, 2) had the HLA‐DR B1*0901 allele and the other patient (pSS‐1) had HLA‐DR B1*1502 and *0803 alleles. The 25mer amino acids contain the anchored motifs that bind to HLA‐DR B1*0901. Thus, IFNγ production by T cells should be due to the recognition of antigen on the HLA molecule by the TCR on T cells.
Figure 1 (A) M3R reactive T cells. (B) Selection of altered peptide ligands.
The results shown in fig 1 were obtained as follows. Blood samples were collected from five Japanese patients with pSS and four Japanese patients with sSS followed up at the University of Tsukuba Hospital. All patients with SS satisfied both the Japanese Ministry of Health criteria for the classification of SS8 and the revised EU‐US criteria9. We also recruited four HC from our university. Approval for this study was granted from the local ethics committee, and written informed consent was obtained from all patients and HC who participated in this study.
Their HLA‐DR allele was examined by the SSOP‐PCR method, as described elsewhere. A 15mer peptide (VPPGECFIQFLSEPT) (M3R AA216–230) corresponding to the sequence of the second extracellular loop domain was also synthesised (Kurabo Industries, Osaka, Japan). PBMCs were purified with Ficoll‐Paque and 5×106 cells were cocultured with 10 μg of M3R peptide (25mer) in 1 ml of RPMI‐1640 with 10% of human AB serum (Sigma, St Louis, MO) for 12 hours at 37°C. As a positive control, 1 μg of staphylococcal enterotoxin B (Toxin Technology Inc, USA) was used. IFNγ‐producing cells were identified by the MACS cytokine secretion assay (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, the cells were incubated with 20 μg of IFNγ detection antibody (Ab; Miltenyi Biotec), 20 μg of anti‐CD4‐FITC Ab (Becton Dickinson, Franklin Lakes, NJ, USA), and 5 μg of anti‐CD3‐APC Ab (Becton Dickinson) for 10 minutes at 4°C. After double washing with a cold buffer (phosphate buffered saline/0.5% bovine serum albumin with 2 mM EDTA), the cells were incubated with 20 μg of anti‐phycoerythrin microbeads (Miltenyi Biotec) for 15 minutes at 4°C. After double washing, the cells were resuspended with 500 μl buffer and then passed through an MS column (Miltenyi Biotec), which was set to mini‐magnet (Miltenyi Biotec). The column was set on the Falcon tube (Becton Dickinson), bead‐binding cells were eluted by 1 ml of cold buffer, and IFNγ‐producing cells were analysed by FACSCalibur (Becton Dickinson).
The 15mer peptide (M3R 216–230) and its nine altered peptide ligand candidates were synthesised (Sigma) (fig 1B). The purity of each peptide was >90%. The anchor positions binding to HLA‐DR B1*0901 are AA222 and AA225, which are indicated as A and B in fig 1B. PBMCs from patient pSS‐2 were used in this experiment; 1×106 cells were cultured with 10 μg of each peptide in 1 ml of RPMI‐1640 with 10% human AB serum. IFNγ‐producing T cells were identified using MACS secretion assay as described in fig 1A.
To determine the altered peptide ligands of M3R in patients with SS, we synthesised nine 15mer peptides (VPPGECFI→E/K/LQFLSEPT, VPPGECFIQ→A/V/MFLSEPT, VPPGECFIQFL→M/E/KSEPT, M3R216–230), in which the anchored motif binding to the HLA‐DR B1*0901 molecule is conserved, although one amino acid to TCR was different. Altered peptide ligands were selected based on inhibition of IFNγ production by M3R reactive T cells. Figure 1B shows that M3R 223I→K and M3R 224Q→A significantly suppressed the number of IFNγ‐producing T cells, suggesting that they are candidates for selection as altered peptide ligands. The inhibition of IFNγ by other cytokines may not be likely, because interleukin 4 producing T cells were not increased (data not shown).
In conclusion, we have provided evidence for the presence of M3R reactive T cells in the serum of patients with SS and shown that VPPGECFKQFLSEPT (M3R 223I→K) and VPPGECFIAFLSEPT (M3R 224Q→A) are candidate altered peptide ligands of the second extracellular domain of M3R. Our findings may provide the basis of a potentially useful antigen‐specific treatment for SS using altered peptide ligands of autoantigens recognised by autoreactive T cells.
References
- 1.Sumida T, Matsumoto I, Maeda T, Nishioka K. T‐cell receptor in Sjögren's syndrome. Br J Rheumatol 199736622–629. [DOI] [PubMed] [Google Scholar]
- 2.Sumida T, Namekawa T, Maeda T, Nishioka K. New T‐cell epitope of Ro/SS‐A 52kD protein in labial salivary glands from patients with Sjögren's syndrome. Lancet 19963481667. [DOI] [PubMed] [Google Scholar]
- 3.Halse A K, Wahren M, Jonsson R. Peripheral blood in Sjogren's syndrome does not contain increased levels of T lymphocytes reactive with the recombinat Ro/SS‐A 52 kD and La/SS‐B 48kD autoantigens. Autoimmunity 19962325–34. [DOI] [PubMed] [Google Scholar]
- 4.Gordon T P, Bolstad A I, Rischmueller M, Jonsson R, Waterman S A. Autoantibodies in primary Sjogren's syndrome: new insights into mechanisms of autoantibody diversification and disease pathogenesis. Autoimmunity 200134123–132. [DOI] [PubMed] [Google Scholar]
- 5.Naito Y, Matsumoto I, Wakamatsu E, Goto D, Sugiyama T, Matsumura R.et al Muscarinic acetylcholine receptor autoantibodies in patients with Sjögren's syndrome. Ann Rheum Dis 200564510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magistris M T D, Alexander J, Coggeshall M, Altman A, Gaeta F C A, Grey H M.et al Antigen analog‐major histo‐compatibility complexes act as antagonists of the T cell receptor. Cell 199268625–634. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med 19951811569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjogren's syndrome (1999): availability and validity. Mod Rheumatol 200414425–434. [DOI] [PubMed] [Google Scholar]
- 9.Vitali C, Bomvardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et al European study group on classification criteria for Sjögren's syndrome. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]