Abstract
Background
Oral glucosamine preparations are widely used as a treatment for osteoarthritis, purportedly functioning by a variety of mechanisms suggested by results of in vitro experiments, and generally using glucosamine concentrations well in excess of 100 μmol/l.
Objective
To use high performance liquid chromatography with a high sensitivity Metrohm‐Peak instrument for pulsed amperometric measurement of human serum glucosamine; a detection limit of 0.5 μmol/l at 1:10 serum dilution allowed measurement of low levels of glucosamine in human serum, which previously has not been possible.
Methods
Eighteen subjects with osteoarthritis were given 1500 mg of commercial glucosamine sulphate after an overnight fast, and serum was then obtained at baseline and every 15–30 minutes over 3 hours, and additionally, from two subjects at 5 and 8 hours. Urine samples were collected at baseline and 3 hours after ingestion from three subjects.
Results
Baseline glucosamine was below the detection limit of 0.5 μmol/l for all subjects, but after ingestion, glucosamine was detected in 17/18 subjects, beginning to rise at 30–45 minutes to a maximum at 90–180 minutes, with a range of 1.9–11.5 μmol/l (0.34–2 μg/ml).
Conclusion
This maximum concentration of 11.5 μmol/l has previously been shown to contribute less than 2% of the galactosamine incorporated into chondroitin sulphate in incubations of glucosamine with cultured human chondrocytes, and is a much lower concentration than the glucosamine concentrations claimed by other investigators to have various significant in vitro effects. This raises questions about current biological rationales for glucosamine use that were based on in vitro effects of glucosamine at much higher concentrations.
Keywords: osteoarthritis, glucosamine, human, serum, cartilage
Osteoarthritis may affect 6–12% of the adult population and up to one third of those over 65 years of age.1 Treatment is largely symptomatic, but it has been claimed that a number of ingested substances modify the disease. Among these, glucosamine supplements such as glucosamine chloride or glucosamine sulphate with or without chondroitin sulphate have been marketed widely. Large industry sponsored randomised clinical controlled trials and meta‐analyses have suggested moderate efficacy,2,3,4,5 but more recent independent investigations have been negative, inviting scepticism.6,7
Initially, stimulation of cartilage chondroitin sulphate formation was proposed as the mechanism of action.8,9 Subsequently, a variety of other mechanisms, affecting chondroitin sulphate or other substances of joints, have been proposed from in vitro experiments with cartilage explants or cultured chondrocytes, or both. These include protection or inhibition of degradation, and a current interest in immunosuppression and other anti‐inflammatory actions. All these mechanisms presuppose that a sufficient amount of glucosamine gets to the cartilage after oral ingestion. However, this would be limited by a first passage from the portal system through the liver before reaching the peripheral circulation.
We have previously measured the amount of exogenous radiolabelled glucosamine that is incorporated into galactosamine of chondroitin sulphate when cultures of mouse and human chondrocytes are incubated with varying levels of radiolabelled glucosamine.10,11 Exogenous glucosamine at 12 μmol/l was found to contribute <2% of the galactosamine incorporated into chondroitin by human chondrocytes, with 98% derived endogenously from glucose. Less than 3.5% was incorporated when glucosamine at 32 μmol/l was used, and less than 10% with 100 μmol/l. There have been no reports of measurable human serum glucosamine levels after oral ingestion. On the contrary, it has been reported12 that no measurable level (detection limit 10 μg/ml or 55 μmol/l) is found in the plasma of healthy volunteers after taking oral doses four times greater than the standard dose. We have now measured serum glucosamine at much lower levels by using high pressure liquid chromatography (HPLC) with an extremely sensitive detection device. Such measurements are critical in order to determine the relevance of the previous reports about mechanisms of action.
Patients and methods
Subjects
Eighteen adults (12 women, 6 men, aged 41–74 years, weight 42–132 kg, body mass index (BMI) 22–40 kg/m2) fulfilling the American College of Rheumatology criteria for hand, hip, or knee osteoarthritis were recruited from the Tufts‐New England Medical Center Rheumatology Clinic. We excluded people with diagnosed diabetes mellitus, chronic kidney or liver disease, acute illness such as infection or myocardial infarction, uncontrolled inflammatory, malignant, or endocrine disorder, current or expected use of corticosteroids, anaemia, and pregnancy. A fasting plasma glucose level was drawn at screening and had to be <6 mmol/l (110 mg/dl) for inclusion in the study. The study was approved by the Human Institutional Review Board of Tufts‐New England Medical Center and the Bedford Veteran's Affairs Hospital.
Subject preparation and ingestion
Subjects took all evening drugs before 8 pm and fasted after 10 pm, with no food, drugs, vitamins, or smoking until after the study visit was completed. They were also instructed to withhold any glucosamine or other nutritional supplements 3 days before the study visit. An intravenous catheter was inserted into an antecubital vein and kept open with normal saline. One 1500 mg purchased packet described as “crystalline glucosamine sulphate” (Rotta Pharmaceuticals), equal to 1175 mg of glucosamine plus 325 mg of sulphate, was mixed in 250 ml of tap water and immediately ingested. Analysis by anionic HPLC for sulphate, and amperometric HPLC for glucosamine, using Metrohm‐Peak units (see “Sample analysis” below) showed that each dose contained a molar ratio of glucosamine:sulphate of 2:1, but also had chloride with a molar ratio of glucosamine:chloride of 1:1. Amounts were consistent with the 1500 mg total.
Sample collection
Blood samples (3 ml) from each participant were obtained at time 0 before ingestion and then 15, 30, 45 minutes, and 1, 1.5, 2, 2.5, and 3 hours after ingestion using normal saline flushes between sampling. Two of the subjects had blood drawn additionally at 5 and 8 hours after ingestion. Blood was collected into tubes containing serum separator and allowed to clot at room temperature for 30 minutes up to 2 hours. Serum was obtained by centrifugation at 1500 rpm at 4°C for 15 minutes, and was stored at −70° to −80°C until analysed. Urine samples were collected from three subjects at times 0 and 3 hours. After measurement of volume, 10 ml was stored at −20°C until analysed.
Sample analysis
Water was purified (>18 MOhm/cm) in a Simplicity 185 water treatment system (Millipore, Bedford, MA, USA), and analysis showed no glucosamine. Chemical grade D glucosamine hydrochloride was obtained from Sigma Chemical Co (St Louis, MO, USA), and chemical grade 5 N sodium hydroxide solution from VWR (Bridgeport, NJ, USA). To measure glucosamine, 200 μl of serum was diluted 1:10 by addition of 1.8 ml of water for automated analysis at 35°C by a Metrohm‐Peak 817 Bioscan (Metrohm‐Peak, Inc, Houston, TX, USA) by pulsed amperometric detection using a 20 μl loop from an ion exchange Metrosep Carb 1 (250×4.5 mm) column. The column was eluted at a flow rate of 1.0 ml/min with 50 mmol/l sodium hydroxide that had been degassed for 10 minutes and open to the atmosphere for 1 hour. Background conductivity was approximately 850 nA.
The instrument as used for these experiments has a detection limit of 0.05 μmol/l with an accuracy up to 0.2 μmol/l of +/−50–25% for automated analysis of glucosamine in 2 ml of water. From 0.2 to 0.5 μmol/l accuracy is ±25–10%, from 0.5 to 1.0 μmol/l accuracy ⩾±10%, and above 1.0 μmol/l accuracy is ⩾±5%. Thus a 1:10 dilution of serum provides a measurable detection limit of glucosamine at 0.5 μmol/l and a quantification limit of ±10% to 5% at 1 to 10 μmol/l. Samples were placed with 1, 2, and 6 μmol/l standards at the beginning, middle, and end of each run. Each sample was introduced automatically for 20 minutes' chromatography, so that as many as 60 samples plus nine standards could be measured within 24 hours. Determinations were made each day with the first set of standards for each run, and the standards during the middle and at the end of the run were compared with the standards at the beginning. The intraday precision was ±2–4%.
Glucosamine concentrations were found to be stable in the serum separator tubes at room temperature for 2 hours or more, and stable in the cryovial tubes at −80°C for a month or more. When glucosamine was added to serum samples, total glucosamine was found to be additive, demonstrating that there was no loss by adsorption or interference by serum components.
Statistical analysis
Absolute values were reported and some expressed as means (SD). Significance between means was assessed by Student's t test (p<0.05).
Results
Table 1 gives data on subjects' age, sex, weight, BMI, location of osteoarthritis (hand, hip, knee), and previous glucosamine usage (seven had been using glucosamine before the study).
Table 1 Serum glucosamine levels and characterisation of the 18 participants.
| Patient No | Age | Weight | BMI* | OA | GLcN use | Maximum | |
|---|---|---|---|---|---|---|---|
| (years) | (kg) | GlcN (μmol/l) | Time (h) | ||||
| Women | |||||||
| 3 | 70 | 96 | 32 | Knee | No | 3.8 | 3 |
| 4 | 74 | 74 | 27 | Hand, knee | Yes | 3.2 | 2 |
| 5 | 56 | 97 | 40 | Knee | No | 4.3 | 3 |
| 6 | 41 | 88 | 34 | Hip | No | 5.9 | 3 |
| 7 | 61 | 42 | 22 | Hand, knee | Yes | 5.8 | 1.5 |
| 11 | 53 | 70 | 26 | Hand, knee | No | 0 | 0 |
| 13 | 63 | 60 | 23 | Hand, hip | Yes | 11.5 | 3 |
| 14 | 49 | 74 | 31 | Hand | Yes | 7.1 | 3 |
| 18 | 59 | 65 | 27 | Hand | No | 3.4 | 2.5 |
| 21 | 52 | 97 | 36 | Knee | No | 3.2 | 3 |
| 22 | 67 | 72 | 26 | Hand, knee | No | 2.6 | 2 |
| 23 | 70 | 118 | 38 | Knee | No | 6.4 | 2.5 |
| Men | |||||||
| 2 | 56 | 95 | 33 | Hand, knee | No | 1.9 | 2.5 |
| 12 | 57 | 107 | 31 | Hand, knee | Yes | 8.3 | 3 |
| 15 | 70 | 84 | 28 | Knee | Yes | 6.1 | 3 |
| 17 | 69 | 95 | 31 | Hand, knee | Yes | 3.9 | 2 |
| 19 | 71 | 73 | 25 | Knee | No | 4.3 | 1.5 |
| 20 | 46 | 132 | 40 | Hand, hip, knee | No | 3.9 | 2 |
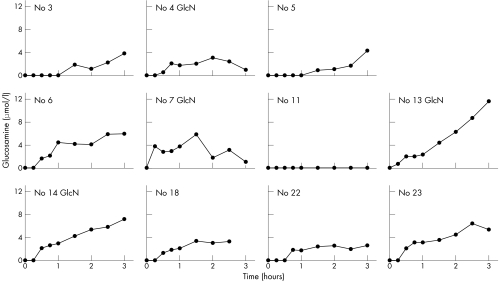
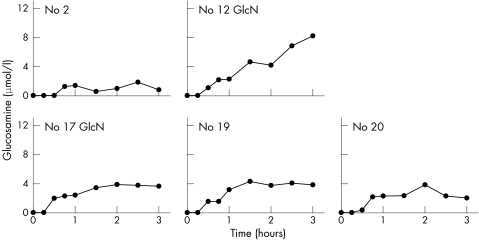
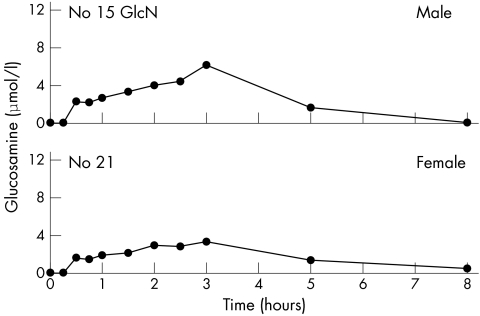
Baseline glucosamine was below the detection limit (0.5 μmol/l for the 1:10 dilution) for all 18 subjects, and was detected after ingestion of the glucosamine in 17/18 subjects (figs 1, 2, and 3). One subject (No 11) had no measurable glucosamine at any time. Mean (SD) maximum levels were the same for women (4.8 (2.9) μmol/l) (fig 1) and men (4.7 (2.2) μmol/l) (fig 2). The overall mean (SD) maximum for all 18 subjects was 4.8 (2.6) μmol/l with a range of 0–11.5 μmol/l. Maximum levels were reached by 1.5–2.5 hours for nine subjects with a range of 1.9 to 6.4 μmol/l, mean (SD) 3.9 (1.4) μmol/l, but not until 3 hours for eight subjects with a range of 3.2 to 11.5 μmol/l, mean (SD) 6.3 (2.7) μmol/l. Glucosamine levels for those who reached maximum at 3 hours were 1.6 times higher than for those who reached maximum in under 3 hours (p = 0.05). However, no 4 hour samples were taken, so it is possible that the maxima could have been somewhat higher and later for some of these. Two of the subjects, one male and one female, had 5 and 8 hour blood levels measured (fig 3) with a considerable decrease in level by 5 hours, and return to baseline by 8 hours.
Figure 1 Serum glucosamine concentrations at time intervals from 0 to 3 hours after ingestion of 1500 mg of glucosamine sulphate by 11 female participants. GlcN = participant taking glucosamine before this study.
Figure 2 Serum glucosamine concentrations at time intervals from 0 to 3 hours after ingestion of 1500 mg of glucosamine sulphate by five male participants. GlcN = participant taking glucosamine before this study.
Figure 3 Serum glucosamine concentrations at time intervals from 0 to 8 hours after ingestion of 1500 mg of glucosamine sulphate by the single male participant and the single female participant who agreed to have 5 and 8 hour blood samples drawn. GlcN = participant taking glucosamine before this study.
Table 1 lists the maximum levels, and time to reach maximum, for each subject after ingestion of the 1500 mg glucosamine sulphate; no correlation with age, weight, and BMI was seen. However, subjects who had previously taken glucosamine products tended to have higher glucosamine levels. Glucosamine levels of all seven participants who had been using glucosamine began to rise by 15 or 30 minutes. In contrast, two of those who had not been taking glucosamine showed no rise until 45 minutes, two at 1.5 hours, and for one no rise occurred. The seven who had been taking glucosamine had maximum levels from 3.2 to 11.5 μmol/l, mean (SD) 6.6 (2.8) μmol/l in comparison (p = 0.03) with the maximum levels from 0 to 6.4 μmol/l, mean (SD) 3.6 (1.78) μmol/l for the 11 participants who had not been taking glucosamine.
Total glucosamine measured in the 3 hour urine of subject No 15 was 5.3 mg, of subject No 21 was 2.5 mg, and of subject No 23 was 7.1 mg (<1% of the ingested glucosamine).
Discussion
The amino sugars, glucosamine and galactosamine, are prominent components of glycoconjugates (glycosaminoglycans, glycoproteins, and glycolipids). Neither of these hexosamines are present in any diet except as part of glycoconjugates, and neither have been described in the blood of any animals. Thus their presence (almost entirely N‐acetylated or N‐sulphated) would appear to be by intracellular production from glucose. Nevertheless, radiolabelled glucosamine can be incorporated experimentally into glycoconjugates and has been a convenient radiolabelled substance for use with tissue cultures to study the formation of glycoconjugates. When [3H]glucosamine together with [35S]sulphate are incubated with cultured cells, it is possible to determine the amount of chondroitin sulphate formed and calculate the percentage of [3H]galactosamine in chondroitin produced from the exogenous [3H]glucosamine relative to the amount produced from endogenous glucose.13 We previously used this technique to demonstrate that cells from multiple tissues including mouse chondrocytes10 and human chondrocytes11 produced more than 99% of the galactosamine of chondroitin sulphate from endogenous glucose when incubated with low concentrations of [3H]glucosamine. With glucosamine at 12 μmol/l, <2% of galactosamine in chondroitin was produced from the added exogenous radiolabelled glucosamine. Thus it is clear from our results that ingestion of 1500 mg of glucosamine sulphate would not provide nearly enough glucosamine to override the endogenous production from glucose.
An alternative rationale might be to achieve a sustained lower level with multiple doses over a long period of time, which could then accumulate to a concentration that might have an effect. However, this would necessitate an availability and elimination times greater than the few hours observed in our experiments. It is of note that serum levels after intravenous administration of [14C]glucosamine (50 μCi added to 400 mg of glucosamine sulphate) to two human subjects were reported14 to peak at 6 minutes and be reduced by 99.8% by 5 hours, indicating that a useful “steady state” by repeated dosage is not feasible without a great increase in dosage. We used a single dose of glucosamine because of this information about its disappearance from human plasma by 5 hours, and also because of a study with dogs that found no significant differences between single dose and multiple dose pharmacokinetics.15
Previously, others12 using a technique with a 55 μmol/l detection limit did not find glucosamine in human plasma before or after 6 g of glucosamine sulphate ingestion, an amount equivalent to four times a standard human dose. Furthermore, little measurable free [14C]glucosamine was demonstrated in the plasma of subjects who ingested the radiolabelled glucosamine.14 This indicates that the ingested glucosamine, being transported by the portal system, is taken up by the liver to be incorporated into glycoconjugates or otherwise metabolised, allowing little if any glucosamine to reach the peripheral circulation. Experiments with rats given 350 mg/kg (17.5 times the human dose) of glucosamine demonstrated maximum glucosamine levels that reached only 100 μmol/l,16 and experiments with dogs given 167 mg/kg (eight times the human dose) reached only 50 μmol/l.15 This suggests that amounts in humans would be considerably lower than the 55 μmol/l detection limit for human experiments after ingestion of 20 mg/kg (1500 mg glucosamine sulphate for a 75 kg person).
Considerable variation in the rise of glucosamine was seen. Most subjects had detectable glucosamine at 30–45 minutes, even as early as 15 minutes, but several only showed a rise at 1.5 hours. Some subjects had maximum concentrations at 1.5–2.5 hours, and others at 3 hours. It is likely that glucosamine levels of some subjects continued to rise beyond the 3 hour period. However, consistent with the report concerning intravenous administration of [14C]glucosamine,14 serum values declined markedly by 5 hours and to baseline by 8 hours in the two subjects who had blood drawn at these time intervals.
There was no correlation of glucosamine levels or time to maximum level with sex, age, weight, or BMI, but the difference found between those who had previously used glucosamine and those who had not, was significant. Those who had previously used glucosamine had an earlier time for detection of glucosamine, a later time to maximum, and higher maximum levels, despite baseline measurements that showed no glucosamine in serum. This suggests that there might be some long term effect of glucosamine usage on the way it is subsequently handled by the liver.
Minor glucosamine stimulation of chondroitin synthesis in cartilage explants and/or chondrocytes has been reported by others, but generally with concentrations of glucosamine 10‐ to a 1000‐fold greater than the serum concentrations we have found. A 20–50% increment in chondroitin sulphate formation was reported when chondrocytes were cultured for 8 or 12 days with glucosamine at 50–500 μmol/l.9 In contrast we found no significant increment when chondrocytes were cultured for 5 hours with glucosamine concentrations below 1 mmol/l, and significantly decreased chondroitin sulphate formation at 1 mmol/l and 10 mmol/l concentrations.10 Incorporation of [13C]glucosamine into chondroitin of cartilage explants was reported to be preferential to incorporation of [13C]glucose, but the 2.5 mmol/l concentration17 was more than 200‐fold higher than the maximum we found in serum. Glucosamine has also been reported to stimulate chondroitin synthesis by cultured chondrocytes or explants after enzymatic, pressure, and heat stressing cartilage explants, but these effects were found at 167–670 μmol/l,18 15–60 fold higher. Another report has indicated some increase in aggrecan by cultured chondrocytes at levels as low as 1 μmol/l with a twofold increase at 100 μmol/l described as “representative” of “six of the 10 separate patients studied”.19 They also reported an increase in the small amounts of cell associated chondroitin, but no increase in the much larger amounts of media chondroitin, when 100–400 μmol/l glucosamine was incubated for several days with chondrocytes.19 There was no indication that replicates rather than single analyses were examined at any glucosamine levels.
Other reports included inhibition of aggrecan degradation at glucosamine concentrations above 2 mmol/l,20 or with 5 mmol/l concentrations,21 or reduction in cartilage degradation at 1.4–140 mmol/l glucosamine,22 protein synthesis and various degradative enzymes in chondrocytes at 100–500 μmol/l,23 and description of a modest decrease in matrix metalloproteinase activity with 50–400 μmol/l glucosamine in 3 day cultures of chondrocytes stimulated by interleukin (IL) 1β.19 Glucosamine at 5.5–25 mmol/l concentrations was reported to be effective in decreasing IL1β suppression of proteoglycan synthesis,24 as well as other IL1β effects at 25 mmol/l glucosamine.25 Inhibition of IL1β‐induced NFκB activation was reported with glucosamine at 5.5 mmol/l,26 but there was little or no effect at 550 or 55 μmol/l. Glucosamine at 28 μmol/l (5 μg/ml) was recently reported to have an effect on gene down regulation by IL1 in cartilage explants over a 6–48 hour period.27 Glucosamine (0.1–1 mmol/l) inhibition of neutrophil functions in osteoarthritis has been reported,28 and 2.5–10 mmol/l glucosamine has been used to decrease activation of T lymphocytes and to decrease mixed leucocyte reactivity, with claims of immunosuppression as a role for glucosamine.29
We conclude that insignificant, trace amounts of glucosamine enter human serum after ingestion of a standard oral dose of glucosamine sulphate (1500 mg), far below any amount that might contribute directly to chondroitin synthesis. Moreover, this level is limited to a few hours after ingestion, with no establishment of any substantial lasting concentration. It is far below most of the concentrations used in in vitro cell or tissue culture incubations by others, usually for days or weeks, in proposing mechanisms to protect chondrocytes, inhibit chondroitin degradation, diminish inflammation, or provide immunosuppression in articular cartilage. Unless consistent actions on cartilage can be demonstrated at the low concentrations and limiting time periods that we found, claims of a meaningful direct effect on cartilage or chondrocytes are questionable.
Acknowledgements
Support was provided by (a) the Medical Research Service of the Department of Veterans Affairs; (b) a grant to JES by the Arthritis Foundation; (c) the Tufts University General Clinical Research Center, funded by the Division of Research Resources of the NIH under grant No MO1‐RR00054, US Department of Health and Human Services, National Institutes of Health and Agency for Healthcare Research and Quality; (d) a Ruth L. Kirschstein National Research Service Award (T‐32).
Abbreviations
BMI - body mass index
HPLC - high performance liquid chromatography
IL - interleukin
Footnotes
Conflict: The authors have no competing interests
References
- 1.Mili F, Helmick C G, Zack M M. Prevalence of arthritis: analysis of data from the US Behavioral Risk Factor Surveillance System, 1996–99. J Rheumatol 2002291981–1988. [PubMed] [Google Scholar]
- 2.Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati L. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3‐year, randomized, placebo‐controlled, double‐blind study. Arch Intern Med 20021622113–2123. [DOI] [PubMed] [Google Scholar]
- 3.Reginster J Y, Deroisy R, Rovati L C, Lee R L, Lejeune E, Bruyere O.et al Long‐term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo‐controlled clinical trial. Lancet 2001357251–256. [DOI] [PubMed] [Google Scholar]
- 4.McAlindon T E, LaValley M P, Gulin J P, Felson D T. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta‐analysis. JAMA 20002831469–1475. [DOI] [PubMed] [Google Scholar]
- 5.Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin Y, Reginster J Y. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta‐analysis. Arch Intern Med 20031631514–1522. [DOI] [PubMed] [Google Scholar]
- 6.Rindone J P, Hiller D, Collacott E, Nordhaugen N, Arriola G. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med 200017291–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes R, Carr A. A randomized, double‐blind, placebo‐controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology (Oxford) 200241279–284. [DOI] [PubMed] [Google Scholar]
- 8.Setnikar I, Pacini M A, Revel L. Antiarthritic effects of glucosamine sulfate studied in animal models. Arzneimittelforschung 199141542–545. [PubMed] [Google Scholar]
- 9.Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage 19986427–433. [DOI] [PubMed] [Google Scholar]
- 10.Mroz P J, Silbert J E. Effects of [3H]glucosamine concentration on [3H]chondroitin sulphate formation by cultured chondrocytes. Biochem J 2003376511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mroz P J, Silbert J E. Use of [3H]glucosamine and [35S]sulfate with cultured human chondrocytes to determine effects of glucosamine concentration on formation of [3H]chondroitin [35S]sulfate. Arthritis Rheum 2004503574–3579. [DOI] [PubMed] [Google Scholar]
- 12.Setnikar I, Giacchetti G, Zanolo G. Pharmacokinetics of glucosamine in the dog and man. Arzneimittelforschung 198636729–735. [PubMed] [Google Scholar]
- 13.Silbert C K, Palmer M E, Humphries D E, Silbert J E. Production of [3H]hexosamine‐labeled proteoglycans by cultures of normal and diabetic skin fibroblasts: dilution of exogenous [3H]glucosamine by endogenous hexosamine from glucose and other sources. Arch Biochem Biophys 1989268393–397. [DOI] [PubMed] [Google Scholar]
- 14.Setnikar I, Palumbo R, Canali S, Zanolo G. Pharmacokinetics of glucosamine in man. Arzneimittelforschung 1993431109–1113. [PubMed] [Google Scholar]
- 15.Adebowale A, Du J, Liang Z, Leslie J L, Eddington N D. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm Drug Dispos 200223217–225. [DOI] [PubMed] [Google Scholar]
- 16.Aghazadeh‐Habashi A, Sattari S, Pasutto F, Jamali F. Single dose pharmacokinetics and bioavailability of glucosamine in the rat. J Pharm Pharm Sci 20025181–184. [PubMed] [Google Scholar]
- 17.Noyszewski E A, Wroblewski K, Dodge G R, Kudchodkar S, Beers J, Sarma A V S.et al Preferential incorporation of glucosamine into the galactosamine moieties of chondroitin sulfate in articular cartilage explants. Arthritis Rheum 2001441089–1095. [DOI] [PubMed] [Google Scholar]
- 18.Lippiello L. Glucosamine and chondroitin sulfate: biological response modifiers of chondrocytes under simulated conditions of joint stress. Osteoarthritis Cartilage 200311335–342. [DOI] [PubMed] [Google Scholar]
- 19.Dodge G R, Jimenez S A. Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase‐3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarthritis Cartilage 200311424–432. [DOI] [PubMed] [Google Scholar]
- 20.Sandy J D, Gamett D, Thompson V, Verscharen C. Chondrocyte‐mediated catabolism of aggrecan: aggrecanase‐dependent cleavage induced by interleukin‐1 or retinoic acid can be inhibited by glucosamine. Biochm J 199833559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilic M Z, Martinac B, Handley C J. Effects of long‐term exposure to glucosamine and mannosamine on aggrecan degradation in articular cartilage. Osteoarthritis Cartilage 200311613–622. [DOI] [PubMed] [Google Scholar]
- 22.Fenton J I, Chlebek‐Brown K A, Peters T L, Caron J P, Orth M W. Glucosamine HCl reduces equine articular cartilage degradation in explant culture. Osteoarthritis Cartilage 20008258–265. [DOI] [PubMed] [Google Scholar]
- 23.Piperno M, Reboul P, Hellio Le Graverand M P, Peschard M J, Annefeld M.et al Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthritis Cartilage 20008207–212. [DOI] [PubMed] [Google Scholar]
- 24.Gouze J ‐ N, Bordji K, Gulberti S, Terlain B, Netter P, Magdalou J.et al Interleukin‐1[beta] down‐regulates the expression of glucuronosyltransferase I, a key enzyme priming glycosaminoglycan biosynthesis: influence of glucosamine on interleukin‐1[beta]‐mediated effects in rat chondrocytes. Arthritis Rheum 200144351–360. [DOI] [PubMed] [Google Scholar]
- 25.Gouze J N, Bianchi A, Becuwe P, Dauca M, Netter P, Magdalou J.et al Glucosamine modulates IL‐1‐induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF‐κB pathway. FEBS Lett 2002510166–170. [DOI] [PubMed] [Google Scholar]
- 26.Largo R, Alvarez‐Soria M A, Diez‐Ortego I, Calvo E, Sanchez‐Pernaute O, Egido J.et al Glucosamine inhibits IL‐1β‐induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 200311290–298. [DOI] [PubMed] [Google Scholar]
- 27.Chan P S, Caron J P, Rosa G J M, Orth M W. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants. Osteoarthritis Cartilage 200513387–394. [DOI] [PubMed] [Google Scholar]
- 28.Hua J, Sakamoto K, Nagaoka I. Inhibitory actions of glucosamine, a therapeutic agent for osteoarthritis, on the functions of neutrophils. J Leucocyte Biol 200271632–640. [PubMed] [Google Scholar]
- 29.Ma L, Rudert W A, Harnaha J, Wright M, Machen J, Lakomy R.et al Immunosuppressive effects of glucosamine. J Biol Chem 200227739343–39349. [DOI] [PubMed] [Google Scholar]