Abstract
Background
The synergistic degradation of cartilage by oncostatin M (OSM) in combination with either interleukin 1 (IL1) or tumour necrosis factor α (TNFα) has been previously demonstrated using bovine nasal cartilage (BNC).
Objectives
(a) To investigate if human nasal cartilage (HNC) responds in the same way as BNC to these cytokine combinations, particularly in collagen degradation. (b) To compare the response of human nasal and articular cartilages.
Methods
Collagen release was assessed by measuring the hydroxyproline content of culture supernatants and proteoglycan release by the dimethylmethylene blue assay. Matrix metalloproteinase (MMP)‐1, MMP‐13, and tissue inhibitor of metalloproteinase 1 release were measured by specific enzyme linked immunosorbent assays (ELISAs), and collagenolytic activity was measured by a bioassay using radiolabelled collagen.
Results
OSM in combination with either IL1 or TNFα acted synergistically to induce collagenolysis from HNC, with a maximum of 79% collagen release. This degradation strongly correlated with MMP‐1 and MMP‐13 levels and collagenolytic activity.
Conclusion
Collagen release from human cartilage is marked and implicates both MMP‐1 and MMP‐13 in the synergistic degradation of human cartilage by OSM in combination with either IL1 or TNFα. HNC responds in the same way as BNC, thus validating the bovine cartilage degradation assay as a model relevant to human disease.
Keywords: collagenase, human cartilage degradation, cytokines, matrix metalloproteinase‐1, matrix metalloproteinase‐13
Cartilage is a specialised tissue, mainly composed of collagen fibrils and proteoglycans. Type II collagen fibrils form an interwoven network providing tensile strength, while the proteoglycans embedded within this matrix draw water into the tissue allowing cartilage to resist compression. The destruction of cartilage involves the loss of both these components. Proteoglycans have a high turnover rate and are readily released from cartilage in response to proinflammatory cytokines such as interleukin 1 (IL1)1 and tumour necrosis factor α (TNFα).2 However, in vivo, when the stimulus is removed, proteoglycans are quickly resynthesised3 by chondrocytes within the collagen network. In contrast, type II collagen has a low turnover rate and when collagen degradation does occur the structural integrity of the tissue is irreversibly lost.4 Although proteoglycan degradation is more commonly studied, it is the degradation of collagen that is a key control point in cartilage resorption. However, finding a system that allows the reproducible study of collagen release has proved difficult.
Chondrocytes within the cartilage maintain a balance between the synthesis and degradation of matrix components. A major characteristic of rheumatoid arthritis (RA) and osteoarthritis (OA) is the progressive loss of the cartilage extracellular matrix5 owing to raised levels of cytokines and growth factors6 that elicit the production of proteolytic enzymes, including the matrix metalloproteinases (MMPs). Of these, the collagenases have been most strongly implicated7 because they specifically cleave collagen, and the fragments produced are present in diseased joints.8 Tissue inhibitors of metalloproteinases (TIMPs), a family of endogenous inhibitors, specifically inhibit active MMPs by forming a 1:1 stoichiometric complex.9
Previous studies investigating the mechanisms of collagen breakdown have been hampered by the difficulty of reproducibly stimulating collagen release. We have previously used bovine nasal cartilage (BNC) as a reliable model for studying collagenolysis, showing that IL1 or TNFα in combination with oncostatin M (OSM) promote a marked synergistic loss of collagen (>90%) accompanied by the induction of collagenolytic activity.10,11 These results can be reproduced in bovine articular cartilage, although the release of collagen is sometimes less extensive than in nasal cartilage. The study of collagenolysis from human articular cartilage (HAC) is hampered by the fact that HAC is particularly resistant to degradation for reasons that are not entirely clear. Only 50% of HAC samples treated with IL1 and OSM respond to cytokine stimulation, with low collagen release (<15%).12 Interestingly, when the HAC samples with low collagen release are examined, all samples treated with combinations of IL1 and OSM responded by releasing proteoglycan and by up regulating MMP‐1.
For the study of human cartilage collagenolysis there is a need for a source of human cartilage that will reproducibly respond to treatment by releasing significant levels of collagen. This would allow the testing of compounds that inhibit release and so could identify new agents that might be therapeutically useful.
Human nasal cartilage (HNC) can be obtained from consenting young and healthy adults who undergo rhinoplasty. The use of HNC for the study of human collagenolysis may overcome the inherent problems with the use of HAC from joint replacement surgery. In this study we have examined whether HNC might be a suitable alternative for the study of cytokine‐induced collagenolysis and compared its response to those of BNC and HAC.
Materials and methods
Reagents
Human IL1α was a gift from GlaxoSmithKline (Greenford, UK). Human OSM was a gift from Professor JK Heath (University of Birmingham, UK). Recombinant human TNFα was obtained from R&D Systems (Abingdon, UK). l‐Ascorbic acid was purchased from Wako Pure Chemical Industries, Ltd (Japan). All other chemicals were commercially available analytical grade reagents obtained from Fisher (Loughborough, UK).
HNC explant cultures
The use of HNC was approved by the Southmead Local Research Ethics Committee. HNC removed from six healthy patients (aged 25–60 years) during rhinoplasty was cut into small pieces (∼2 mm3); all patients gave their consent. Each piece was placed into a well of a 96 well microtitre plate (Corning/Costar, Netherlands) and cultured in 200 μl/well of Dulbecco's modified Eagle medium supplemented with glutamine (2 mmol/l), kanamycin (100 μg/ml), streptomycin (200 μg/ml), penicillin (200 IU/ml), and nystatin (40 U/ml). Serum was excluded from stimulated explant cultures because it can markedly alter cell metabolism in the absence of exogenous cytokine(s).13 Because this represents a model of cartilage breakdown, we avoided using chondroprotective agents such as insulin‐like growth factor‐1,14 which are known to be present in serum and which can replace it.15 Cultures were stimulated with IL1 (1 and 5 ng/ml), OSM (10 and 50 ng/ml), TNFα (10 ng/ml), or combinations thereof. The cytokine concentrations used have previously been shown to promote synergistic collagen release from BNC.11,12 For each patient tissue sample 4–6 replicate explant cultures were set up for each control and cytokine treatment, depending on the amount of tissue available. For one patient there was insufficient HNC sample to test the effect of IL1+OSM at 5/50 ng/ml and only IL1+OSM at 1/10 ng/ml was tested in this sample. The media were harvested and replaced with treatments (identical to those at day 0) at days 7, 14, or 21 days at 37°C in a humidified atmosphere of 95% air and 5% CO2. The remaining cartilage was papain digested.12 Harvested media and papain digests were stored at −20°C until assayed.
HAC explant cultures
The use of HAC was approved by the Newcastle and North Tyneside Local Research Ethics Committee. The HAC, macroscopically normal, was from 57 consenting patients with OA (aged >60 years) who underwent joint replacement surgery. The explant cultures were maintained under the same conditions as the HNC cultures except that they contained three cartilage pieces (∼2 mm3) cultured in 600 μl of medium per well of a 24 well plate.12 For each patient tissue sample, four replicate explant cultures were set up for each cytokine treatment.
Proteoglycan degradation
Media samples and papain digests were assayed for sulphated glycosaminoglycans (as a measure of proteoglycan release) using the 1,9‐dimethylmethylene blue dye binding assay.16,17 Results were expressed as the percentage of total proteoglycan released into the medium by day 14 for HNC (explants from four patients) and by day 21 for HAC (explants from 43 patients) because release was slower in HAC.
Collagen degradation
Hydroxyproline release (a measure of collagen degradation) was assayed in media samples and papain digests as described previously.17,18 Results were expressed as the percentage of total collagen released into the medium by day 14 (explants from five patients) and day 21 (explant cultures from two patients were maintained longer to demonstrate larger significant differences between treatments) for HNC and by day 21 for HAC (explants from 55 patients).
Enzyme assays
MMP‐1 (both active and pro‐forms, and MMP‐1 bound to TIMP‐1) and TIMP‐1 (total) were measured by specific enzyme linked immunosorbent assays (ELISAs).19,20
MMP‐13 was measured by sandwich ELISA using a pair of monoclonal anti‐MMP‐13 IgG antibodies (No 46 and No 61), a gift from Daiichi Fine Chemical Co, Ltd (Japan). Human recombinant pro‐MMP‐13 was used for the standard. The ELISA followed a previously described protocol20 using anti‐MMP‐13 IgG at 1.25 μg/ml (No 61) and biotin labelled anti‐MMP‐13 IgG (No 46) at 0.125 μg/ml. A signal was developed using horseradish peroxidase linked streptavidin (Dako, Denmark) with ο‐phenylenediamine substrate, and plates read at 490 nm. The linear range of the assay was 0.6–20 ng/ml. The assay was specific for both the pro‐ and active forms of MMP‐13.
Collagenase activity was determined by the 3H‐acetylated collagen diffuse fibril assay.21 One unit of collagenase activity degraded 1 μg of collagen per minute at 37°C.
Statistical analysis
Data are shown as the mean (SD) for multiple experiments for each cytokine treatment. Statistical significance was assessed using analysis of variance with post hoc Bonferroni's comparison test using SPSS version 11.0 (SPSS, Chicago, IL). Values of p<0.05 were considered significant. Spearman's rank correlation was used to analyse the relationships between collagen release, collagenase activity, and MMP levels.
Results
Effect of IL1+OSM on proteoglycan and collagen release from HNC
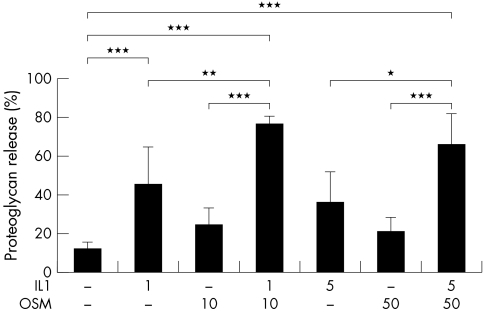
HNC was treated with IL1+OSM at high and low concentration combinations. Significant and synergistic cumulative proteoglycan release from HNC was reproducibly detected in four separate experiments performed with explants from four patients when stimulated for 14 days with IL1+OSM. Both concentrations of IL1+OSM (1/10 ng/ml, 5/50 ng/ml) stimulated proteoglycan release (mean (SD) 62.4 (16.9)%, 69.2 (4.4)%, respectively) that was significantly raised (p<0.001) compared with control (12.5 (2.0)%) or stimulation with either cytokine alone (fig 1).
Figure 1 Significant proteoglycan release from human cartilage induced by IL1 in combination with OSM. HNC was stimulated with IL1 (1 or 5 ng/ml) and/or OSM (10 or 50 ng/ml) in serum‐free medium for 14 days with serum‐free medium only as control. Day 7 media were collected and replaced with identical test reagents. Proteoglycan release was assayed as described in “Materials and methods”. Cumulative proteoglycan release by day 14 is expressed as a percentage of the total. Each bar is the mean (SD) of 4–6 explant cultures for each treatment. The response shown is reproducible and representative of experiments performed with explants from four patients. *p<0.05, **p<0.01, ***p<0.001.
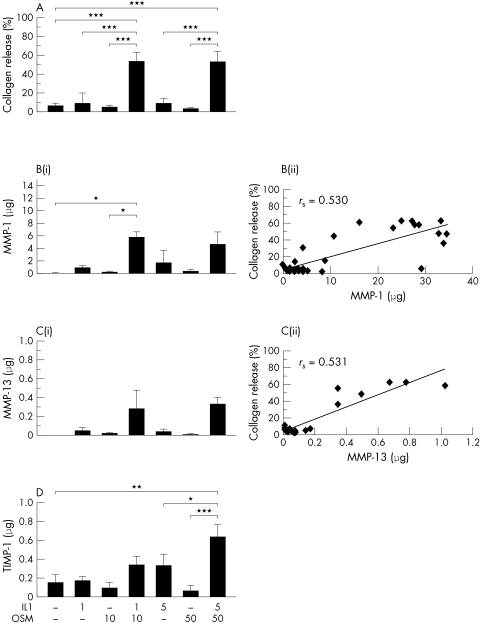
A synergistic cumulative collagen release was also seen with IL1+OSM (1/10 ng/ml, 26.4 (13.6)%; 5/50 ng/ml, 25.1 (8.0)%; control, 2.5 (0.8)%) from HNC that was significantly increased compared with control (p<0.005), and this was reproducibly detected in five separate experiments performed with explants from five patients when stimulated for 14 days. However, in two experiments performed with explants from two patients (six replicates each) extended to 21 days, IL1+OSM (1/10 ng/ml) stimulated further collagen release (mean 59.6%); again, no further increase above this was seen for higher cytokine concentrations. Thus, collagen release mediated by this cytokine combination was significantly higher than control (mean 6.2%; p<0.001) or stimulation with either cytokine alone (fig 2A).
Figure 2 Significant human cartilage collagen release and increased MMP‐1, MMP‐13, and TIMP‐1 levels are induced by IL1 in combination with OSM. HNC was stimulated with IL1 (1 or 5 ng/ml) and/or OSM (10 or 50 ng/ml) in serum‐free medium for 21 days with serum‐free medium only as control. Day 7 and 14 media were collected and replaced with identical test reagents. Collagen release and MMP‐1, MMP‐13, and TIMP‐1 levels were assayed as described in “Materials and methods”. (A) Cumulative collagen release by day 21 is expressed as a percentage of the total. (Bi) Cumulative MMP‐1 levels released by day 21. (Bii) Correlation between cumulative MMP‐1 and cumulative collagen release by day 21. (Ci) Cumulative MMP‐13 levels released by day 21. (Cii) Correlation between cumulative MMP‐13 and cumulative collagen release by day 21. (D) Cumulative TIMP‐1 levels released by day 21. Each bar is the mean (SD) for 4–6 explant cultures for each treatment. The response shown is reproducible and representative of experiments performed with explants from two patients. *p<0.05, **p<0.01, ***p<0.001. rs = Spearman's rank correlation coefficient.
Cumulative MMP‐1 levels were significantly higher (p<0.05) than control for those explants stimulated with IL1+OSM (fig 2B(i)). The highest mean MMP‐1 level of 4.5 μg was detected in the low concentration IL1+OSM stimulated explants. Cumulative MMP‐13 levels were also increased compared with control in the IL1+OSM stimulated explants, but these differences did not reach significance (fig 2C(i)). The highest mean MMP‐13 level of 0.3 μg was detected in the high concentration IL1+OSM stimulated explants. However, MMP levels in the high IL1+OSM combination were not significantly higher than in the low concentration combination. MMP‐1 levels were at least 10 times greater than MMP‐13 levels. Total collagen release correlated with levels of MMP‐1 (rs = 0.530, p<0.005) and MMP‐13 (rs = 0.531, p<0.05) (figs 2B(ii) and 2C(ii)).
Cumulative TIMP‐1 levels were raised for HNC explant cultures stimulated with IL1 (5 ng/ml) alone and IL1+OSM at both concentrations compared with control. However, this difference was only significant when the high concentration of IL1+OSM was used for stimulation (fig 2D).
When explant cultures were assayed for collagenase activity against a collagen substrate no collagenase activity was detected in the control samples, and ⩽0.3 U/ml was measured in the OSM stimulated samples. IL1 stimulated samples had 1–1.6 U/ml of collagenase activity while samples stimulated with IL1+OSM (at both concentrations) had 2.6–3.1 U/ml of collagenase activity (data not shown). Collagenase activity significantly correlated with collagen release (rs = 0.953, p<0.001).
Effect of TNFα+OSM on proteoglycan and collagen release from HNC
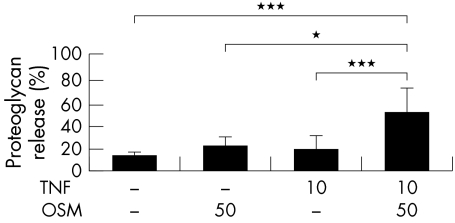
A synergistic cumulative proteoglycan release from HNC was reproducibly detected in three separate experiments performed with explants from three patients when stimulated for 14 days with the TNFα+OSM combination (10/50 ng/ml, mean (SD) 48.0 (1.3)%). This cytokine combination also stimulated proteoglycan release that was significantly raised (p<0.001) compared with control (12.5 (2.0)%) or stimulation with either cytokine alone (fig 3).
Figure 3 Significant proteoglycan release from human cartilage induced by TNFα (in combination with OSM). HNC was stimulated with TNFα (10 ng/ml) and/or OSM (50 ng/ml) in serum‐free medium for 14 days, with serum‐free medium only as control. Day 7 media were collected and replaced with identical test reagents. Proteoglycan release was assayed as described in “Materials and methods”. Cumulative proteoglycan release by day 14 is expressed as a percentage of the total. Each bar is the mean (SD) for 4–6 explant cultures for each treatment. The response shown is reproducible and representative of experiments performed with explants from three patients. *p<0.05, ***p<0.001.
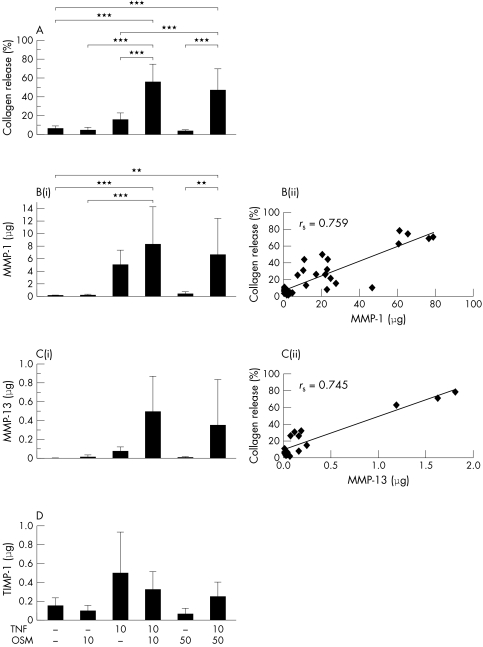
When TNFα and OSM were combined a synergistic cumulative release of collagen from HNC was also reproducibly detected at day 14 (10/50 ng/ml; mean (SD) 29.7 (12.2)%) and significantly increased compared with control (2.5 (0.8)%; p<0.005) in five separate experiments performed with explants from five patients. Two experiments performed with explants from two patients, extended to day 21 (six replicates each), showed that significantly higher collagen release was observed from the cartilage stimulated with TNFα+OSM (10/10 ng/ml, 67.0%; 10/50 ng/ml, 61.2%) than control (6.2%; p<0.001) or either cytokine alone (fig 4A). The highest value of collagen release (79%) was observed for explant cultures from one patient when stimulated with 10/10 ng/ml. The higher OSM concentration did not increase this collagen release.
Figure 4 Significant human cartilage collagen release and increased MMP‐1 and MMP‐13 levels are induced by TNFα (in combination with OSM). HNC was stimulated with TNFα (10 ng/ml) and/or OSM (10 or 50 ng/ml) in serum‐free medium for 21 days, with serum‐free medium only as control. Day 7 and 14 media were collected and replaced with identical test reagents. Collagen release and MMP‐1, MMP‐13, and TIMP‐1 levels were assayed as described in “Materials and methods”. (A) Cumulative collagen release by day 21 is expressed as a percentage of the total. (Bi) Cumulative MMP‐1 levels released by day 21. (Bii) Correlation between cumulative MMP‐1 and cumulative collagen release by day 21. (Ci) Cumulative MMP‐13 levels released by day 21. (Cii) Correlation between cumulative MMP‐13 and cumulative collagen release by day 21. (D) Cumulative TIMP‐1 levels released by day 21. Each bar is the mean (SD) for 4–6 explant cultures for each treatment. The response shown is reproducible and representative of experiments performed with explants from two patients. **p<0.01, ***p<0.001. rs = Spearman's rank correlation coefficient.
Cumulative MMP‐1 levels were significantly higher than control for those explant cultures stimulated with TNFα+OSM (fig 4B(i)). The highest mean MMP‐1 level of 7.7 μg was detected in TNFα+OSM (both at 10 ng/ml) stimulated samples. TNFα+OSM‐induced MMP‐13 levels were also increased compared with control (fig 4C(i)), but these differences did not reach significance. The highest mean MMP‐13 level of 0.5 μg was detected in TNFα+OSM (both at 10 ng/ml) stimulated samples. The higher concentration of TNFα+OSM did not increase MMP‐1 or MMP‐13 release. MMP‐1 levels were more than 10 times greater than MMP‐13 levels. Total collagen release correlated well with MMP‐1 (rs = 0.759, p<0.001) and MMP‐13 (rs = 0.745, p<0.001) (figs 4B(ii) and 4C(ii)).
Cumulative TIMP‐1 levels were raised for HNC samples stimulated with TNFα alone and TNFα+OSM, at both concentrations, compared with control. However, these differences were not significant (fig 4D).
Collagenase activity was not detected in the control explant cultures. TNFα stimulated cultures had 2.2–2.5 U/ml of collagenase activity but when in combination with OSM (10 or 50 ng/ml), stimulated cultures had 4–4.7 U/ml (data not shown). Collagenase activity significantly correlated with collagen release (rs = 0.941, p<0.005).
HAC responses to IL1+OSM stimulation and comparison with HNC responses
Table 1 summarises the data collected after stimulation of HAC explant cultures with IL1+OSM. These data show that when HAC was stimulated with IL1+OSM (the most potent cytokine combination in BNC studies), nearly all explants responded by releasing proteoglycan and up regulating MMP‐1 (table 1). Interestingly, the basal level of proteoglycan release from control cultures was substantial, half the cytokine stimulated release. This is presumably because these cartilage samples were taken from diseased subjects before joint replacement and could indicate that aggrecanase activity is present in the tissue. Collagen release in articular cartilage was typically low (mean (SD) 7.0 (7.5)%) with a 55% response rate (30/55 joints, data not shown), similar to that previously reported.12
Table 1 Comparison of human articular cartilage and human nasal cartilage responses to IL1+OSM stimulation.
| Cumulative release | Proteoglycan (%) | Collagen (%) | MMP‐1 (ng) | TIMP‐1 (ng) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | IL1+ OSM | Control | IL1 + OSM | Control | IL1 + OSM | Control | IL1 + OSM | |
| HAC (21 days) | 39.0 (13.9) | 79.5 (10.9) | 3.3 (4.0) | 7.0 (7.5) | 146 (141) | 2264 (1325) | 149 (111) | 81 (54.3) |
| n = | 43 | 55 | 10 | 9 | ||||
| HNC (14 days) | 12.5 (2.0) | 69.2 (4.4) | 2.5 (0.8) | 25.1 (8.0) | 74.4 | 2668 | 125.7 | 391 |
| n = | 3 | 5 | 2 | 2 | ||||
Human articular cartilage (HAC) and human nasal cartilage (HNC) were stimulated with IL1+OSM (5 and 50 ng/ml) in serum‐free medium for 14 or 21 days, with serum‐free medium only as control. Day 7 and 14 media were collected and replaced with identical test reagents. Cumulative release, over 14 or 21 days, of proteoglycan, collagen, MMP‐1, and TIMP‐1 were assayed as described in “Materials and methods”. n = the number of patients whose samples were used in explant culture experiments.
We also compared the responses of HAC explant culture with those of the HNC explant cultures. Day 14 data are shown for HNC whereas day 21 data are shown for HAC because HAC responds less quickly to cytokine stimulation. When HNC was stimulated with IL1+OSM, all explants (from five patients) responded by releasing proteoglycan and collagen. An increased production of MMP‐1 was detected compared with control as seen with HAC. However, an increase in TIMP‐1 was only seen with HNC. Proteoglycan release was similar between the two tissues, although slower in HAC. However, the basal level of proteoglycan release was a third of the cytokine stimulated release seen in nasal cartilage where explants were from normal healthy subjects and represent normal cartilage. TIMP‐1 cytokine stimulated release was raised in HNC compared with HAC. MMP‐1 (both pro‐ and active forms detected) release in HNC was similar in HAC, but collagen release was less in HAC than in HNC (table 1), probably because most of the MMP‐1 produced by HAC was not activated (data not shown).
Discussion
This study clearly demonstrates that OSM in combination with IL1 or TNFα synergistically induces proteoglycan, but more importantly collagen release from human cartilage as we have previously reported for porcine and bovine cartilages.10,11,12 Indeed, we found the highest extent of collagen release (79%) from human cartilage yet reported. Furthermore, this high release correlated significantly with raised collagenolytic activity levels, and specifically with MMP‐1 and MMP‐13. This strongly supports the involvement of these MMPs in cytokine stimulated collagenolysis as we, and others, have found.7,10,11,12,22
Previously we reported that OSM in combination with IL1 or TNFα potently induces synergistic collagen release from BNC explants. We have also reported the up regulation of MMP‐1, MMP‐3, and MMP‐13 (and TIMP‐1) by northern blot analysis when bovine nasal chondrocytes were stimulated with IL1+OSM or TNFα+OSM.10,11 In the present study we found that human cartilage also responds to these combinations by synergistically releasing collagen, as well as producing raised protein levels of MMP‐1 and MMP‐13, and also TIMP‐1. These cytokine combinations stimulated the production by HNC of MMP‐1 levels that were >10 times those of MMP‐13, levels that reflect the ratio found in joint fluids from patients with RA and OA where MMP‐1 levels are substantially higher than MMP‐13 levels.23 The correlation of MMP‐1 and MMP‐13 with collagen release and collagenolytic activity strongly supports the involvement of these two collagenases in cytokine stimulated cartilage destruction, as previously described by others.7,22 We have previously demonstrated that raised levels of MMP‐1 and MMP‐13 produced by BNC correlate with the generation of one quarter and three quarter fragments of collagen,12 clearly indicating classical collagenase activity. Moreover, inclusion of TIMPs blocks this collagenolysis, clearly supporting a role for these collagenolytic MMPs17 in IL1+OSM mediated collagen release.
The responses to cytokine stimulation were similar in HNC and BNC explants, but delayed in HNC. For BNC, proteoglycan release was increased when stimulated by IL1+OSM compared with either cytokine alone. This effect could only be detected early, at day 3, because by day 7 differences between IL1 and IL1+OSM stimulated proteoglycan release were no longer significant as the stimulated release approached 100%.12,24 However, for HNC, significant differences in proteoglycan release due to cytokines alone versus combinations with OSM were detected at day 7 (only when IL1+OSM were used at 5/50 ng/ml) but also at day 14, which was more similar to the release in HAC.25 This slower response was also evident for collagen release. Significant collagen release in HNC was observed after 14 days of stimulation, similar to BNC, but HNC cultured for 21 days displayed collagen release more similar to that of BNC at 14 days. The difference in tissue response can be attributed to species differences and tissue cellularity, because bovine tissue is more cellular than human tissue.26
Although it is possible to demonstrate proteoglycan release of >80% from HAC, in the past we have found it difficult to show significant and reproducible collagen release from HAC when stimulated with an array of cytokines. Factors such as age and the use of diseased tissue (and disease type), with prolonged cytokine exposure of tissue with low cellularity, increased collagen cross links and advanced glycation end products, can all contribute to the lack of response of the tissue. Only 55% of the tissue samples responded to IL1+OSM by releasing collagen, with typically low (mean (SD) 7.0 (7.5%)) collagen release, and activation of the procollagenases produced was limited.12 All samples studied show up regulation of pro‐MMP‐1 which is not found by either cytokine alone. In contrast, we found HNC was readily stimulated to resorb with IL1+OSM or TNFα+OSM with ⩾60% collagen release at day 21.
Differences in response to IL1+OSM stimulation between HAC and HNC may be due to cell density, tissue age, health, and tissue permeability. Although HNC (24.9×106 cells/g) is 25 times more cellular than HAC (1×106 cells/g),27,28 we saw similar levels of MMP‐1 produced by both human tissues, as shown in table 1, but at different time points (14 days for HNC compared with 21 days for HAC). Moreover, the MMP‐1 produced by HAC was mainly inactive while that produced by HNC was more than 50% active when stimulated with IL1+OSM, leading to marked collagenolysis. In addition, joint diseases have a prolonged duration of onset because cartilage destruction typically begins many years before surgical intervention. HAC is routinely obtained from diseased (OA) joints of elderly patients (>60 years) at the time of joint replacement when the cartilage has already lost significant amounts of proteoglycan and collagen. Articular cartilage accumulates high levels of advanced glycation end product cross links with age that contribute to the tissue's resistance to proteolysis.29 HAC is also less permeable than HNC,30 so it is more difficult for enzymes to penetrate through the tissue and for collagen fragments to be released from within the matrix.
Although many studies have used BNC to assess cartilage degradation,10,11,12,24,31,32,33,34,35,36 criticism of these studies (especially the dramatic responses reported to various cytokines) centres on the use of animal cartilage, suggesting there is no proven relevance to human cartilage destruction and disease. The present study clearly shows that the responses of BNC can be mirrored in HNC and demonstrates that both of these nasal cartilages are suitable models of cartilage destruction for investigating the mechanisms of cartilage catabolism.
We have clearly demonstrated that cartilage alone has the ability to degrade its matrix,10,11,12,17 and in order to study the mechanisms of human cartilage collagenolysis it is essential that a responsive cartilage is used. HNC can be obtained from young, healthy adults and thus provides a reliable and alternative source of human cartilage for study. New microtechnologies will enable the generation of valuable data to help identify the mechanism of cartilage collagenolysis.
In conclusion, we have shown the highest collagen release reported in human cartilage in response to cytokine combinations. Both MMP‐1 and MMP‐13 are up regulated, implicating both collagenases in human cartilage resorption.
Acknowledgements
This work was supported by the Anne Coleman Arthritis Fund, the Arthritis Research Campaign, UK, and the Dunhill Medical Trust. Daiichi Fine Chemicals provided the antibodies to measure MMP‐13.
Abbreviations
BNC - bovine nasal cartilage
ELISA - enzyme linked immunosorbent assay
HAC - human articular cartilage
HNC - human nasal cartilage
IL1 - interleukin 1
MMP - matrix metalloproteinase
OA - osteoarthritis
OSM - oncostatin M
RA - rheumatoid arthritis
TIMP - tissue inhibitor of metalloproteinases
TNFα - tumour necrosis factor α
Footnotes
Conflicting interest: There were no conflicting interests.
References
- 1.Dingle J T, Page‐Thomas T D P, King B, Bard D R. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis 198746527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986322547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page‐Thomas D P, King B, Stephens T, Dingle J T. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin‐1. Ann Rheum Dis 19915075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jubb R W, Fell H B. The breakdown of collagen by chondrocytes. J Pathol 1980130159–162. [DOI] [PubMed] [Google Scholar]
- 5.Cawston T E, Rowan A D. Tissue destruction and repair. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. London: Mosby, 2003127–134.
- 6.Moos V, Fickert S, Muller B, Weber U, Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol 199926870–879. [PubMed] [Google Scholar]
- 7.Shlopov B V, Lie W R, Mainardi C L, Cole A A, Chubinskaya S, Hasty K A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum 1997402065–2074. [DOI] [PubMed] [Google Scholar]
- 8.Fraser A, Fearon U, Billinghurst R C, Ionescu M, Reece R, Barwick T.et al Turnover of type II collagen and aggrecan in cartilage matrix at the onset of inflammatory arthritis in humans: relationship to mediators of systemic and local inflammation. Arthritis Rheum 2003483085–3095. [DOI] [PubMed] [Google Scholar]
- 9.Cawston T E. Metalloproteinase inhibitors. In: Barrett AJ, Salversson G, eds. Proteinase inhibitors. Amsterdam, The Netherlands: Elsevier, 1986589–610.
- 10.Rowan A D, Hui W, Cawston T E, Richards C D. Adenoviral gene transfer of interleukin‐1 in combination with oncostatin M induces significant joint damage in a murine model. Am J Pathol 20031621975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui W, Rowan A D, Richards C D, Cawston T E. Oncostatin M in combination with tumor necrosis factor induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum 2003483404–3418. [DOI] [PubMed] [Google Scholar]
- 12.Cawston T E, Curry V A, Summers C A, Clark I M, Riley G P, Life P F.et al The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum 1998411760–1771. [DOI] [PubMed] [Google Scholar]
- 13.Sah R L, Chen A C, Grodzinsky A J, Trippel S B. Differential effects of bFGF and IGF‐1 on matrix metabolism in calf and adult bovine cartilage experiments. Arch Biochem Biophys 1994308137–147. [DOI] [PubMed] [Google Scholar]
- 14.Tyler J A. Insulin‐like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J 1989260543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luyten F P, Hascall V C, Nissley S P, Morales T I, Reddi A H. Insulin‐like growth factors maintain steady‐state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys 1988267416–425. [DOI] [PubMed] [Google Scholar]
- 16.Farndale R W, Buttle D J, Barrett A J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986883173–177. [DOI] [PubMed] [Google Scholar]
- 17.Ellis A J, Curry V A, Powell E K, Cawston T E. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP‐2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun 199420194–101. [DOI] [PubMed] [Google Scholar]
- 18.Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 1963351961–1965. [Google Scholar]
- 19.Clark I M, Powell L K, Wright J K, Cawston T E, Hazleman B L. Monoclonal antibodies against human fibroblast collagenase and the design of an enzyme‐linked immunosorbent assay to measure total collagenase. 199212475–480. [DOI] [PubMed] [Google Scholar]
- 20.Clark I M, Powell L K, Wright J K, Cawston T E. Polyclonal and monoclonal antibodies against human tissue inhibitor of metalloproteinases (TIMP) and the design of an enzyme‐linked immunosorbent assay to measure TIMP. Matrix 19911176–85. [DOI] [PubMed] [Google Scholar]
- 21.Koshy P J T, Rowan A D, Life P F, Cawston T E. 96 well plate assays for measuring collagenase activity using 3H‐acetylated collagen. Anal Biochem 1999275202–207. [DOI] [PubMed] [Google Scholar]
- 22.Billinghurst R C, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C.et al Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997991534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K.et al Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis 200059455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cawston T E, Ellis A J, Humm G, Lean E, Ward D, Curry V. Interleukin‐1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun 1995215377–385. [DOI] [PubMed] [Google Scholar]
- 25.Cawston T, Billington C, Cleaver C, Elliott S, Hui W, Koshy P.et al The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci 1999878120–129. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Cui Z, Urban J P G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage‐bone interface. Arthritis Rheum 2004503915–3924. [DOI] [PubMed] [Google Scholar]
- 27.Homicz M R, McGowan K B, Lottman L M, Beh G, Sah R L, Watson D. A compositional analysis of human nasal septal cartilage. Arch Fac Plast Surg 2003553–58. [DOI] [PubMed] [Google Scholar]
- 28.Chaipinyo K, Oakes B W, Van Damme M I. The use of debrided human articular cartilage for autologous chondrocyte implantation: maintenance of chondrocyte differentiation and proliferation in type I collagen gels. J Orthop Res 200422446–455. [DOI] [PubMed] [Google Scholar]
- 29.DeGroot J, Verzijl N, Wenting‐van Wijk M J G, Bank R A, Lafeber F P, Bijlsma J W.et al Age‐related decrease in susceptibility of human articular cartilage to matrix metalloproteinase‐mediated degradation: the role of advanced glycation end products. Arthritis Rheum 2001442562–2571. [DOI] [PubMed] [Google Scholar]
- 30.Rotter N, Tobias G, Lebl M, Roy A K, Hansen M C, Vacanti C A.et al Age‐related changes in the composition and mechanical properties of human nasal cartilage. Arch Biochem Biophy 2002403132–140. [DOI] [PubMed] [Google Scholar]
- 31.Arner E C, Hughes C E, Decicco C P, Caterson B, Tortorella M D. Cytokine‐induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage 19986214–228. [DOI] [PubMed] [Google Scholar]
- 32.Gendron C, Kashiwagi M, Hughes C, Caterson B, Nagase H. TIMP‐3 inhibits aggrecanase‐mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett 2003555431–436. [DOI] [PubMed] [Google Scholar]
- 33.Kozaci L D, Brown C J, Adcocks C, Galloway A, Hollander A P, Buttle D J. Stromelysin 1, neutrophil collagenase, and collagenase 3 do not play major roles in a model of chondrocyte mediated cartilage breakdown. Mol Pathol 199851282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto K, Iizawa T, Harada H, Yamada K, Katsumata M, Takahashi M. Cartilage degradation independent of MMP/aggrecanases. Osteoarthritis Cartilage 2004121006–1014. [DOI] [PubMed] [Google Scholar]
- 35.Saxne T, Heinegard D, Wollheim F A. Human arthritic synovial fluid influences proteoglycan biosynthesis and degradation in organ culture of bovine nasal cartilage. Coll Relat Res 19888233–247. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg J J, Kincaid S B, Sledge C B. Inhibition of cartilage breakdown by hydrocortisone in a tissue culture model of rheumatoid arthritis. Ann Rheum Dis 198342323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]