Abstract
Objective
To describe the efficacy and safety of adalimumab in patients with rheumatoid arthritis (RA) who had previously discontinued infliximab treatment.
Methods
24 patients with RA who discontinued treatment with infliximab (switchers) were treated with adalimumab (40 mg every 2 weeks, subcutaneously) for 12 months. The results were compared with those for 25 patients with RA receiving adalimumab who had not previously used an anti‐tumour necrosis factor α inhibitor (controls). Disease activity was measured with the 28 joint count Disease Activity Score (DAS28), and clinical response with the American College of Rheumatology (ACR) 20% response criteria.
Results
At baseline there were no differences in demographic, clinical, and laboratory features between the two groups. After 12 months' adalimumab treatment, clinical improvement was similar in both groups. More specifically, ACR 20% response criteria were achieved by 18/24 (75%) switchers and by 19/25 (76%) subjects in the control group. Four switchers discontinued the study—two because of adverse events and two because of lack of efficacy, while three control patients discontinued the study—one because of lack of efficacy and two owing to side effects.
Conclusion
Adalimumab is a well tolerated and effective treatment for patients with RA, even when infliximab has been discontinued.
Keywords: rheumatoid arthritis, infliximab, adalimumab
The anti‐tumour necrosis factor α (TNFα) agent, infliximab, a chimeric monoclonal antibody, is highly effective in the treatment of rheumatoid arthritis (RA).1 However, a subset of patients with RA experience adverse drug reactions or drug failure, requiring infliximab to be stopped.1 RA is a chronic progressive disease needing continuous treatment, and thus when infliximab is stopped the disease may flare up. Therefore, for practising physicians an obvious question is: how effective and safe is switching from one anti‐TNFα agent to another? To answer this question, we investigated the efficacy and safety of adalimumab, a humanised anti‐TNFα monoclonal antibody, in patients with RA who had previously discontinued infliximab treatment.
Materials and methods
This 12 month, open label, comparative study was conducted in a single university centre in Greece. The clinical outcome of adalimumab in patients who had previously used infliximab (switchers) was compared with the efficacy of adalimumab in patients who had not previously received anti‐TNFα inhibitors (controls).
Inclusion criteria
Patients were eligible if they had (a) RA according to the American College of Rheumatology (ACR) criteria2; (b) active disease defined as ⩾6 tender joints and ⩾6 swollen joints and erythrocyte sedimentation rate ⩾40 mm/1st h; (c) no active infectious diseases, and had recently received infliximab infusions.
Study design
Patients had been treated with standard dosage of infliximab as previously reported.3 At least 4 weeks but no more than 10 weeks had to have elapsed between the last infliximab infusion and the first adalimumab administration. Patients were instructed by a specialised nurse in the self administration of adalimumab (40 mg every 2 weeks subcutaneously). Twenty four patients (switchers) received adalimumab for 12 months and were compared with 25 patients with RA treated with adalimumab who had not previously used infliximab (controls). The two groups were matched according to age, sex, disease duration, and 28 joint count Disease Activity Score (DAS28). For each patient in the switcher group a patient from the control group was selected (individual matching). Each pair was matched for age (±3 years), sex, disease duration (±1 year), and DAS28. Concomitant drugs, such as disease modifying antirheumatic drugs and/or prednisone (⩽7.5 mg/day), were allowed and remained stable during the study. The institutional review board and the ethics committee of the university hospital approved the protocol and all patients gave written informed consent before entering into the study.
Evaluation
The clinical response was evaluated according to the ACR 20% and European League Against Rheumatism (EULAR) response criteria,4,5 while disease activity was measured with the DAS28.6
Monitoring
A complete blood count with differential and platelet count, as well as serum values for liver enzymes, bilirubin, albumin, glucose, creatinine, and urine analysis were obtained before treatment and at each patient's visit, every 2 months for a total period of 12 months.
Results
Of 84 patients who were being treated with infliximab, 28 had to stop the treatment.3 Switcher patients had received infliximab for a mean (SD) period of 18.5 (3.8) months. Nine patients had discontinued treatment owing to lack of efficacy, 16 owing to adverse drug reactions, and three had been lost from the follow up. Of those patients with side effects, nine discontinued treatment owing to hypersensitivity reactions, six owing to infections (including two with pulmonary tuberculosis), and one owing to paraesthesias.3 Twenty four of these 28 patients were eligible to enter the study and began treatment with adalimumab. They were compared with 25 patients receiving adalimumab who had not previously received anti‐TNFα treatment. Table 1 presents the patients' characteristics. There were no differences in mean age, disease duration, seropositivity, and DAS28.
Table 1 Clinical characteristics of patients with RA treated with adalimumab.
| Variables | Switchers | Controls |
|---|---|---|
| (n = 24) | (n = 25) | |
| Age (years), mean (SD) | 56.7 (11.2) | 55.9 (10.8) |
| Female, No (%) | 22 (92) | 22 (88) |
| Disease duration (years), mean (SD) | 16.6 (7.0) | 15.8 (7.5) |
| Seropositivity, No (%) | 15 (63) | 16 (64) |
| DAS28, mean (SD) | 5.6 (0.8) | 5.9 (0.9) |
| DMARD treatment, No (%) | 24 (100) | 25 (100) |
| Methotrexate | 20 (83) | 22 (88) |
| Ciclosporin | 1 (4) | – |
| Leflunomide | 3 (13) | 3 (12) |
| Prednisone treatment, No (%) | 24 (100) | 25 (100) |
| Prednisone dosage (mg/day), mean (SD) | 6.8 (2.1) | 7.0 (2.5) |
DMARD, disease modifying antirheumatic drug.
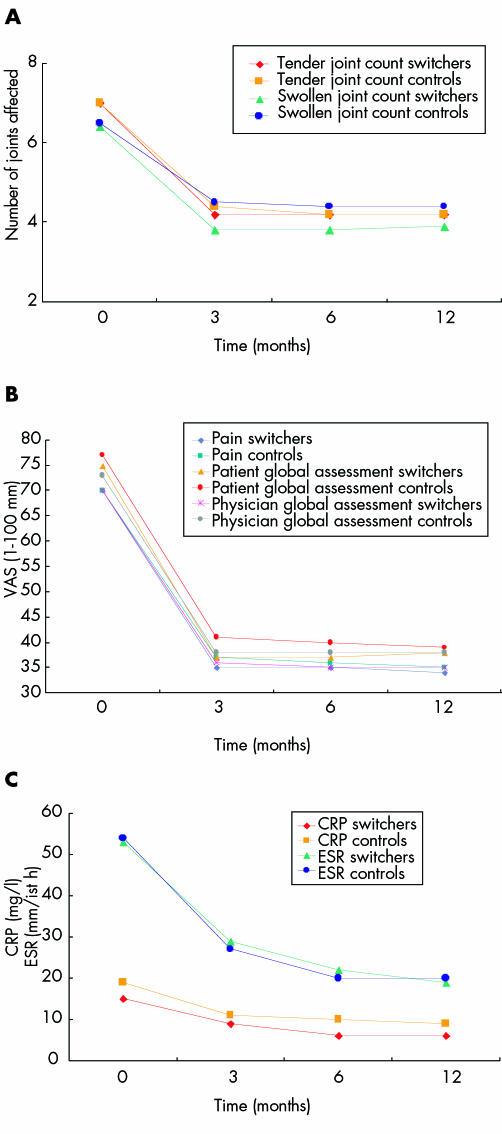
After 12 months' treatment with adalimumab a significant reduction in the tender and swollen joint counts (fig 1A), and improvement in the pain score, patient global assessment, and physician global assessment were noted in both groups (fig 1B). In addition, a reduction of acute phase reactants was noted in both groups (fig 1C). No statistical differences were found between switchers and controls (figs 1A, B, and C). Table 2 outlines the clinical response of adalimumab treatment. Eighteen (75%) of the 24 switchers achieved the ACR 20% response criteria, while 19/25 (76%) of the control group attained the ACR 20% criteria. A similar response was noted for the EULAR response criteria. A significant improvement in the DAS28 was found in both groups. It is of interest to note that of the 18 patients in the switcher group who achieved the ACR 20% response criteria, 8 had previously discontinued infliximab treatment owing to lack of efficacy, while 10 had stopped infliximab treatment owing to side effects (table 2).
Figure 1 Clinical and laboratory features at entry and at 3, 6, and 12 months in switchers and control patients with RA treated with adalimumab.
Table 2 Clinical response after 12 months of adalimumab treatment in patients with RA.
| Switchers | ||||
|---|---|---|---|---|
| Drug failure | Adverse events | All switchers | Controls | |
| (n = 9) | (n = 15) | (n = 24) | (n = 25) | |
| No (%) patients achieving: | ||||
| ACR 20% | 8 (89) | 10 (67) | 18 (75) | 19 (76) |
| ACR 50% | 5 (56) | 7 (47) | 12 (50) | 14 (56) |
| ACR 70% | 3 (33) | 5 (33) | 8 (33) | 9 (36) |
| EULAR | 7 (78) | 10 (67) | 17 (71) | 18 (72) |
| DAS28, mean (SD) | ||||
| Baseline | 5.4 (0.7) | 5.7 (0.8) | 5.6 (0.8) | 5.9 (0.9) |
| 12 Months | 3.3 (0.6) | 3.2 (0.6) | 3.2 (0.6) | 3.2 (0.7) |
No significant differences were seen between any group of switchers and the control group according to χ2 test for categorical parameters and Wilcoxon's test for continuous variables.
Eleven (46%) of the switchers and 11 (44%) of the control group developed adverse drug reactions, most of which resolved without sequelae. However, four switcher patients discontinued the study—two because of adverse events and two because of lack of efficacy, while three patients from the control group discontinued the study—one because of lack of efficacy and the other two owing to side effects. Among the switchers who discontinued the study because of side effects, one stopped owing to herpes zoster infection and the other owing to an immediate hypersensitivity reaction. This last patient had developed a similar reaction when treated with infliximab. Among the controls who discontinued the study owing to side effects, one patient developed herpes zoster and the other recurrent lower respiratory tract infections.
Discussion
TNFα inhibitors represent a class of biological agents that have gained significant attention for their rapid onset of action and disease modifying properties. Studies show that etanercept, a recombinant TNFα receptor fusion protein, is equivalent to methotrexate (MTX) in RA.7 Infliximab, a chimeric monoclonal IgG1 antibody against TNFα is normally used in combination with MTX for those with an insufficient response to MTX alone.1 A third TNFα inhibitor adalimumab, a human monoclonal antibody, is now available for the treatment of RA.8 Little information is available about the clinical benefit of changing from one TNFα inhibitor to another when the first agent has demonstrated a lack of efficacy or caused adverse events.
A French study described the usefulness of switching TNFα inhibitors among 131 patients with RA receiving either etanercept or infliximab.9 Eight patients switched from infliximab to etanercept, with five reporting improvement in RA symptoms, while six switched from etanercept to infliximab with clinical improvement in three. A retrospective study reported that patients with RA who do not respond to etanercept might experience improved disease control with a switch to infliximab. The efficacy of infliximab was clinically and statistically similar in subjects who had never received anti‐TNFα treatment. Indeed, disease activity improved significantly in both groups.10 In a recent study, 25 patients who discontinued infliximab were subsequently treated with etanercept in a prospective, open label, 12 week study. It was shown that etanercept was well tolerated and effective in treating patients with RA, even when infliximab was stopped.11 Another study from Stockholm showed that for patients with insufficient efficacy from etanercept, treatment with infliximab provided better results, suggesting that a trial of infliximab is reasonable in such patients. On the other hand, for patients who discontinued infliximab owing to adverse events, treatment with etanercept gave at least a similar clinical efficacy.12 Thus, in these two clinical situations: when etanercept fails owing to a lack of efficacy, and when infliximab fails owing to adverse events, trying the alternative of these two TNFα blockers does make clinical sense.13
Our study adds to the existing data by comparing the response of patients with RA who switch from infliximab to adalimumab, with that of patients receiving adalimumab with no previous TNFα treatment. After 12 months' adalimumab treatment, the degree of clinical response was similar in both groups. In addition, no significant difference was found in the safety profile of both groups. No specific side effects due to infliximab treatment are predictors for adverse events or response to treatment by switching to adalimumab. As far as we know, no previous studies have described the efficacy of adalimumab in patients with RA who previously discontinued infliximab treatment. However, there are some pilot and case report studies in patients with Crohn's disease who discontinued infliximab and were treated with adalimumab. They showed that adalimumab was well tolerated and appears to be a clinically beneficial option for patients with Crohn's disease who have lost their response to, or cannot tolerate, infliximab.14,15,16 The results of this study reinforce the above and provide strong evidence that adalimumab is a well tolerated and effective treatment option for patients with RA, even when infliximab has been discontinued. It is interesting to note that treatment with adalimumab in our study was started for some patients 4 weeks after the last infliximab infusion. There may have been a carry over effect of infliximab. This may strengthen the conclusion of our study, because patients who switched to adalimumab tolerated the treatment well.
A weakness of this study is the small number of patients in each group, which might result in a lower power, or ability to detect differences in the efficacy of adalimumab between groups. Thus, research is needed with larger numbers of patients to determine which patients' characteristics predict a response to different TNFα inhibitors, such as pharmacokinetics, TNFα polymorphisms, and cytokine profile.
Abbreviations
ACR - American College of Rheumatology
DAS 28 - 28 joint count Disease Activity Score
MTX - methotrexate
RA - rheumatoid arthritis
TNFα - tumour necrosis factor α
References
- 1.Maini R, St Clair E W, Breedveld F, Kalden J, Weisman M, Smolen J.et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. ATTRACT Study Group. Lancet 19993541932–1939. [DOI] [PubMed] [Google Scholar]
- 2.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 3.Voulgari P V, Alamanos Y, Nikas S N, Bougias D V, Temekonidis T I, Drosos A A. Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med 2005118515–520. [DOI] [PubMed] [Google Scholar]
- 4.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 5.Paulus H E, Egger M J, Ward J R, Williams H J. Analysis of improvement in individual rheumatoid arthritis patients treated with disease‐modifying antirheumatic drugs, based on the findings in patients treated with placebo. The Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis Rheum 199033477–484. [DOI] [PubMed] [Google Scholar]
- 6.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 7.Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I.et al A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999340253–259. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt M E, Keystone E C, Furst D E, Moreland L W, Weisman M H, Birbara C A.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 20034835–45. [DOI] [PubMed] [Google Scholar]
- 9.Brocq O, Plubel Y, Breuil V, Grisot C, Flory P, Mousnier A.et al Etanercept‐infliximab switch in rheumatoid arthritis: 14 out of 131 patients treated with anti TNF alpha. Presse Med 2002311836–1839. [PubMed] [Google Scholar]
- 10.Hansen K E, Hildebrand J P, Genovese M C, Cush J J, Patel S, Cooley D A.et al The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol 2004311098–1102. [PubMed] [Google Scholar]
- 11.Haraoui B, Keystone E C, Thorne C, Pope J E, Chen I, Asare C G.et al Clinical outcomes of patients with rheumatoid arthritis after switching from infliximab to etanercept. J Rheumatol 2004312356–2359. [PubMed] [Google Scholar]
- 12.van Vollenhoven R, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 2003621195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraoui B. Is there a rationale for switching from one anti‐tumor necrosis factor agent to another? J Rheumatol 2004311021–1022. [PubMed] [Google Scholar]
- 14.Youdim A, Vasiliauskas E A, Targan S R, Papadakis K A, Ippoliti A, Dubinsky M C.et al A pilot study of adalimumab in infliximab‐allergic patients. Inflamm Bowel Dis 200410333–338. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn W J, Hanauer S, Loftus E V, Jr, Tremaine W J, Kane S, Cohen R.et al An open‐label study of the human anti‐TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol 2004991984–1989. [DOI] [PubMed] [Google Scholar]
- 16.Stallmach A, Giese T, Schmidt C, Meuer S C, Zeuzem S S. Severe anaphylactic reaction to infliximab: successful treatment with adalimumab—report of a case. Eur J Gastroenterol Hepatol 200416627–630. [DOI] [PubMed] [Google Scholar]