Abstract
Objectives
To assess the clinical implications of autoantibodies directed against different parts of the Mi‐2β autoantigen in patients with myositis.
Methods
A systematic assessment of the clinical, laboratory, and histological characteristics of 48 anti‐Mi‐2 positive patients from six European centres was made. Anti‐Mi‐2 autoantibodies were determined with an ELISA using four overlapping fragments spanning the entire amino acid sequence of the autoantigen. Data were compared with results for a large group of anti‐Mi‐2 negative patients with myositis published previously.
Results
Anti‐Mi‐2 autoantibodies were found in dermatomyositis, polymyositis, and inclusion body myositis. In general, myositis with anti‐Mi‐2 autoantibodies was characterised by relatively mild disease, sometimes accompanied by extramuscular symptoms, including arthralgia, arthritis, Raynaud's phenomenon, and interstitial lung disease. Cardiac disease was not seen, and treatment response was fair. No differences were found between patients with autoantibodies to different fragments of the Mi‐2β antigen, except for a potentially increased risk of cancer in patients with antibodies directed to the N‐terminal fragment of the autoantigen.
Conclusions
Anti‐Mi‐2 autoantibodies are not a marker of a specific subtype of myositis. No significant differences between patients with autoantibodies to different fragments of the Mi‐2β autoantigen are found, with the possible exception of an increased risk of cancer in patients with antibodies to the N‐terminal fragment.
Keywords: autoantibodies, anti‐Mi‐2, polymyositis, dermatomyositis, inclusion body myositis
A subset of patients with myositis have unique autoantibodies that are considered to be specific for this disorder.1 One of these so‐called myositis‐specific autoantibodies (MSAs) recognises the Mi‐2 antigen, a 220 kDa protein in a nuclear protein complex containing histone deacetylase and nucleosome remodelling activities.2 These anti‐Mi‐2 autoantibodies are detected in about 20% of myositis sera, and are reported to occur primarily in patients with dermatomyositis (DM).3,4,5
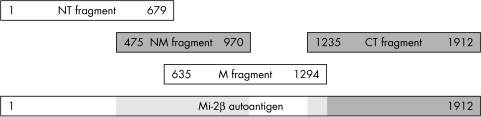
Recently, we showed that anti‐Mi‐2 autoantibodies are present in the sera of patients with polymyositis (PM) and inclusion body myositis (IBM).6,7 An explanation for this finding may be that we used an enzyme linked immunosorbent assay (ELISA) technique in which four overlapping fragments were used spanning the entire amino acid sequence of the Mi‐2β autoantigen (fig 1), whereas previous studies only used the NM fragment that is thought to contain the major epitope region.
Figure 1 Schematic representation of the Mi‐2β autoantigen, and the fragments used for detection of anti‐Mi‐2 autoantibodies.
To elucidate the clinical implications of the presence of autoantibodies directed against different parts of the Mi‐2β autoantigen, we systematically studied the clinical, laboratory, and histological characteristics of the largest cohort of anti‐Mi‐2 positive patients ever studied.
Patients and methods
Patients and patient evaluation
Serum samples were collected from 417 patients with myositis from several European neurological and rheumatologic institutes. The autoantibody profile of this large group has been reported previously.6 In addition, serum samples were collected from all patients with myositis seen between 2000 and 2003 at the Neuromuscular Centre Nijmegen. All patients with anti‐Mi‐2 autoantibodies were included in the study. In total, 48 patients were studied from five European countries (Czech Republic 11, Greece 8, Italy 7, The Netherlands 17, Sweden 5).
Before the collection of clinical data, a standardised questionnaire was devised, and study definitions and conventions for evaluation were agreed upon by all evaluators. The questionnaires were sent to the treating physicians and included detailed clinical and laboratory information. Clinical data of all Dutch patients was obtained by questioning and examining the patients, reviewing their charts, and consulting their treating physician.
Control group
Whenever possible, data were compared with data from a large group of patients with myositis published previously.7 This group of 125 patients included seven patients with anti‐Mi‐2 autoantibodies; all other patients had negative test results for this autoantibody. These anti‐Mi‐2 positive patients were excluded from the comparator myositis group.
Serological techniques
The presence of anti‐Mi‐2 autoantibodies was determined by ELISA, as previously described.6 In short, four overlapping fragments spanning the complete amino acid sequence of the Mi‐2β autoantigen were used (fig 1). Optical densities were measured bichromatically at 492 nm/620 nm (Titertek Multiscan MCC340 Mkll; Flow, Meckenheim, Germany). Tests were regarded as positive at ⩾2.5‐fold optical densities compared with that of three negative control sera. Positive and negative controls as well as patients' sera were run in duplicate on every test plate.
Serum samples were also tested for the presence of the other most common MSAs (anti‐Jo‐1 and anti‐SRP), as previously described.6 All samples tested negative for these MSAs.
Statistical analysis
Besides comparing data from anti‐Mi‐2 positive patients with those from anti‐Mi‐2 negative patients, a comparison was made between patients with autoantibodies directed against different fragments of the Mi‐2β antigen. Discontinuous grouped data were analysed by χ2 frequency distribution. Fisher's exact test was used in cases of a predicted frequency ⩽2. Continuous data were analysed using the Wilcoxon‐Mann‐Whitney test. A p value <0.05 was considered significant. Patients whose data for a particular variable were not available were excluded from the analysis of that variable, and the number used for the calculation of percentages was adjusted accordingly.
Results
The anti‐Mi‐2 group, in general, did not differ significantly from the comparator myositis group, with the exception of a slightly better treatment response (table 1). The disease onset was rarely acute (data not shown). Most patients complained of insidious onset of slight muscle weakness and myalgia, usually preceded by arthralgia or the occurrence of a rash (data not shown).
Table 1 Clinical and laboratory characteristics of 48 patients with anti‐Mi‐2 autoantibodies, divided into three groups based on the reactivity of the antibodies to different components of the Mi‐2β autoantigen, compared with a large group of patients with myositis published previously.7.
| Characteristics | Type A* | Type B* | Type C* | Total Mi‐2 | Control | p Value |
|---|---|---|---|---|---|---|
| (n = 12) | (n = 28) | (n = 7) | (n = 48)† | (n = 118) | ||
| Myositis diagnosis12,13 | ||||||
| DM | 33 | 54 | 57 | 50 | 36 | NS |
| PM | 58 | 39 | 14 | 40 | 57 | NS |
| IBM | 8 | 10 | 0 | 8 | 22 | <0.05¶ |
| Demographics | ||||||
| Sex (F:M) | 1.4:1 | 2.1:1 | 1:1.3 | 1.5:1 | 2.6:1 | NS |
| Signs and symptoms | ||||||
| Dysphagia | 33 | 39 | 14 | 33 | 43 | NS |
| Muscle atrophy | 25 | 53 | 57 | 46 | 25 | NS |
| Dry eyes/mouth | 8 | 17 | 42 | 14 | 16 | NS |
| CTS | 11 | 0 | 25 | 6 | 17 | NS |
| Dyspnoea | 33 | 30 | 14 | 28 | 39 | NS |
| Arthralgia | 58 | 34 | 28 | 39 | 45 | NS |
| Arthritis | 25 | 30 | 14 | 26 | 22 | NS |
| Raynaud's phenomenon | 33 | 25 | 14 | 25 | 26 | NS |
| Interstitial lung disease | 25 | 14 | 14 | 16 | 20 | NS |
| Dermatological signs and symptoms | ||||||
| Heliotrope rash | 33 | 37 | 57 | 40 | 18 | NS |
| Gottron's sign | 33 | 48 | 57 | 47 | 17 | <0.01§ |
| Shawl sign | 8 | 7 | 14 | 9 | NA | NS |
| V sign | 17 | 30 | 43 | 28 | NA | NS |
| Nailfold lesions | 33 | 24 | 43 | 29 | 15 | NS |
| Calcinosis | 0 | 7 | 0 | 4 | NA | NS |
| Associated disorders | ||||||
| Cancer | 25 | 4 | 0 | 8 | 3 | NS |
| Rheumatic disorders | 33 | 17 | 14 | 20 | 12 | NS |
| Laboratory investigations | ||||||
| Creatine kinase‡ | 1118 | 3726 | 2883 | 2864 | 1495 | NS |
| Treatment response (clinical) | ||||||
| Complete | 40 | 34 | 80 | 41 | 15 | <0.01§, <0.05¶ |
| Partial | 50 | 53 | 20 | 48 | 71 | <0.01§ |
| None | 10 | 11 | 0 | 9 | 14 | NS |
Numbers are percentages of total.
DM, dermatomyositis; PM, polymyositis; IBM, inclusion body myositis; F, female; M, male; CTS, carpal tunnel syndrome; NA, not assessed; NS, not significant.
*See text for explanation; †one patient could not be classified into one of the three groups; ‡mean creatine kinase upon presentation in U/l; §control group v total Mi‐2 group; ¶type B v type C.
There were no specific histological characteristics for the anti‐Mi‐2 group in general. A large proportion of muscle biopsy specimens showed atypical abnormalities, whereas others had the distinct features of DM (perifascicular atrophy, perivascular inflammatory infiltrates) or IBM (basophilic rimmed vacuoles, endomysial inflammatory infiltrates with invasion of non‐necrotic muscle fibres). The muscle biopsy was normal in three patients. None of the patients without DM associated dermatological abnormalities had histological features suggestive of DM (membrane attack complex deposition in capillaries, perivascular inflammatory infiltrates, perifascicular atrophy).
Several different patterns of autoantibody reactivity to fragments of the Mi‐2β autoantigen were found. In sera with reactivity to more than one fragment, the reactivity was always to adjacent or overlapping fragments. In total, three different patterns of reactivity could be distinguished: type A with reactivity solely to the NT fragment (thus indicating that the antigenic site was between amino acid 1 and 475), type B with the strongest reactivity to the NM and/or M fragment, and type C with reactivity only to the CT fragment (antigenic site between amino acid 1294 and 1912).
No clear differences were found between the three patterns of reactivity for age of onset (data not shown), season of onset (data not shown), clinical signs and symptoms at onset (data not shown), or histological characteristics (data not shown). Several clinical differences were seen between the three patterns, although none of them were statistically significant (table 1). Patients with pattern A were more frequently diagnosed with cancer than patients with pattern B or C (table 1). In total, four patients with anti‐Mi‐2 autoantibodies were diagnosed with a malignancy (one vaginal carcinoma, one non‐Hodgkin's lymphoma, one renal adenocarcinoma, and one gastric cancer). Three of these patients (one diagnosed with PM, and two diagnosed with DM) had pattern A, whereas only one patient (diagnosed with gastric cancer several years before the diagnosis of PM) had pattern B.
Discussion
Most studies have used the NM fragment of the Mi‐2β autoantigen, which is thought to contain the major antigenic epitope. By using four different fragments spanning the entire amino acid sequence of the Mi‐2β autoantigen, we were able to detect the presence of anti‐Mi‐2 autoantibodies in a relatively large number of serum samples from patients with PM and IBM.6,7 To elucidate the clinical implications of the presence of autoantibodies directed against different parts of the Mi‐2β autoantigen, we systematically studied the clinical, laboratory, and histological characteristics of the largest cohort of anti‐Mi‐2 positive patients ever.
Anti‐Mi‐2 positive myositis
Unlike other MSAs, anti‐Mi‐2 autoantibodies do not appear to be a marker for a specific subtype of myositis. The anti‐Mi‐2 positive patients did not differ from a large control group of patients with myositis, with the exception that the clinical treatment response was better in the anti‐Mi‐2 group. This feature might be explained by the fact that the control group included a larger number of patients with IBM. In general, myositis with anti‐Mi‐2 autoantibodies in this study was characterised by a relatively mild myositis (mild muscle weakness) that can be accompanied by extramuscular signs and symptoms, including arthralgia, arthritis, Raynaud's phenomenon, and interstitial lung disease. Cardiac disease was not seen, and the treatment response appeared to be fair.
We were unable to confirm the fact that anti‐Mi‐2 is specific for DM. Only half of the anti‐Mi‐2 positive patients were diagnosed with DM and the other patients did not have DM associated dermatological abnormalities, or histological characteristics of DM. The high prevalence of anti‐Mi‐2 autoantibodies in non‐DM patients cannot be explained by the serological technique used because non‐DM patients were also identified with autoantibodies mainly directed to the NM fragment, the main antigenic fragment used in other studies.
Anti‐Mi‐2 fragments
Several differences were noticed between patients with antibodies to different fragments of the autoantigen, but none of these were statistically significant. The most remarkable difference was the more frequent occurrence of cancer in patients with autoantibodies directed to the N‐terminal fragment. It has been suggested that anti‐Mi‐2 autoantibodies may serve as an exclusion criterion for paraneoplastic myositis.8,9 Only a few patients with a malignancy and anti‐Mi‐2 autoantibodies have been described.3,4 The prevalence of malignancy in anti‐Mi‐2 positive patients appears to be lower than for patients with DM in general (3% v 32%).3,4,6,7,8,9,10,11 In our study we found a slightly higher prevalence of malignancy in anti‐Mi‐2 positive patients than previously reported (8% v 3%). The finding that patients with autoantibodies directed to the N‐terminal fragment of the Mi‐2β antigen appear to have a higher risk for malignancy raises the question of whether the presence of autoantibodies to different fragments of the Mi‐2β autoantigen may serve as a marker of an increased risk of paraneoplastic myositis. Future studies are needed to answer this clinically relevant question.
From these data we conclude that anti‐Mi‐2 autoantibodies are not a marker of a specific subtype of myositis. Testing for anti‐Mi‐2 autoantibodies may be helpful in establishing the diagnosis myositis, but once this diagnosis has been made we do not advise routine testing for anti‐Mi‐2 autoantibodies. Future studies are required to examine further the potential association between the presence of autoantibodies directed against certain fragments of the Mi‐2β antigen and an increased risk for cancer.
Acknowledgements
The work of GJDH was supported by NWO‐MW grant 940‐37‐009. The work of WJvV and WTMVE was in part supported by the Council of Chemical Sciences of the Netherlands Organisation for Scientific Research (NWO‐CW) with financial aid from the Netherlands Technology Foundation (grant 349–3699). JV obtained institutional support MSM 0021620812 from the Ministry of Education, Youth and Sports.
We are grateful to Dr M Renz (Karlsruhe, Germany) for the purified recombinant Mi‐2 fragments and thank Dr M Bluethner (Karlsruhe, Germany) for his useful suggestions.
Abbreviations
DM - dermatomyositis
ELISA - enzyme linked immunosorbent assay
IBM - inclusion body myositis
MSAs - myositis‐specific autoantibodies
PM - polymyositis
Footnotes
Conflict: None of the authors have a conflict of interest to declare.
References
- 1.Hengstman G J D, Van Engelen B G M, Vree Egberts W T M, Van Venrooij W J. Myositis specific autoantibodies: overview and recent developments. Curr Opin Rheumatol 200113476–482. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, LeRoy G, Seelig H P, Lane W S, Reinberg D. The dermatomyositis‐specific autoantigen Mi‐2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 199895279–289. [DOI] [PubMed] [Google Scholar]
- 3.Targoff I N, Reichlin M. The association between Mi‐2 antibodies and dermatomyositis. Arthritis Rheum 198528796–803. [DOI] [PubMed] [Google Scholar]
- 4.Love L A, Leff R L, Fraser D D, Targoff I N, Dalakas M, Plotz P H.et al A new approach to the classification of idiopathic inflammatory myopathy: myositis‐specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 199170360–374. [DOI] [PubMed] [Google Scholar]
- 5.Hausmanowa‐Petrusewicz I, Kowalska‐Oledzka E, Miller F W, Jarzabek‐Chorzelska M, Targoff I N, Blaszczyk‐Kostanecka M.et al Clinical, serologic, and immunogenetic features in Polish patients with idiopathic inflammatory myopathies. Arthritis Rheum 1997401257–1266. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer R, Hengstman G J D, Vree Egberts W, Ehrfeld H, Bozic B, Ghirardello A.et al Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 200160116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hengstman G J D, Brouwer R, Vree Egberts W T M, Seelig H P, Jongen P J H, Van Venrooij W J.et al Clinical and serological characteristics of 125 Dutch myositis patients. Myositis specific autoantibodies aid in the differential diagnosis of the idiopathic inflammatory myopathies. J Neurol 200224969–75. [DOI] [PubMed] [Google Scholar]
- 8.Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Gambari P F, Doria A. Anti‐Mi‐2 antibodies. Autoimmunity 20053879–83. [DOI] [PubMed] [Google Scholar]
- 9.Hill C L, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A.et al Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study. Lancet 200135796–100. [DOI] [PubMed] [Google Scholar]
- 10.Roux S, Seelig H P, Meyer O. Significance of Mi‐2 autoantibodies in polymyositis and dermatomyositis. J Rheumatol 199825395–396. [PubMed] [Google Scholar]
- 11.Hirakata M, Suwa A, Sato S, Nojima T, Okada T, Kawakami Y.et al New profiles of autoantibodies in the sera of Japanese patients with myositis [abstract]. Arthritis Rheum 200348(suppl 9)S103 [Google Scholar]
- 12.Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975292344–347. [DOI] [PubMed] [Google Scholar]
- 13.Verschuuren J J, Badrising U A, Van Engelen B G M, Van der Hoeven H, Hoogendijk J, Wintzen A R. Inclusion body myositis. In: Emery AEH, ed. Diagnostic criteria for neuromuscular disorders. London: Royal Society Medicine Press, 199781–84.