Abstract
Background
Sjögren's syndrome (SS), an autoimmune exocrinopathy mainly affecting lachrymal and salivary glands, results in ocular and oral dryness (keratoconjunctivitis sicca and xerostomia). The aetiology and pathogenesis are largely unknown; currently, only palliative treatment is available.
Objective
To determine whether gene transfer of vasoactive intestinal peptide (VIP), based on its immunomodulatory properties, might be useful in the management of SS.
Methods
A recombinant serotype 2 adeno‐associated virus encoding the human VIP transgene (rAAV2hVIP) was constructed and its efficacy tested in the female non‐obese diabetic (NOD) mouse model for SS after retrograde instillation in submandibular glands (SMGs). 1010 particles/gland of rAAV2hVIP or rAAV2LacZ (encoding β‐galactosidase; control vector) were administered at 8 weeks of age (before sialadenitis onset). Salivary flow rates were determined before vector delivery and at time of death (16 weeks). After death, saliva, serum, and SMGs were harvested. Salivary output, inflammatory infiltrates (focus scores), VIP protein expression, cytokine profile, and serum anti‐VIP antibodies were analysed.
Results
rAAV2hVIP significantly improved the salivary flow, increased SMG and serum expression of VIP, and reduced SMG cytokines interleukin (IL) 2, IL10, IL12 (p70), and tumour necrosis factor α, and serum RANTES, compared with the control vector. No difference in focus scores or apoptotic rates was found; neutralising antibodies were not detected.
Conclusions
Local delivery of rAAV2hVIP can have disease modifying and immunosuppressive effects in SMGs of the NOD mouse model of SS. The new strategy of employing VIP prophylactically may be useful for both understanding and managing the salivary component of SS.
Keywords: vasoactive intestinal peptide, Sjögren's syndrome, gene transfer, adeno‐associated virus, autoimmune disease
Sjögren's syndrome (SS) is an autoimmune exocrinopathy of unknown aetiology, predominantly affecting peri‐ and post‐menopausal women.1 Although the main symptoms consist of ocular and oral dryness (xerophthalmia and xerostomia), there are also systemic effects. Features of affected glands are infiltrating, apoptosis resistant CD4+ T cells, and to a lesser extent, CD8+ T cells, B cells, and macrophages, and proinflammatory cytokines secreted from both lymphocytes and epithelial cells, together leading to inflammatory infiltrates, acinar atrophy, and destruction.2,3 At present, patients are only offered symptomatic treatment, which is often unsatisfactory.
Vasoactive intestinal peptide (VIP), initially discovered as a gastrointestinal hormone, exhibits abundant functions, ranging from neurotransmitter, vasodilator, and bronchodilator effects to acting as a trophic agent, secretagogue, and immunomodulator.4,5,6,7 VIP belongs to the glucagon/secretin superfamily.8 Its precursor protein, prepro‐VIP/PHM‐27, encoded on human chromosome 6,9 is an 8837 bp gene, containing seven exons and six introns and yielding the 28‐amino acid VIP.10 The amino acid sequence has been completely preserved in humans and mice11,12 and transgenic human VIP (hVIP) has been shown to act through mouse VIP receptors in transgenic mice.13
Based on its immunomodulatory properties, VIP possibly may be useful in the management of several autoimmune disorders,14 but its short half life may limit the applications of protein based treatment. In contrast, gene transfer potentially offers a means of sustained expression of a transgene like VIP.7 Because high serum VIP levels are associated with secretory diarrhoea in patients with a VIPoma,15 local treatment with VIP is preferable. Salivary glands provide an excellent target site for localised gene transfer after retrograde ductal infusion of vectors.16
Recently, we have constructed a recombinant serotype 5 adenovirus encoding the human VIP cDNA (rAd5CMVhVIP) and shown expression and function of the transgene.17 Recombinant adenoviral vectors offer strong, but short term, protein expression due to a potent immune response by the host.18 Adeno‐associated virus (AAV), a small, single stranded DNA, non‐pathogenic virus, has shown considerable promise as a viral vector for gene therapy. For recombinant serotype 2 AAV vectors (rAAV2) this is attributable to the ability to infect numerous mammalian cells, dividing as well as non‐dividing, and a minimal immune response.18,19 In previous in vivo studies we have demonstrated the therapeutic effects of different transgenes encoded by rAAV2 vectors, currently the most widely used serotype, when delivered to murine submandibular glands (SMGs),20,21,22 including local delivery of an rAAV2 encoding human interleukin 10 (hIL10) to the non‐obese diabetic (NOD) mouse.23 The NOD mouse develops, besides type I insulin dependent diabetes mellitus, exocrine gland infiltrates and decreased glandular secretion, which are dependent on age and sex,2,24,25 making it the most useful, commonly available animal model to study the disease properties of SS.
In this study we have constructed the vector, recombinant serotype 2 adeno‐associated virus encoding the human VIP transgene (rAAV2hVIP), and examined its ability to alter the progressive SS‐like dysfunction in NOD mice after local SMG delivery before disease onset.
Materials and methods
Construction of a viral vector encoding functional hVIP
We previously reported construction of the hVIP cDNA and the generation of a recombinant serotype 5 adenoviral vector rAd5CMVhVIP.17 The cytomegalovirus (CMV) promoter/enhancer, hVIP cDNA and simian virus 40 (SV40) polyadenylation signal from pAC‐CMV‐hVIP were subcloned into the rAAV2 plasmid pDT1.1 (containing the ampicillin resistance gene; described as pAAV‐MCS2.7 in Braddon et al20), resulting in pAAV2CMVhVIP (6391 bp, 1937 bp between inverted terminal repeats). The correct construct was verified by restriction digests and sequencing, and the presence of the inverted terminal repeats was confirmed by an SmaI digest (not shown). rAAV2hVIP was generated by subsequently cotransfecting this plasmid with the adenoviral helper packaging plasmid, pDG (a generous gift of Professor JA Kleinschmidt), at a ratio of 1:3 in 15 cm plates of ∼40% confluent 293 T cells using a calcium phosphate precipitation procedure.26 Additional details can be found in the supplementary information (available at http://www.annrheumdis.com/supplemental).
Infectious vectors were demonstrated by transducing 1.4×104 293 HEK cells on 96 well plates with serial dilutions of each aliquoted CsCl fraction in the presence of 1.5×107 particles of wild‐type adenovirus. After 24 hours of incubation, supernatants from infected cells were analysed with an enzyme linked immunosorbent assay (ELISA) for VIP (see below).
Construction of rAAV2LacZ
Previously, we reported construction, expression, and function of rAAV2LacZ (encoding β‐galactosidase; described as rAAVRnLacZ in Chiorini et al).23,27
Mice
Animal studies were approved by the National Institute of Dental and Craniofacial Research (NIDCR) Animal Care and Use Committee and the National Institutes of Health (NIH) Biosafety Committee. All procedures were conducted in accordance with the International Association for the Study of Pain (IASP) standards. Female NOD/LtJ mice (stock 001976) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Additional details can be found in the supplementary information (http://www.annrheumdis.com/supplemental).
Mice were maintained throughout the course of the study in the NIDCR animal facility (Bethesda, MD, USA) in accordance with Federal guidelines. Starting at age 10 weeks, body weights were measured weekly, as well as blood glucose levels (obtained by tail cut), using a OneTouch monitor (LifeScan, Milpitas, CA, USA). All mice with blood glucose levels ⩾22.2 mmol/l were given Ultra‐Lente insulin (Eli Lilly, Indianapolis, IN, USA) injections subcutaneously (5 U/mouse, every 24 hours) to limit diabetes related dehydration, as described.23
Gene transfer, and salivary, SMG, and serum collection
Initially, to investigate the effects of rAAV2hVIP administration on salivary gland function in NOD mice, we tested two rAAV2hVIP vector doses (109 and 1010 particles, seven animals/group) for each SMG (both glands targeted); the latter dose yielded the best results. For the second study, we instilled only one dose, 1010 particles in each gland (eight animals/group). The experiments described herein report the 1010 particles/SMG data from the two separate studies, over the indicated 8–16 week time course. Both studies gave comparable results. Vector delivery and saliva collection (at 8 weeks of age), and saliva, SMG, and blood collections (16 weeks) were performed as described in previous reports from our laboratory.20,21,22,23 Additional details can be found in the supplementary information (http://www.annrheumdis.com/supplemental).
Transgene expression
VIP expression and secretion were measured by an ELISA (Peninsula Laboratories, San Carlos, CA, USA) of cell supernatants, and murine saliva, serum, and SMG extracts. The assay sensitivity was ∼0.016 ng/ml; the polyclonal rabbit antibody was known to cross react with mouse and rat VIP. Murine samples were spiked with standard concentrations of recombinant VIP (Peninsula Laboratories), and different dilutions in assay buffer were used to investigate assay interference in biological samples.
Determination of serum antibodies against VIP
Serum antibodies to VIP were determined as follows. Aliquots of undiluted mouse serum were obtained at 16 weeks and pooled by vector group. To 50 μl of standard concentrations (0.016–10 ng/ml) of recombinant VIP (Peninsula Laboratories), 2 μl of pooled serum was added to obtain a final serum dilution of 1:26. Next, the samples were incubated at 37°C for 30 minutes. The VIP ELISA, containing polyclonal rabbit anti‐VIP antibodies (Peninsula Laboratories), was then used to detect unbound VIP protein, according to the manufacturer's instructions. A change in the ELISA standard curve, where the optical density is inversely related to the protein concentration, was used to detect the presence of anti‐VIP antibodies in NOD mouse sera.
Quantification of cytokines
IL2, IL4, IL6, IL10, IL12 (p70), interferon γ (IFNγ), tumour necrosis factor α (TNFα), and RANTES were measured commercially in SearchLight proteome arrays (Pierce Biotechnology, Woburn, MA, USA), which are multiplexed assays involving a sandwich ELISA procedure.23 Additional details can be found in the supplementary information (http://www.annrheumdis.com/supplemental).
Histological assessment of SMGs
Part of each SMG was fixed in 10% formalin overnight. Thereafter, the tissues were dehydrated in a series of graded ethanol solutions and embedded in paraffin. Three sections (5 μm thick), each 50 μm apart from the previous one, were mounted on poly‐l‐lysine coated slides and stained with haematoxylin‐eosin. Histopathological scoring of all three sections was performed by counting the number of foci present (one focus is an aggregate of 50 or more lymphocytes or histiocytes per 4 mm2).23,28,29 The scoring was done blindly by three examiners (BML, FM, APC), and the mean of all focus scores for each animal was then calculated.
Apoptosis of SMG epithelial cells
VIP reportedly can protect cells from apoptosis.30 Apoptotic epithelial cells were identified in SMG paraffin embedded sections by a terminal deoxynucleotidyl transferase (TdT)‐mediated dUTP‐biotin nick‐end labelling (TUNEL) assay using a cell death detection kit (Chemicon, Temecula, CA, USA), according to the manufacturer's instructions and as previously described.31 During subsequent visualisation by light microscopy, the number of apoptotic epithelial cells was counted blindly by three examiners (BML, FM, APC), and the apoptotic index determined as the percentage of apoptotic cells divided by the total amount of epithelial cells (counted with Scion Image Software (Scion Corporation, Frederick, MD, USA)). For each animal, three different fields in one section were examined. The mean of all apoptotic indices for each animal was then calculated.
Statistical analysis
Data analysis consisted of descriptive statistics, reported as means (SEM) or medians, unpaired Student's t tests, and Mann‐Whitney rank sum tests, as appropriate.
Results
VIP expression by rAAV2hVIP in vitro
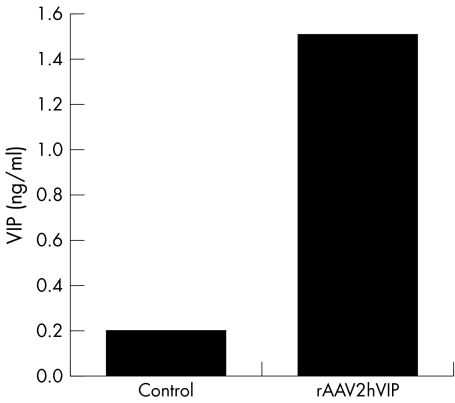
The highest particle titres of rAAV2hVIP were typically found in fractions with a refractive index of ∼1.372, equivalent to a buoyant density of ∼1.4 g/ml, and corresponded to 1.4×1013 particles/ml. VIP expression after transduction of 293 HEK cells with these fractions showed that 4.9×103 particles rAAV2hVIP per cell resulted in 1.5 ng VIP/ml culture medium (sevenfold increase over background) (fig 1). Functional activity of the hVIP transgene expressed from this transgene cassette has been previously described.17
Figure 1 In vitro VIP expression directed by rAAV2hVIP. Transduction of 293 HEK cells with rAAV2hVIP (multiplicity of infection (MOI) = 4.9×103), in the presence of wild‐type adenovirus (MOI = 1.0×103); control represents identical amount of 293 HEK cells infected with wild‐type adenovirus alone. This experiment was analysed in duplicate and is representative of four separate experiments.
VIP expression in vivo
VIP expression was measured in saliva, serum, and protein extracts of SMGs. Reliable results were obtained for serum and SMG extracts (see supplementary table 1 (http://www.annrheumdis.com/supplemental)). Animals given the rAAV2hVIP vector exhibited significantly higher levels of VIP (ng/ml, mean (SEM)) than controls in SMG extracts (0.49 (0.03) v 0.36 (0.05), p = 0.039), and in serum (0.16 (0.01) v 0.13 (0.01), p = 0.030). It was not possible to discern salivary VIP expression accurately because of a complex cross reactivity in salivary samples (data not shown).
Table 1 Levels of inflammatory molecules in SMG extracts.
| Cytokine | LacZ | hVIP | p Value |
|---|---|---|---|
| IL2 | 0.13 (0.03) | 0.01 (0.01) | 0.001 |
| IL4* | 0.00 | 0.00 | 1.000 |
| IL6* | 0.00 | 0.00 | 0.397 |
| IL10* | 0.05 | 0.00 | 0.054 |
| IL12 (p70) | 0.10 (0.00) | 0.05 (0.01) | 0.008 |
| TNFα* | 0.05 | 0.00 | 0.021 |
| IFNγ | 6.46 (1.19) | 5.97 (0.59) | 0.708 |
| RANTES | 3.75 (0.39) | 2.68 (0.54) | 0.142 |
Protein expression of immunomodulatory molecules (pg/mg wet weight) in SMG extracts after administration of rAAV2LacZ (LacZ, n = 7) or rAAV2hVIP (hVIP, n = 8). Means (SEM) are shown unless otherwise noted.
*Mann‐Whitney rank sum test with median values.
Serum anti‐VIP antibodies
To determine the presence of serum anti‐VIP antibodies, pooled serum samples of 16 week old mice treated with rAAV2LacZ or rAAV2hVIP were incubated with standard concentrations of recombinant VIP and assessed by an ELISA. No differences were seen between the two vector groups, indicating that local delivery of rAAV2hVIP did not result in the development of serum anti‐VIP antibodies 8 weeks after vector delivery (see supplementary fig 2 (http://www.annrheumdis.com/supplemental)).
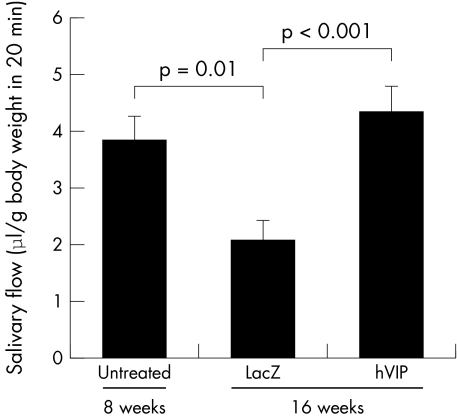
Figure 2 Effect of rAAV2hVIP and rAAV2LacZ on salivary flow rate in NOD mice. Mice were anaesthetised and pilocarpine stimulated whole saliva was collected, as described in “Materials and methods”. Salivary flow rate (μl/body weight (grams) in 20 minutes) at 8 weeks (n = 19; randomly selected from both groups, untreated) and mice at 16 weeks of age either treated with rAAV2LacZ (LacZ, n = 13) or rAAV2hVIP (hVIP, n = 15) is shown. Bars represent means (SEM). Student's t tests were performed and the p values for the differences are indicated.
Effect of rAAV2hVIP and rAAV2LacZ on salivary function in NOD mice
Female NOD mice show a progressive decline in salivary flow rates starting between 8 and 12 weeks of age.28 We examined salivary flow before virus administration at 8 weeks of age (baseline) and at the time of death (16 weeks). At baseline, the mean (SEM) salivary flow rate was 3.81 (0.47) μl/body weight (grams) in 20 minutes. At 16 weeks of age, 8 weeks after vector delivery, the salivary flow rates for the rAAV2LacZ and rAAV2hVIP group were, respectively, 2.05 (0.36) and 4.32 (0.47) (p<0.001; fig 2). The decrease in flow rate of mice treated with rAAV2LacZ at 16 weeks compared with 8 weeks was also significant (p = 0.01).
Effect of rAAV2hVIP and rAAV2LacZ on inflammatory infiltrates in SMGs of NOD mice
Female NOD mice start developing characteristic focal inflammatory infiltrates in SMGs after 8 weeks of age28; such local changes also form an important clinical feature in patients with SS. SMG sections of 16 week old rAAV2hVIP treated mice showed no difference in focus scores (mean (SEM)) from the group treated with rAAV2LacZ (1.93 (0.11) v 1.85 (0.26), p = 0.75).
Effect of rAAV2hVIP and rAAV2LacZ on apoptotic rate in SMGs of NOD mice
The median apoptotic index, as an indication of the apoptotic rate, of epithelial cells in SMGs did not differ between the rAAV2LacZ and rAAV2hVIP groups (medians 0.15 v 0.16, respectively, p = 0.45).
Effect of rAAV2hVIP and rAAV2LacZ on cytokine expression in SMG extracts and sera of NOD mice
VIP can act as an immunomodulator, inhibiting levels of proinflammatory cytokines32,33 and increasing levels of anti‐inflammatory cytokines.34 We examined protein expression of several pro‐ and anti‐inflammatory cytokines and one chemokine, locally in extracts of SMGs, as well as systemically in serum, in treated mice at 16 weeks of age. Aqueous SMG extracts from NOD mice treated with rAAV2hVIP showed lower levels of IL2 (p = 0.001), IL10 (p = 0.054), IL12 (p70) (p = 0.008), and TNFα (p = 0.021) than those from mice treated with rAAV2LacZ (table 1). IL4, IL6, IFNγ, and RANTES levels in SMG extracts did not differ significantly between these groups. Serum levels of all measured cytokines did not differ between the two vector groups (supplementary table 2 (http://www.annrheumdis.com/supplemental)). However, levels of RANTES in serum were significantly lower in rAAV2hVIP treated mice (6.8 v 12.2 pg/ml, p = 0.040).
Discussion
As far as we know, this is the first report examining the efficacy of transgenic VIP in a model of SS. Additionally, we are not aware of any publications using protein based VIP treatment to alter the disease course of SS, although administration of the protein VIP has been successfully employed in other models of autoimmune diseases, such as collagen‐induced arthritis35,36 and experimental autoimmune uveoretinitis.37 An rAAV2hVIP was constructed and its immunomodulatory and clinical efficacy tested in the NOD mouse model for SS. For the present study, we chose to treat the animals early before disease onset—that is, a prevention, not treatment, model. Based on the results, in the future, we will test the effects of rAAV2hVIP upon administration after disease onset, more closely resembling a clinical situation. Instillation of rAAV2hVIP in the SMGs of NOD mice led to higher salivary flow rates, no difference in focus scores or apoptotic rates, increased SMG and serum expression of VIP, a reduction of cytokines IL2, IL10, IL12 (p70), and TNFα in SMG extracts and of serum RANTES, compared with the results with the control vector.
Salivary gland cells are polarised and can secrete transgene encoded proteins either via the constitutive pathway across the basolateral membrane, or on stimulation via the regulated pathway into saliva.38,39,40 Pre/pro‐VIP protein contains a recognition signal directing it into secretory granules during post‐translational processes.41 Because the hVIP cDNA used here should contain that signal,17 salivary cells should secrete hVIP mostly via the regulated pathway into saliva, with only a small proportion exiting to the interstitium and serum.38,39,42 VIP is a small neuropeptide, quickly degraded and inactivated, giving it a limited bioavailability.43 However, by adding protease inhibitors/EDTA to samples herein, as well as by performing multiple dilution and spiking experiments, we were able to determine accurate VIP levels in both serum and SMG extracts. rAAV2hVIP treated mice showed significantly higher VIP expression in SMG extracts and serum than control rAAV2LacZ treated mice. Unfortunately, owing to a complex cross reactivity pattern, we were unable to discern reliable salivary VIP expression despite multiple controls and precautions. We have not studied VIP levels in human saliva, but this was previously done in studies employing the same precautions as we did.44,45 The protein levels expressed from the rAAV2hVIP vector in vivo, while low, are reasonable and comparable to those of other transgenic secretory proteins in rAAV2 experiments.21,22
We observed a decrease in the levels of several proinflammatory cytokines, IL2, IL12 (p70), and TNFα, but also in the anti‐inflammatory cytokine IL10, in SMG extracts after hVIP gene transfer. Importantly, serum cytokines were not different between the two rAAV vector groups, with only levels of the chemokine RANTES being lower in the serum of rAAV2hVIP treated animals. Several previous studies with untreated NOD/LtJ mice, of about the same age as the NOD mice studied by us, showed comparable serum cytokine levels to our data (for example, see Yang et al46,47). To our knowledge, SMG cytokine protein levels of 16 week old NOD/LtJ mice have not been previously reported.
In the immune system VIP acts as an autocrine regulator.32 The VIP/PACAP receptor family consists of three G‐protein coupled receptors, two of which, VPAC1 and VPAC2, are present on macrophages, and CD4+ and CD8+ T lymphocytes.48,49 The effects of VIP are wide ranging, including the inhibition of several macrophage functions, T cell proliferation, lymphocyte migration, and the expression of chemokines and proinflammatory cytokines.7,32,33 Conversely, the production of IL10 is reportedly stimulated by VIP.34 Pozo et al postulated that VIP is a Th2 cytokine with a key role in neuroimmunology34,50—that is, VIP production by Th2 cells, as well as VIP stimulation of Th2, and inhibition of Th1, functions. Although VIP may exert these effects in some autoimmune diseases with a balance shifted towards Th1 response, such as rheumatoid arthritis,51 our results here suggest it may be a different case in the NOD mouse model and SS. Upon gene transfer of hVIP, we did not observe a significant shift from Th1 to Th2 cytokine production. In fact, hVIP gene transfer led to an unexpected down regulation of SMG IL10 levels and may indicate that VIP acts as a more overall immunosuppressant than strict Th2 cytokine in this SS model. In patients with SS, salivary gland and serum IL10 levels are actually increased, depending on the disease stage and activity, and might be a sign of B cell activation and lymphoma development.52,53,54,55 As recently stated by Delaleu et al, “further approaches modulating cytokines are warranted in SS”.56
Although the exact immunopathogenesis still waits to be elucidated, SS is thought to resemble an imbalance in cytokine production, locally as well as systemically, depending on disease stage and severity.3,56 However, the cytokine profile in SMG biopsies with simultaneous expression of IFNγ, IL2, IL4, and IL13 provides an argument against a simple Th1 or Th2 predominance in SS.3 Clearly, hVIP gene transfer to SMGs in the present study resulted in local immunomodulatory activity.
Salivary flow rates were increased in the rAAV2hVIP group, but lymphocytic infiltrates (focus scores) were unaffected. Although historically xerostomia in patients with SS was solely attributed to a destruction of glandular tissue, a well recognised incongruity exists between the reduction in salivary flow and the extent of focal lymphoid infiltration.57,58 Hypofunction of salivary epithelial cells might be, in addition, due to the effects of cytokines or autoantibodies (for example, anti‐muscarinic receptor antibodies) in patients with SS.59,60 Thus, it is not entirely unexpected that a dissociation was seen in rAAV2hVIP effects on salivary flow rate and focal SMG inflammatory infiltrates.
Salivary glands consist of acinar cells, forming the primary saliva, and ductal cells, which primarily reabsorb NaCl, but are relatively impermeable to water.16 rAAV2 vectors appear to transduce only ductal cells, not acinar cells.21,22 Therefore, we suggest that the transgenic hVIP secreted by the murine transduced ductal cells will bind to VIP receptors on surrounding lymphocytes, as well as conceivably membranes of adjacent acinar cells, thereby influencing the immune milieu and/or directly facilitating enhanced salivary secretion.
In human SMGs, VIP receptors are found on both luminal and basal membranes of mucous acinar cells, as well as intercalated ducts.61 In human labial salivary glands, VIP binding sites are detected on the basal membranes of mucous acini,62 and the presence of a functional VIP receptor system in vitro has been demonstrated on isolated acinar cells.63 There are no data on VIP receptors in epithelial cells of mouse SMGs. In addition, several studies of patients with SS have shown the existence of immunoreactive VIP nerve fibres in human SMGs61 and human labial salivary glands,63,64 where VIP containing nerves are in close contact with the acinar cells, inside the acinar basement membrane.44 Taken together, it is thought that VIP has a role in several components of reflex salivary secretion.62,65
Conclusion
This is the first study describing the effect of VIP gene transfer in a model of SS. We have employed local administration of an rAAV2 vector encoding hVIP to murine SMGs and shown local disease modifying and immunosuppressive effects. The data suggest that VIP may be useful in the management of the salivary component of SS.
Supplementary Material
Acknowledgements
We thank Dr Christine Delporte for her expert knowledge on VIP and helpful suggestions.
Abbreviations
AAV - adeno‐associated virus
ELISA - enzyme linked immunosorbent assay
IFNγ - interferon γ
IL - interleukin
NOD - non‐obese diabetic
SMG - submandibular gland
SS - Sjögren's syndrome
TNFα - tumour necrosis factor α
VIP - vasoactive intestinal peptide
Footnotes
Conflict: No conflict of interest has been declared by the authors.
References
- 1.Jonsson R, Hagan H‐J, Gordon T. Sjögren's syndrome. In: Koopman WJ, ed. Arthritis and allied conditions ‐ a textbook of rheumatology. 14th ed. Philadelphia: Lippincott Williams & Wilkins, February20011736–1759.
- 2.Cha S, Peck A B, Humphreys‐Beher M G. Progress in understanding autoimmune exocrinopathy using the non‐obese diabetic mouse: an update. Crit Rev Oral Biol Med 2002135–16. [DOI] [PubMed] [Google Scholar]
- 3.Borchers A T, Naguwa S M, Keen C L, Gershwin M E. Immunopathogenesis of Sjögren's syndrome. Clin Rev Allergy Immunol 20032589–104. [DOI] [PubMed] [Google Scholar]
- 4.Said S I. Vasoactive intestinal peptide. J Endocrinol Invest 19869191–200. [DOI] [PubMed] [Google Scholar]
- 5.Gozes I, Furman S. VIP and drug design. Curr Pharm Des 20039483–494. [DOI] [PubMed] [Google Scholar]
- 6.Voice J K, Dorsam G, Chan R C, Grinninger C, Kong Y, Goetzl E J. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept 2002109199–208. [DOI] [PubMed] [Google Scholar]
- 7.Delgado M, Abad C, Martinez C, Juarranz M G, Arranz A, Gomariz R P.et al Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med 20028016–24. [DOI] [PubMed] [Google Scholar]
- 8.Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI‐27‐like peptide, PHM‐27. Nature 1983304547–549. [DOI] [PubMed] [Google Scholar]
- 9.Gozes I, Avidor R, Yahav Y, Katznelson D, Croce C M, Huebner K. The gene encoding vasoactive intestinal peptide is located on human chromosome 6p21‐‐‐‐6qter. Hum Genet 19877541–44. [DOI] [PubMed] [Google Scholar]
- 10.Yamagami T, Ohsawa K, Nishizawa M, Inoue C, Gotoh E, Yanaihara N.et al Complete nucleotide sequence of human vasoactive intestinal peptide/PHM‐27 gene and its inducible promoter. Ann N Y Acad Sci 198852787–102. [DOI] [PubMed] [Google Scholar]
- 11.Brenneman D E, Hill J M, Gozes I. Vasoactive intestinal peptide in the central nervous system. Psychopharmacology ‐ the fourth generation of progress 2000: [27 screens], Available from: http://www.acnp.org/g4/GN401000057/CH057.html (accessed 1 November 2005)
- 12.Lamperti E D, Rosen K M, Villa‐Komaroff L. Characterization of the gene and messages for vasoactive intestinal polypeptide (VIP) in rat and mouse. Brain Res Mol Brain Res 19919217–231. [DOI] [PubMed] [Google Scholar]
- 13.Kato I, Suzuki Y, Akabane A, Yonekura H, Tanaka O, Kondo H.et al Enhancement of glucose‐induced insulin secretion in transgenic mice overexpressing human VIP gene in pancreatic beta cells. Ann N Y Acad Sci. 1996;805: 232–42; discussion 42–3, [DOI] [PubMed]
- 14.Firestein G S. VIP: a very important protein in arthritis. Nat Med 20017537–538. [DOI] [PubMed] [Google Scholar]
- 15.Park S K, O'Dorisio M S, O'Dorisio T M. Vasoactive intestinal polypeptide‐secreting tumours: biology and therapy. Baillieres Clin Gastroenterol 199610673–696. [DOI] [PubMed] [Google Scholar]
- 16.Baum B J, Wellner R B, Zheng C. Gene transfer to salivary glands. Int Rev Cytol 200221393–146. [DOI] [PubMed] [Google Scholar]
- 17.Lodde B M, Delporte C, Goldsmith C M, Tak P P, Baum B J. A recombinant adenoviral vector encoding functional vasoactive intestinal peptide. Biochem Biophys Res Commun 2004319189–192. [DOI] [PubMed] [Google Scholar]
- 18.Lai C M, Lai Y K, Rakoczy P E. Adenovirus and adeno‐associated virus vectors. DNA Cell Biol 200221895–913. [DOI] [PubMed] [Google Scholar]
- 19.Conlon T J, Flotte T R. Recombinant adeno‐associated virus vectors for gene therapy. Expert Opin Biol Ther 200441093–1101. [DOI] [PubMed] [Google Scholar]
- 20.Braddon V R, Chiorini J A, Wang S, Kotin R M, Baum B J. Adenoassociated virus‐mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther 199892777–2785. [DOI] [PubMed] [Google Scholar]
- 21.Yamano S, Huang L Y, Ding C, Chiorini J A, Goldsmith C M, Wellner R B.et al Recombinant adeno‐associated virus serotype 2 vectors mediate stable interleukin 10 secretion from salivary glands into the bloodstream. Hum Gene Ther 200213287–298. [DOI] [PubMed] [Google Scholar]
- 22.Voutetakis A, Kok M R, Zheng C, Bossis I, Wang J, Cotrim A P.et al Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A 20041013053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok M R, Yamano S, Lodde B M, Wang J, Couwenhoven R I, Yakar S.et al Local adeno‐associated virus‐mediated interleukin 10 gene transfer has disease‐modifying effects in a murine model of Sjögren's syndrome. Hum Gene Ther 2003141605–1618. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys‐Beher M G, Peck A B. New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model. Arch Oral Biol 199944(suppl 1)S21–S25. [DOI] [PubMed] [Google Scholar]
- 25.Kok M R, Baum B J, Tak P P, Pillemer S R. Use of localised gene transfer to develop new treatment strategies for the salivary component of Sjögren's syndrome. Ann Rheum Dis 2003621038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski R J. Production of high‐titer recombinant adeno‐associated virus vectors in the absence of helper adenovirus. J Virol 1998722224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiorini J A, Wendtner C M, Urcelay E, Safer B, Hallek M, Kotin R M. High‐efficiency transfer of the T cell co‐stimulatory molecule B7‐2 to lymphoid cells using high‐titer recombinant adeno‐associated virus vectors. Hum Gene Ther 199561531–1541. [DOI] [PubMed] [Google Scholar]
- 28.Yamano S, Atkinson J C, Baum B J, Fox P C. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol 199992265–275. [DOI] [PubMed] [Google Scholar]
- 29.Greenspan J S, Daniels T E, Talal N, Sylvester R A. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 197437217–229. [DOI] [PubMed] [Google Scholar]
- 30.Said S I, Dickman K G. Pathways of inflammation and cell death in the lung: modulation by vasoactive intestinal peptide. Regul Pept 20009321–29. [DOI] [PubMed] [Google Scholar]
- 31.Kiatipattanasakul W, Nakayama H, Nakamura S, Doi K. Lectin histochemistry in the aged dog brain. Acta Neuropathol (Berl) 199895261–268. [DOI] [PubMed] [Google Scholar]
- 32.Pozo D, Delgado M, Martinez M, Guerrero J M, Leceta J, Gomariz R P.et al Immunobiology of vasoactive intestinal peptide (VIP). Immunol Today 2000217–11. [DOI] [PubMed] [Google Scholar]
- 33.Grimm M C, Newman R, Hassim Z, Cuan N, Connor S J, Le Y.et al Cutting edge: vasoactive intestinal peptide acts as a potent suppressor of inflammation in vivo by trans‐deactivating chemokine receptors. J Immunol 20031714990–4994. [DOI] [PubMed] [Google Scholar]
- 34.Delgado M, Munoz‐Elias E J, Gomariz R P, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide enhance IL‐10 production by murine macrophages: in vitro and in vivo studies. J Immunol 19991621707–1716. [PubMed] [Google Scholar]
- 35.Delgado M, Abad C, Martinez C, Leceta J, Gomariz R P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 20017563–568. [DOI] [PubMed] [Google Scholar]
- 36.Williams R O. Therapeutic effect of vasoactive intestinal peptide in collagen‐induced arthritis. Arthritis Rheum 200246271–273. [DOI] [PubMed] [Google Scholar]
- 37.Keino H, Kezuka T, Takeuchi M, Yamakawa N, Hattori T, Usui M. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol 20041221179–1184. [DOI] [PubMed] [Google Scholar]
- 38.Baum B J, Berkman M E, Marmary Y, Goldsmith C M, Baccaglini L, Wang S.et al Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther 1999102789–2797. [DOI] [PubMed] [Google Scholar]
- 39.Hoque A T, Baccaglini L, Baum B J. Hydroxychloroquine enhances the endocrine secretion of adenovirus‐directed growth hormone from rat submandibular glands in vivo. Hum Gene Ther 2001121333–1341. [DOI] [PubMed] [Google Scholar]
- 40.Castle J D, Castle A M. Sorting and secretion of salivary proteins. Crit Rev Oral Biol Med 19934393–398. [DOI] [PubMed] [Google Scholar]
- 41.Steiner D F, Quinn P S, Chan S J, Marsh J, Tager H S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci 19803431–16. [DOI] [PubMed] [Google Scholar]
- 42.Marmorstein A D, Csaky K G, Baffi J, Lam L, Rahaal F, Rodriguez‐Boulan E. Saturation of, and competition for entry into, the apical secretory pathway. Proc Natl Acad Sci U S A 2000973248–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnadas A, Onyuksel H, Rubinstein I. Interactions of VIP, secretin and PACAP(1‐38) with phospholipids: a biological paradox revisited. Curr Pharm Des 200391005–1012. [DOI] [PubMed] [Google Scholar]
- 44.Tornwall J, Konttinen Y T, Hietanen J, Sorsa T, Hukkanen M, Uusitalo H. VIP in salivary glands in Sjögren's syndrome. Br J Rheumatol 199534891–893. [DOI] [PubMed] [Google Scholar]
- 45.Santavirta N, Konttinen Y T, Tornwall J, Segerberg M, Santavirta S, Matucci‐Cerinic M.et al Neuropeptides of the autonomic nervous system in Sjögren's syndrome. Ann Rheum Dis 199756737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z D, Chen M, Wu R, McDuffie M, Nadler J L. The anti‐inflammatory compound lisofylline prevents type I diabetes in non‐obese diabetic mice. Diabetologia 2002451307–1314. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Chen M, Ellett J D, Fialkow L B, Carter J D, Nadler J L. The novel anti‐inflammatory agent lisofylline prevents autoimmune diabetic recurrence after islet transplantation. Transplantation 20047755–60. [DOI] [PubMed] [Google Scholar]
- 48.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept 2002108165–173. [DOI] [PubMed] [Google Scholar]
- 49.Goetzl E J, Sreedharan S P. Mediators of communication and adaptation in the neuroendocrine and immune systems. FASEB J 199262646–2652. [DOI] [PubMed] [Google Scholar]
- 50.Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J 2004181325–1334. [DOI] [PubMed] [Google Scholar]
- 51.Vervoordeldonk M J, Tak P P. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep 20024208–217. [DOI] [PubMed] [Google Scholar]
- 52.Garcic‐Carrasco M, Font J, Filella X, Cervera R, Ramos‐Casals M, Siso A.et al Circulating levels of Th1/Th2 cytokines in patients with primary Sjögren's syndrome: correlation with clinical and immunological features. Clin Exp Rheumatol 200119411–415. [PubMed] [Google Scholar]
- 53.Anaya J M, Correa P A, Herrera M, Eskdale J, Gallagher G. Interleukin 10 (IL‐10) influences autoimmune response in primary Sjögren's syndrome and is linked to IL‐10 gene polymorphism. J Rheumatol 2002291874–1876. [PubMed] [Google Scholar]
- 54.Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, Fujimura T.et al Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren's syndrome. Arthritis Rheum 1996391376–1384. [DOI] [PubMed] [Google Scholar]
- 55.Mitsias D I, Tzioufas A G, Veiopoulou C, Zintzaras E, Tassios I K, Kogopoulou O.et al The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjögren's syndrome. Clin Exp Immunol 2002128562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delaleu N, Jonsson M V, Jonsson R. Disease mechanisms of Sjögren's syndrome. Drug Discovery Today: Disease Mechanisms 20041329–336. [Google Scholar]
- 57.Lindahl G, Hedfors E. Lymphocytic infiltrates and epithelial HLA‐DR expression in lip salivary glands in connective tissue disease patients lacking sicca: a prospective study. Br J Rheumatol 198928293–298. [DOI] [PubMed] [Google Scholar]
- 58.Jonsson R, Kroneld U, Backman K, Magnusson B, Tarkowski A. Progression of sialadenitis in Sjögren's syndrome. Br J Rheumatol 199332578–581. [DOI] [PubMed] [Google Scholar]
- 59.Fox R I, Stern M. Sjögren's syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl 20023–13. [PubMed]
- 60.Hocevar A, Tomsic M, Praprotnik S, Hojnik M, Kveder T, Rozman B. Parasympathetic nervous system dysfunction in primary Sjögren's syndrome. Ann Rheum Dis 200362702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kusakabe T, Matsuda H, Gono Y, Kawakami T, Kurihara K, Tsukuda M.et al Distribution of VIP receptors in the human submandibular gland: an immunohistochemical study. Histol Histopathol 199813373–378. [DOI] [PubMed] [Google Scholar]
- 62.Tornwall J, Uusitalo H, Hukkanen M, Sorsa T, Konttinen Y T. Distribution of vasoactive intestinal peptide (VIP) and its binding sites in labial salivary glands in Sjögren's syndrome and in normal controls. Clin Exp Rheumatol 199412287–292. [PubMed] [Google Scholar]
- 63.Pedersen A M, Dissing S, Fahrenkrug J, Hannibal J, Reibel J, Nauntofte B. Innervation pattern and Ca2+ signalling in labial salivary glands of healthy individuals and patients with primary Sjögren's syndrome (pSS). J Oral Pathol Med 20002997–109. [DOI] [PubMed] [Google Scholar]
- 64.Konttinen Y T, Hukkanen M, Kemppinen P, Segerberg M, Sorsa T, Malmstrom M.et al Peptide‐containing nerves in labial salivary glands in Sjögren's syndrome. Arthritis Rheum 199235815–820. [DOI] [PubMed] [Google Scholar]
- 65.Martin S C, Shuttleworth T J. The control of fluid‐secreting epithelia by VIP. Ann N Y Acad Sci. 1996;805: 133–47; discussion 47–8, [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.