Abstract
Objective
To investigate the diagnostic reliability of anti‐CCP antibodies (anti‐CCP Ab) in distinguishing hepatitis C virus (HCV) associated rheumatological manifestations and Sjögren's syndrome from rheumatoid arthritis.
Methods
147 HCV infected patients (HCV RNA positive) were compared with 64 patients with definite rheumatoid arthritis in a retrospective study. Anti‐CCP Ab were detected using the Immunoscan ELISA kit (second generation) and rheumatoid factor (RF) by the FIDIS™ Rheuma kit.
Results
Among the 147 HCV infected patients (77 women; mean (SD) age 58 (16) years), 77 (52%) had a mixed cryoglobulin (MC), 38 (26%) an MC associated systemic vasculitis, 35 (24%) arthralgia/arthritis, and seven (5%) definite Sjögren's syndrome. HCV infected patients with arthralgia were more often RF positive than those without arthralgia (54% v 27%; p = 0.003), but less often than patients with rheumatoid arthritis (54% v 81%; p = 0.009). Anti‐CCP Ab were detected in only two HCV infected patients with arthralgia (5.7%), in none without arthralgia or with Sjögren's syndrome, and in 78% of patients with rheumatoid arthritis. With a specificity of 93.5% and a positive predictive value of 96% for rheumatoid arthritis, anti‐CCP Ab were the most specific biological marker.
Conclusions
Anti‐CCP antibodies are very rarely found in HCV infected patients with rheumatological manifestations or Sjögren's syndrome. They are reliable serological markers to distinguish these from patients with rheumatoid arthritis.
Keywords: hepatitis C virus, arthritis, Sjögren's syndrome, anti‐CCP, rheumatoid factor
The hepatitis C virus (HCV) infection is a worldwide disease that affects more than 170 million people. Besides liver complications, mainly chronic hepatitis and cirrhosis, many extrahepatic manifestations have been found to be HCV associated.1 Polyarthralgia is the most common rheumatological symptom in HCV infected patients (20–83%). Arthritis is rarely reported and is characterised by an intermittent, mono‐ or oligoarticular, non‐destructive arthritis or symmetrical polyarthritis mimicking rheumatoid arthritis.2,3,4 A sicca syndrome is also often reported in HCV infected patients,5,6 as it is in rheumatoid arthritis.7 Some HCV infected patients with a sicca syndrome have true Sjögren's syndrome, with prevalences ranging from 9% to 56% depending on the diagnostic criteria.6 HCV infection is, moreover, commonly associated with the detection of IgM rheumatoid factor (RF), with prevalences ranging from 30% to 68% depending on the presence of mixed cryoglobulinaemia.1,3 In the light of these features, the distinction between HCV associated arthropathy and recent onset rheumatoid arthritis, when articular damage and deformities have not yet occurred, may be difficult. As a possible way of distinguishing between these two conditions, anti‐keratin antibodies (AKA) have been investigated. These share a high specificity for rheumatoid arthritis8 and were found scarce among HCV infected patients with arthropathy.9 However, there are technical difficulties in standardising AKA, and poor sensitivity for their detection in rheumatoid arthritis. Several studies have shown that anti‐cyclic citrullinated peptide antibodies (anti‐CCP Ab) in rheumatoid arthritis have high sensitivity (41–70%) and specificity (91–98%), depending on the population under study and the generation of an enzyme linked immunosorbent assay (ELISA) kit.10,11 Anti‐CCP Ab now appear to be the most specific antibodies for the diagnosis of rheumatoid arthritis, and the easiest to detect.
To our knowledge, there has been only one small study investigating anti‐CCP Ab in patients with HCV associated arthropathy.12 Our aim in the present study was to evaluate the prevalence of the anti‐CCP Ab in a large population of HCV infected patients with rheumatological manifestations or Sjögren's syndrome, and to determine its diagnostic reliability in discriminating such cases from rheumatoid arthritis.
Methods
Patients
The study included 147 HCV infected patients (positive anti‐HCV antibodies and HCV RNA), followed in the same internal medicine department. Epidemiological, clinical, immunochemical, virological, and liver histology features were collected retrospectively for each patient.
Sjögren's syndrome was defined, according to the European classification criteria,13 by the presence of a sicca syndrome (ocular or buccal) and severe histological damage on labial salivary gland biopsy, corresponding to stages III and IV of Chisholm and Mason's scoring system. The presence of anti‐SSA or anti‐SSB antibodies was assessed (detected by ELISA) but was not required for the diagnosis of Sjögren's syndrome.
A control group for the diagnostic reliability of the anti‐CCP Ab and RF comprised 64 patients with a definite diagnosis of rheumatoid arthritis (78.5% female), fulfilling the American College of Rheumatology criteria for this disease.14
Anti‐CCP Ab and RF determination
Sera previously stored at −80°C were used for the detection of RF and anti‐CCP Ab. The anti‐CCP Ab were detected using an Immunoscan rheumatoid arthritis ELISA kit (second generation; Euro‐Diagnostica, provided by DiaSorin, Antony, France) with a manufacturer's cut off of positivity of 25 U/ml; IgM‐RF was detected using the FIDIS™ Rheuma kit (BMD, Marne la Vallée, France) with a manufacturer's cut off of positivity of 30 IU/ml.
Statistical analysis
Qualitative values between two groups were compared by the χ2 test and quantitative values by the non‐parametric Mann–Whitney test using EPI INFO 2002 software (CDC, Atlanta, Georgia, USA). Significance was assumed at p<0.05. The MedCalc® software (version 7.5.0.0) was used for the evaluation of the diagnostic features (receiver operating characteristic (ROC) curve, sensitivity, specificity, and positive and negative predictive values).
Results
Epidemiological and clinical features of the patients studied
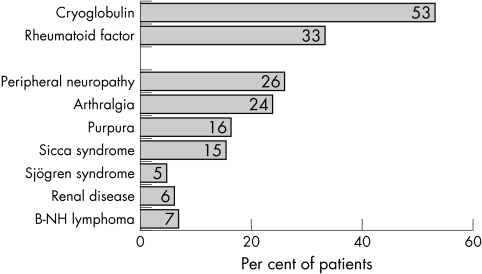
We enrolled 147 HCV infected patients with a mean (SD) age of 58 (15.6) years; 52% were women and 56% of HCV genotype 1. At liver biopsy (analysed using the Metavir scoring system), 47% had significant fibrosis (F2F3F4) and 13.6% had cirrhosis (F4). Seventy eight patients (53%) had a mixed cryoglobulin and 38 (26%) presented with clinical manifestations related to systemic vasculitis. Overall, 64 HCV infected patients (43.5%) had at least one of the clinical extrahepatic manifestations detailed in fig 1. Thirty five had rheumatological involvement (23.8%) and seven had Sjögren's syndrome (4.8%); none of the latter were positive for anti‐SSA or anti‐SSB antibodies.
Figure 1 Hepatitis C virus associated extrahepatic manifestations among infected patients. B‐NH lymphoma, non‐Hodgkin B cell lymphoma.
The 35 HCV infected patients with arthralgia tended to be older (62.2 (16) v 56.6 (15) years; p = 0.07) and more often female (66% v 48%; p = 0.07) than the patients without arthralgia. They more often had mixed cryoglobulins (86% v 43%; p<10−4), mostly type II MC (60% v 17.9%; p<10−4). There was no difference in the severity of liver disease (table 1).
Table 1 Comparative analysis of hepatitis C virus infected patients according to the presence of arthralgia.
| HCV with arthralgia (n = 35) | HCV without arthralgia (n = 112) | p Value | ||||
|---|---|---|---|---|---|---|
| Female | 23 (65.7%) | 54 (48.2%) | 0.07 | |||
| Age (years) (mean (SD)) | 62.2 (16.0) | 56.6 (15.0) | 0.07 | |||
| Metavir fibrosis score (mean (SD)) | 1.8 (1.1) | 1.7 (1.3) | 0.5 | |||
| Mixed cryoglobulin, n (%) | 30 (85.7%) | 48 (42.9%) | <10−4 | |||
| Type II | 21 (60%) | 20 (17.9%) | <10−4 | |||
| Type III | 8 (22.9%) | 22 (19.6%) | 0.86 | |||
| Oligoclonal type | 1 (2.9%) | 6 (5.4%) | 0.9 | |||
| RF positive | 19 (54.3%) | 30 (26.8%) | 0.003 | |||
| Anti‐CCP Ab | 2 (5.7%) | 0 (0%) | 0.01 |
Values are n (%) unless stated otherwise.
Anti‐CCP Ab, anti‐cyclic citrullinated peptide antibodies; HCV, hepatitis C virus; RF, rheumatoid factor.
The 64 patients with definite rheumatoid arthritis were mainly female (87.5%). Their mean (SD) age was 58.6 (14.1) years, similar to the HCV infected patients (57.7 (15.5) years) (p = 0.9). None of them had definite Sjögren's syndrome.
Diagnostic values of the rheumatoid factor assay
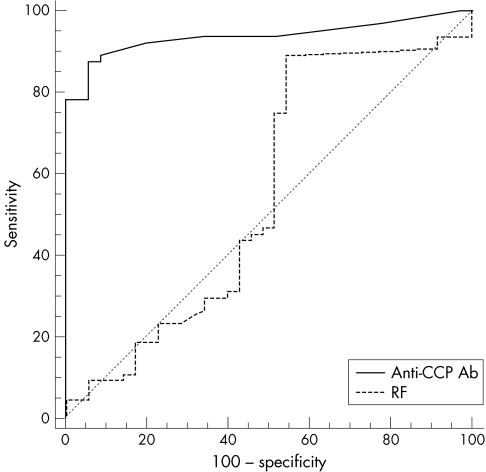
RF was more often positive in patients with rheumatoid arthritis (81%) than in the HCV infected patients as a whole (33%) (p<10−5) or in those with arthralgia (54%) (p = 0.009). HCV infected patients with arthralgia were more likely to be RF positive than those without arthralgia (54% v 27%; p = 0.003). Among the seven HCV infected patients with Sjögren's syndrome, only one was RF positive (14%). Using an ROC curve analysis to assess the reliability of RF to distinguish rheumatoid arthritis from HCV associated arthropathy, the area under the ROC curve (AUC) was 0.55 (95% confidence interval (CI), 0.45 to 0.65). The greatest accuracy of RF positivity for the diagnosis of rheumatoid arthritis was reached at a cut off value of 15 IU/ml, with a sensitivity of 89%, a specificity of 46%, a positive predictive value (PPV) of 75%, and a negative predictive value (NPV) of 70%. Taking into account the manufacturer's cut off at 30 IU/ml, the diagnostic values of RF positivity for rheumatoid arthritis were defined by a sensitivity of 81%, a specificity of 46%, a PPV of 73%, and an NPV of 57% (fig 2).
Figure 2 Diagnostic value of the anti‐cyclic citrullinated peptide antibodies (anti‐CCP Ab) and rheumatoid factor (RF) for discriminating between rheumatoid arthritis and hepatitis C virus associated arthropathy, assessed by the area under the receiver operating characteristic curve (AUC): anti‐CCP (bold line), AUC = 0.94 (95% confidence interval, 0.87 to 0.98); RF (dotted line), AUC = 0.55 (0.45 to 0.65) (p<10−3).
Diagnostic value of the anti‐CCP antibodies assay
Anti‐CCP Ab were found positive in only two HCV infected patients among those presenting with arthralgia (5.7%), compared with none of the 112 HCV infected patients without arthralgia and none of the seven HCV infected patients with Sjögren's syndrome. In contrast, 50 patients with rheumatoid arthritis were positive for anti‐CCP Ab (78%). The AUC was 0.94 (95% CI, 0.87 to 0.98), and the greatest accuracy of anti‐CCP Ab positivity for the diagnosis of rheumatoid arthritis was reached at a cut off of 19 U/ml, with a sensitivity of 87.5%, a specificity of 94.3%, a PPV of 96.6%, and an NPV of 80.5%. Using the manufacturer's cut off at 25 U/ml, anti‐CCP Ab positivity had a sensitivity of 78%, a specificity of 94.3%, a PPV of 96%, and an NPV of 70% for the diagnosis of rheumatoid arthritis (fig 2).
Discussion
Rheumatological manifestations are common during HCV infection and in some cases may mimic the onset of rheumatoid arthritis. In the absence of deforming lesions or articular destruction—both of which are uncommon in recent onset rheumatoid arthritis—a definite diagnosis and specific treatment are usually delayed. Our results showed that anti‐CCP antibodies were rarely positive among HCV infected patients with arthralgia (5.7%) or Sjögren's syndrome (0%), whereas they were present in 78% of patients with rheumatoid arthritis.
The detection of RF is not a reliable diagnostic tool, as many cases of recent onset rheumatoid arthritis are seronegative, while many non‐rheumatoid conditions—such as connective tissue diseases and HCV infection—may be seropositive. Among HCV infected patients, 30–68% are RF positive.1,3 In the present study, we found that RF was more commonly present in patients with arthralgia (54%) than those without arthralgia (27%) (p = 0.003). Despite a high prevalence of RF among patients with rheumatoid arthritis (81%), the PPV remained low (73%).
With regard to the prevalence of the anti‐CCP Ab, a previous study among HCV infected patients using a second generation ELISA kit (cut off = 20 U/ml) reported a prevalence of 6.9% (2/29) in patients with mixed cryoglobulins and 0% (0/50) in those without.15 No correlation with the presence of rheumatological manifestations was reported, but neither of the two patients who were positive for anti‐CCP Ab had definite or suspected rheumatoid arthritis.
In relation to the prevalence of anti‐CCP Ab in HCV associated arthropathy, only one small study has been published.12 In a cohort of 39 HCV infected patients, including 31 with arthropathy, compared with 30 patients with definite rheumatoid arthritis, anti‐CCP Ab was present in 77% of the patients with rheumatoid arthritis and in none of HCV infected patients. In the present study, anti‐CCP Ab detection was carried out in a larger population of 147 HCV infected patients. Our study population, moreover, reflects the classical characteristics of HCV infected patients, with about 50% of them having mixed cryoglobulins and 24% arthralgia.1 Anti‐CCP Ab, detected by a second generation ELISA kit, were found positive in only two HCV infected patients with arthralgia (5.7%). These two patients did not develop any further signs of rheumatoid arthritis. Interestingly, none of the seven HCV infected patients with definite Sjögren's syndrome was positive for anti‐CCP Ab. In a larger cohort of patients with primary Sjögren's syndrome, only 7.5% were positive for anti‐CCP Ab.16 Conversely, anti‐CCP Ab positivity was found in 78% of patients with rheumatoid arthritis, in accordance with previously reported prevalences.10,11
Conclusions
The distinction between HCV associated arthropathy and rheumatoid arthritis has great relevance for clinicians. Our results in a large patient cohort showed that anti‐CCP antibodies were rarely present in HCV infected patients and were a reliable serological marker to discriminate between patients with HCV associated rheumatological manifestations and patients with rheumatoid arthritis.
Abbreviations
anti‐CCP Ab - anti‐cyclic citrullinated peptide antibodies
AUC - area under the curve
HCV - hepatitis C virus
MC - mixed cryoglobulin
NPV - negative predictive value
PPV - positive predictive value
RF - rheumatoid factor
References
- 1.Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, Loustaud‐Ratti V.et al Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d'Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l'Hepatite C. Medicine (Baltimore) 20007947–56. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y H, Ji J D, Yeon J E, Byun K S, Lee C H, Song G G. Cryoglobulinaemia and rheumatic manifestations in patients with hepatitis C virus infection. Ann Rheum Dis 199857728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sène D, Ghillani‐Dalbin P, Thibault V, Guis L, Musset L, Duhaut P.et al Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol 2004312199–2206. [PubMed] [Google Scholar]
- 4.Buskila D, Shnaider A, Neumann L, Lorber M, Zilberman D, Hilzenrat N.et al Musculoskeletal manifestations and autoantibody profile in 90 hepatitis C virus infected Israeli patients. Semin Arthritis Rheum 199828107–113. [DOI] [PubMed] [Google Scholar]
- 5.Verbaan H, Carlson J, Eriksson S, Larsson A, Liedholm R, Manthorpe R.et al Extrahepatic manifestations of chronic hepatitis C infection and the interrelationship between primary Sjogren's syndrome and hepatitis C in Swedish patients. J Intern Med 1999245127–132. [DOI] [PubMed] [Google Scholar]
- 6.Loustaud‐Ratti V, Riche A, Liozon E, Labrousse F, Soria P, Rogez S.et al Prevalence and characteristics of Sjogren's syndrome or Sicca syndrome in chronic hepatitis C virus infection: a prospective study. J Rheumatol 2001282245–2251. [PubMed] [Google Scholar]
- 7.Andonopoulos A P, Drosos A A, Skopouli F N, Acritidis N C, Moutsopoulos H M. Secondary Sjogren's syndrome in rheumatoid arthritis. J Rheumatol 1987141098–1103. [PubMed] [Google Scholar]
- 8.Aho K, Palusuo T, Kurki P. Marker antibodies of rheumatoid arthritis: diagnostic and pathogenetic implications.Semin Arthritis Rheum 199423379–387. [DOI] [PubMed] [Google Scholar]
- 9.Kessel A, Rosner I, Zuckerman E, Golan T D, Toubi E. Use of antikeratin antibodies to distinguish between rheumatoid arthritis and polyarthritis associated with hepatitis C infection. J Rheumatol 200027610–612. [PubMed] [Google Scholar]
- 10.Bas S, Perneger T V, Seitz M, Tiercy J M, Roux‐Lombard P, Guerne P A. Diagnostic tests for rheumatoid arthritis: comparison of anti‐cyclic citrullinated peptide antibodies, anti‐keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 200241809–814. [DOI] [PubMed] [Google Scholar]
- 11.Vittecoq O, Incaurgarat B, Jouen‐Beades F, Legoedec J, Letourneur O, Rolland D.et al Autoantibodies recognizing citrullinated rat filaggrin in an ELISA using citrullinated and non‐citrullinated recombinant proteins as antigens are highly diagnostic for rheumatoid arthritis. Clin Exp Immunol 2004135173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bombardieri M, Allessandri C, Labbadia G, Lannuccelli C, Carlucci F, Riccieri V.et al Role of anti‐cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection‐associated polyarticular involvement. Arthritis Res Ther 20046137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitali C, Bombardieri S, Moutsopoulos H M, Coll J, Gerli R, Hatron P Y.et al Assessment of the European classification criteria for Sjogren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren's Syndrome. Ann Rheum Dis 199655116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 15.Wener M H, Hutchinson K, Morishima C, Gretch D R. Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C virus infection and cryoglobulinemia. Arthritis Rheum 2004502305–2308. [DOI] [PubMed] [Google Scholar]
- 16.Gottenberg J E, Mignot S, Nicaise‐Rolland P, Cohen‐Solal J, Aucouturier F, Goetz J.et al Prevalence of anti‐cyclic citrullinated peptide and anti‐keratin antibodies in patients with primary Sjogren's syndrome. Ann Rheum Dis 200564114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]