Abstract
Objectives
To determine the long term safety profile of the tumour necrosis factor (TNF) antagonist etanercept in subjects with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS) aged ⩾65 years in comparison with subjects aged <65 years.
Methods
Safety data from an integrated database of 4322 subjects enrolled in 18 RA trials, 2 PsA trials, and 2 AS trials were analysed. Safety end points included subject incidence of all adverse events (AE), serious adverse events (SAE), infectious events (IE), medically important infections (MII), and deaths. Events of particular interest in subjects treated with TNF modulating biological treatments, including demyelinating diseases, tuberculosis, lymphomas, and cardiovascular diseases, were also evaluated.
Results
The incidence of AE, SAE, IE, MII, and malignancies was not significantly raised in elderly subjects in comparison with subjects aged <65 years. No cases of tuberculosis were reported in the trials. Demyelinating diseases were seen only in subjects aged <65 years. The incidence and types of death in the elderly subjects were consistent with the expected rates for subjects of comparable age.
Conclusions
Etanercept is a generally safe and well tolerated biological agent for treatment of rheumatological diseases in the elderly, and the risk of AE in these studies was no greater in subjects aged ⩾65 years than in younger subjects.
Keywords: geriatric, elderly, etanercept, safety, rheumatological diseases
Epidemiological studies have indicated that the incidence of rheumatoid arthritis (RA) increases with age, reaching an annual rate of about 130 cases per 100 000 population for women over the age of 65 in the United States.1 Despite the high incidence of this disease in the elderly, patients who are ⩾65 years of age have been consistently underrepresented in clinical trials of arthritis treatments.2 Older patients tend to present with more severe disease than younger subjects,3 and advancing age is a predictor of poor radiographic outcome4 and risk of permanent work disability.5 In contrast with RA, the age of onset of subjects with inflammatory spondyloarthritides, which include psoriatic arthritis (PsA) and ankylosing spondylitis (AS), is generally under the age of 40.6 However, elderly subjects with PsA tend to present with a more severe onset of disease than younger subjects, and have a more destructive outcome.7 Similarly, subjects with late onset AS are more likely to present with systemic symptoms, inflammatory upper spinal pain, and peripheral arthritis than younger subjects.8 In general, older subjects also present with a greater number of comorbidities, resulting in higher levels of polypharmacy and increased risk of adverse pharmaceutical interactions. Older people therefore represent a rapidly growing population of rheumatology patients with unique challenges, requiring special considerations to achieve desirable clinical outcomes safely.
Many rheumatic diseases, including RA, PsA, and AS, are autoimmune conditions, characterised by dysregulation and chronic activation of T cell responses.9,10 The ultimate outcome is the overproduction of proinflammatory cytokines, including tumour necrosis factor (TNF) and interleukin 1, which have been postulated to mediate the joint destruction seen in RA.11,12 TNF blockade is currently the most effective biological approach to the treatment of RA, with demonstrated efficacy and safety.12,13 Etanercept is a fully human, soluble, TNF receptor‐IgG1 fusion protein that binds to both soluble and membrane bound TNF, thereby inhibiting its interaction with cell surface receptors and preventing TNF mediated cellular responses. Etanercept has been approved by the Federal Drug Administration for the treatment of subjects with moderately to severely active RA, PsA, polyarticular juvenile RA (JRA), AS, and psoriasis.14 Long term extension studies in subjects with RA have been performed for up to 7 years.15 In addition, more than 262 000 patients have been treated with etanercept outside clinical trials world wide, representing over 515 000 patient‐years of experience.
This study aimed at determining the incidence of important adverse reactions in a database of subjects with RA, PsA, and AS enrolled in clinical trials who were 65 years of age and older and contrasting the results with the incidence of adverse events reported in subjects under the age of 65 years who were taking etanercept. Although subjects in clinical trials are carefully screened for the absence of multiple, clinically significant comorbidities, this database should suggest whether such older subjects are more likely to have significant adverse events than younger patients when treated with etanercept.
Subjects and methods
Subjects
Subjects with active rheumatic diseases enrolled in all clinical trials performed to evaluate the safety and efficacy of etanercept in the treatment of RA (18 trials), PsA (2 trials), and AS (2 trials) were included. Safety data were collected from all subjects who had received at least one dose of etanercept, and pooled for this integrated analysis. Safety data for patients receiving etanercept who are not in clinical trials are not systematically collected, and therefore are not included in this analysis.
Study designs
RA trials
Subjects were enrolled in one phase I trial,16 one phase II trial,17 two phase II/III trials,18,19 four phase III trials,20,21,22 one phase IV trial, two pharmacokinetic studies,23 four open label trials,24,25 one investigator initiated trial, and two trials designed to examine the safety and efficacy of lyophilised and liquid26 formulations of etanercept were included in this safety analysis. All the trials enrolled subjects with moderate to severe RA, and two of the trials examined only subjects with JRA.19
PsA trials
Subjects with PsA who had both skin and joint symptoms were enrolled in two clinical trials designed to examine the safety and efficacy of etanercept in the treatment of PsA symptoms.27,28
AS trials
Subjects with moderate to severe AS who were enrolled in a randomised, placebo controlled trial29 and an open label extension of that trial30 were included in this safety analysis.
Study drug
Etanercept (Enbrel; Immunex‐Wyeth Research) was supplied to subjects in syringes, each containing the contents of one reconstituted vial of etanercept or placebo. The study drug was self administered by subcutaneous injection. In some studies, subjects were initially given reconstituted drug, and were later given vials to be reconstituted at home.
Safety end points
The integrated dataset used to evaluate safety included data from all subjects who received at least one dose of etanercept in Amgen sponsored studies (completed and continuing) in approved rheumatological indications through 31 December 2003. Safety end points included subject incidence rates of all adverse events (AE), serious adverse events (SAE), infectious events (IE), medically important infections (MII), and deaths. MII are defined as infections that required the use of intravenous antibiotics or admission to hospital. The reporting of MII was chosen for this analysis in order to capture infections that are of clinical interest, rather than serious IE, which is a regulatory definition and may underreport clinically relevant events.
AE that are of particular interest in TNF modulating biological treatments, including tuberculosis, demyelinating disorders, and cardiovascular diseases, were also evaluated. Assignment of the term congestive heart failure (CHF) included the following preferred terms that could represent CHF: CHF, aggravated CHF, oedema lung, effusion pleural, fluid overload, heart failure, right heart failure, and peripheral oedema. Preferred terms used to identify potential cases of demyelinating disorders included paraesthesia, multiple sclerosis, optic neuritis, and neuropathy. All AE were classified using the current Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) dictionary for each trial except for the trial of the liquid formulation of etanercept, which used the Medical Dictionary for Drug Regulatory Affairs (MedDRA) coding. Intensities of AE were classified according to a modified National Cancer Institute Common Toxicity Criteria Scale.
Statistical methods
The purpose of these integrated safety analyses was to explore various profiles of AE during exposure to etanercept, and to compare AE in elderly subjects aged ⩾65 and subjects aged <65. Subjects from the 22 rheumatology trials were divided into two cohorts: those aged ⩾65 years and those aged <65, where age is calculated relative to the date a subject first received etanercept. Within each age cohort, the subject incidence of events that occurred during exposure to etanercept (including treatment during the initial randomised trials and up to 5 years in extension studies) and during the control period are summarised. The incidence of treatment related events was determined by subtracting event rates in the control groups from event rates in the treated groups. Differences between incidence in the etanercept treatment group and the control group are reported with 95% confidence intervals (CIs). No differences were found in the incidence of most AE between subjects aged <65 and subjects aged ⩾65, and no major differences in the point estimates for the events that were attributable to etanercept treatment; hence, the reported events were not corrected for the different durations of exposure to etanercept or control. As the observation time is longer in the etanercept treated group, this approach is considered to be biased towards a more conservative view of safety. The statistical analysis of serious cardiovascular events was the exception to this analytical approach, and rates of these events were adjusted for exposure.
Results
Subjects
A total of 3893 unique subjects with RA, JRA, PsA, and AS were included in this safety analysis: 3296 subjects aged <65 and 597 subjects ⩾65 years of age (table 1). A greater proportion of subjects aged ⩾65 years took part in the RA trials (17.3%) than in the PsA (5.3%) and AS (1.4%) trials. Subjects in the RA trials were predominantly female (76.3% of all subjects) and the proportion of women was similar in both age cohorts. In contrast, women represented 47.5% of all subjects in the PsA trials, yet 71.4% of patients ⩾65 were female. In the AS trials, women comprised 24.2% of all patients, and all the elderly subjects were men. Subjects in all of the trials were predominantly white. Data from these trials represent 6798 subject‐years of exposure to etanercept in the setting of rheumatic diseases (table 2).
Table 1 Subject demographics at baseline in all studies.
| Characteristics | RA | PsA | AS | |||
|---|---|---|---|---|---|---|
| Age <65 | Age ⩾65 | Age <65 | Age ⩾65 | Age <65 | Age ⩾65 | |
| (n = 2772) | (n = 579) | (n = 251) | (n = 14) | (n = 273) | (n = 4) | |
| % of total subjects in group | 82.7 | 17.3 | 94.7 | 5.3 | 98.6 | 1.4 |
| Sex, No (% female) | 2134 (77.0) | 424 (73.2) | 116 (46.2) | 10 (71.4) | 67 (24.5) | 0 (0.0) |
| Race, No (% white) | 2180 (78.6) | 518 (89.5) | 224 (89.2) | 14 (100.0) | 253 (92.7) | 4 (100.0) |
| Age (years) | ||||||
| Median | 47 | 70 | 46 | 70 | 42 | 65 |
| Range | 1–64 | 65–90 | 18–64 | 65–76 | 18–64 | 65–70 |
RA, rheumatoid arthritis; PsA, psoriatic arthritis; AS, ankylosing spondylitis.
Table 2 Subject‐years of exposure to etanercept.
| Age | ||
|---|---|---|
| <65 Years | ⩾65 Years | |
| RA | 5592 | 887 |
| PsA | 245 | 15 |
| AS | 58 | 1 |
| Total | 5895 | 903 |
RA, rheumatoid arthritis; PsA, psoriatic arthritis; AS, ankylosing spondylitis.
Adverse events
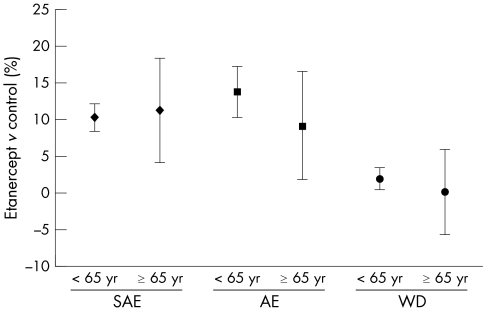
Table 3 presents a summary of AE reported in all 22 trials. The proportion of subjects reporting AE and SAE was apparently higher in subjects aged ⩾65, whether they were treated with etanercept or not (table 3). However, as shown in fig 1, when the data were normalised by subtracting background AE and SAE (the rate observed in the control group, whether receiving placebo or methotrexate (MTX)), there were no significant differences based on the observed CI of AE or SAE between the two age groups. A higher proportion of elderly subjects than subjects under 65 years of age withdrew from the studies owing to an AE, regardless of treatment group (table 3).
Table 3 No (%) of subjects reporting an adverse event.
| Age <65 years | Age ⩾65 years | |||
|---|---|---|---|---|
| Control* | Etanercept† | Control | Etanercept | |
| (n = 1020) | (n = 2652) | (n = 170) | (n = 480) | |
| Adverse event | 647 (63.4) | 2046 (77.1) | 126 (74.1) | 400 (83.3) |
| Serious adverse event | 41 (4.0) | 378 (14.3) | 30 (17.6) | 139 (29.0) |
| Infectious event | 406 (39.8) | 1470 (55.4) | 87 (51.2) | 234 (48.8) |
| Medically important event | 13 (1.3) | 105 (4.0) | 12 (7.1) | 50 (10.4) |
| Withdrawal owing to adverse event | 36 (3.5) | 144 (5.4) | 21 (12.4) | 60 (12.5) |
*Subjects in control groups received either placebo or methotrexate; †subjects in the etanercept groups may have previously been included in control groups, and entered the etanercept group at a later stage of the same trial or a different trial. Data represent events occurring during etanercept treatment.
Figure 1 AE and subject withdrawals owing to AE are not significantly higher in elderly subjects. Data points represent the difference in the proportion of subjects in treatment and control groups (percentage of subjects in etanercept treatment group – percentage of subjects in control groups) reporting AE, SAE, and subject withdrawals owing to AE (WD). Error bars represent 95% CIs.
Infectious events
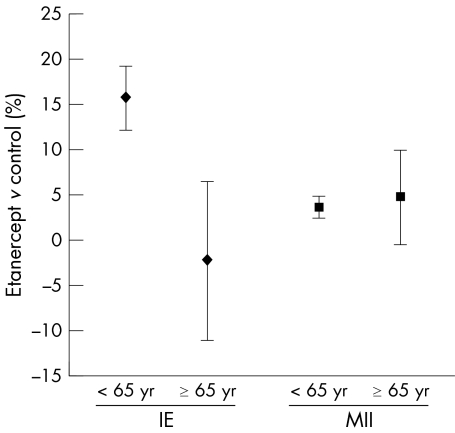
The proportion of subjects reporting an IE was higher in younger subjects receiving etanercept than in elderly subjects (table 3), which was primarily related to the higher rates of upper respiratory track infections in younger subjects. In contrast, a higher percentage of elderly subjects reported an MII compared with younger subjects. Figure 2 shows that when the data were normalised by subtracting the event rates of subjects in the control groups, overlapping CI indicated that there was no significant difference between subjects aged <65 and those aged ⩾65 in reporting an MII. No cases of tuberculosis were seen in either age group.
Figure 2 IE are higher in subjects aged <65. Data points represent the difference in the proportion of subjects in the treatment and control groups (percentage of subjects in etanercept treatment group – percentage of subjects in control groups) reporting IE and MII. Error bars represent 95% CIs.
Cardiovascular diseases
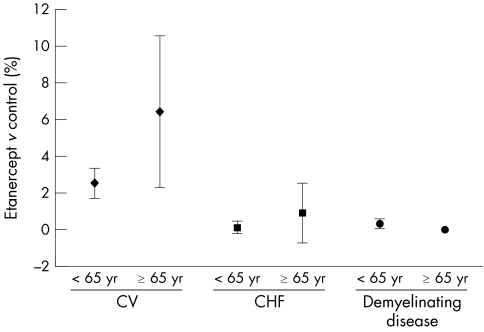
Rates of cardiovascular events were no higher in elderly subjects than in younger subjects when adjusted for the longer duration of exposure to etanercept (table 4) and after the data were normalised by subtracting the background cardiovascular events (fig 3). A total of 57/650 (8.8%) subjects aged ⩾65 reported a serious cardiovascular event; 50 of these subjects received at least one dose of etanercept and seven subjects received either placebo or MTX. In contrast, 83/3672 (2.3%) of subjects aged <65 reported a cardiovascular event: 78 subjects received etanercept and five received either placebo or MTX.
Table 4 Exposure adjusted rates of cardiovascular events.
| Age <65 years | Age ⩾65 years | |||
|---|---|---|---|---|
| Control* | Etanercept | Control* | Etanercept | |
| Number of events | 10 | 110 | 11 | 74 |
| Exposure (subject‐years) | 699 | 5895 | 75 | 903 |
| Exposure adjusted rate† | 1.43 | 1.87 | 14.67 | 8.19 |
| SIR (95% CI) | 1.30 (0.08 to 4.48) | 0.56 (0.24 to 1.07) | ||
SIR, standardised incidence ratio; CI, confidence interval.
*Subjects in control groups received either placebo or methotrexate; †exposure adjusted rate = number of events per subject‐year.
Figure 3 Cardiovascular disease, congestive heart failure, and demyelinating disorders were not increased in elderly subjects. Data points represent the difference in the proportion of subjects in treatment and control groups (percentage of subjects in etanercept treatment group – percentage of subjects in control groups) reporting cardiovascular (CV) events, CHF, and demyelinating disease. Error bars represent 95% CIs.
Demyelinating disorders
In the pooled safety analysis of rheumatic diseases reported here, eight cases of demyelinating disorders were seen in subjects aged <65, which included six subjects in the RA trials and two subjects with PsA. No cases of demyelinating disease in subjects aged ⩾65 were reported (fig 3).
Malignancies
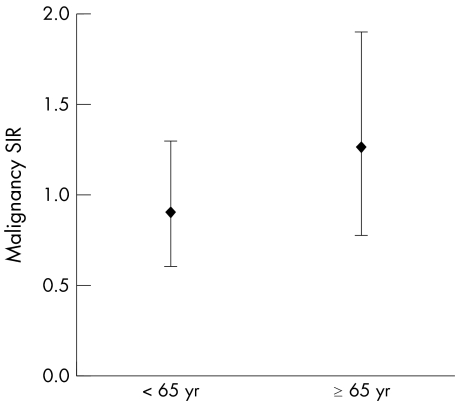
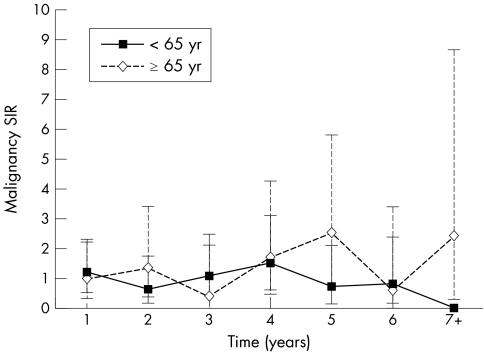
Malignancy standardised incidence ratios (SIR), adjusted for age and sex, were similar for subjects aged <65 and elderly patients who had received at least one dose of etanercept (fig 4). Figure 5 shows that the incidence of overall malignancies (including lymphoma) remained stable over time and was comparable with, or less than, that expected in the RA population as the duration of exposure to etanercept increased.
Figure 4 Malignancy rates are similar in young and elderly subjects. Data points represent malignancy standardised incidence ratios (SIR) adjusted for age and sex in subjects receiving at least one dose of etanercept. Error bars represent 95% CIs.
Figure 5 Malignancy rates are stable over time. Data points represent age and sex adjusted malignancy standardised incidence ratios (SIR) for subjects aged <65 and ⩾ 65. Error bars represent 95% CIs for younger (solid error bars) and elderly (dashed error bars) subjects.
Deaths
A total of 41 deaths were reported in the integrated database: 21 in subjects under the age of 65, and 20 in subjects ⩾65 years of age (table 5). Half of the deaths in the elderly subjects (10/20) were due to cardiac events, whereas gastrointestinal disorders and neoplasms were the most common cause of death in the younger subjects. The number of expected deaths, based on the number of subject‐years of exposure to etanercept, was 29 and 30 for subjects aged ⩾65 and subjects aged <65, respectively (table 6).
Table 5 Numbers and causes of death.
| Causes of death | Age | |
|---|---|---|
| <65 Years | ⩾65 Years | |
| (n = 3296) | (n = 597) | |
| Cardiac disorders | 2 | 10 |
| Gastrointestinal disorders | 4 | 1 |
| General disorders | 1 | 1 |
| Hepatobiliary disorders | 1 | 0 |
| Infections and infestations | 1 | 4 |
| Injury | 1 | 0 |
| Neoplasms (benign, malignant, unspecified) | 4 | 1 |
| Nervous system disorders | 3 | 0 |
| Respiratory (thoracic and mediastinal disorders) | 3 | 2 |
| Vascular disorders | 1 | 1 |
| Total deaths | 21 | 20 |
Table 6 Expected deaths in etanercept trials for rheumatic conditions.
| Age <65 years | Age ⩾65 years | |||||
|---|---|---|---|---|---|---|
| Expected* | Observed | SIR† (95% CI) | Expected* | Observed | SIR† (95% CI) | |
| RA | 28.9 | 20 | 0.69 (0.42 to 1.07) | 29.0 | 20 | 0.69 (0.42 to 1.07) |
| PsA | 1.1 | 1 | 0.91 (0.02 to 5.07) | 0.4 | 0 | 0.00 (0.00 to 9.22) |
| AS | 0.2 | 0 | 0.00 (0.00 to 18.44) | 0.0 | 0 | N/A |
SIR, standardised incidence ratio; CI, confidence interval; RA, rheumatoid arthritis; PsA, psoriatic arthritis; AS, ankylosing spondylitis; N/A, not applicable
*Expected number of deaths per National Vital Statistics Report for 199951; †standardised incidence ratio for observed deaths/expected deaths.
Discussion
The subjects included in this analysis represent several rheumatic diseases: RA, PsA, and AS. Elderly subjects who were aged ⩾65 were a minority population in all studies, particularly in the PsA and AS trials. Nevertheless, the integrated dataset reported here represented considerable duration of exposure to etanercept in elderly subjects. The multistudy database of safety events from these trials also included 357 subjects with JRA, with 370 subject‐years of exposure to etanercept. These young subjects comprised 11% of the subjects under the age of 65 described here, representing a minority of the subjects in the analysis. It is likely that the inclusion of these subjects improved the safety profile of the group of subjects under the age of 65, and would have accentuated differences between the two age groups. Similarly, the inclusion of subjects from the AS and PsA trials, who were predominately younger, would also have accentuated the differences between the two age groups.
Differences in AE and SAE between treatment and control groups were consistent across age cohorts; however, IE were raised in the younger subjects receiving etanercept relative to subjects receiving placebo or MTX. This difference was due primarily to an increased incidence of upper respiratory infections14 in younger subjects receiving etanercept. This result is consistent with safety data in trials of etanercept in subjects with psoriasis. In the pooled analysis described here, a higher percentage of elderly subjects than younger subjects reported an MII. Although not significantly different from the results for the age matched control group, these data are clinically significant, and suggest that the severity of IE may be greater in subjects aged ⩾65.
Biological treatments that target TNF have been associated with the reactivation of tuberculosis (and other opportunistic infections), increased mortality of subjects with CHF, and the development and exacerbation of demyelinating diseases, and lymphomas.14,31,32,33
The association between etanercept and reactivation of tuberculosis is weaker than that seen with other TNF antagonists, and the reason for this discrepancy is unclear. Possible explanations may be the shorter half life of etanercept, the lower peak serum concentration after subcutaneous administration, or the area under the etanercept serum concentration‐time curve between administrations. It has been suggested that more sustained neutralisation of TNF with the monoclonal antibodies infliximab or adalimumab may place the patient at higher risk for opportunistic infections such as tuberculosis.34 No cases of tuberculosis were seen in the etanercept trials reported here.
Recent studies have shown that patients with RA have a higher rate of cardiovascular disease, which can remain silent,35,36 and a higher likelihood of developing CHF.37 The reasons for these observations may well be related to the chronic inflammation associated with RA.35 Patients with PsA also appear to be at increased risk of cardiovascular disease associated with inflammation.38,39 Recent reports of heart failure in patients receiving TNF modulating treatments40,41 must therefore be interpreted in the context of the increased risk of heart disease in this patient population. There have also been reports42 that indicated that there was no exacerbation of CHF or increased mortality in patients treated with anti‐TNF agents. As cardiovascular disease also increases with age, our statistical approach of normalising the data by subtracting the rate of cardiovascular events in control subjects from the rates seen in elderly subjects receiving etanercept and adjusting for exposure was used to determine whether these events were associated with the use of the study drug in elderly subjects. The data in this multistudy analysis suggest that the use of etanercept is not associated with higher rates of serious cardiovascular events in either age cohort.
Although the causal relationship is unclear, TNF antagonists have been associated with rare cases of new onset or exacerbation of demyelinating diseases. Animal models have suggested a role for TNF in the development of inflammatory demyelinating disease.43 A recent review suggested that the rate of new cases of multiple sclerosis in patients receiving TNF antagonists did not differ from the rate of cases in the general population.43 A total of eight cases of demyelinating disease were noted in the studies presented here, and none was seen in subjects aged 65 years and older.
Patients with RA have not been shown to have an increased risk of malignancies,31 with the exception of lymphomas.44,45 The SIR of lymphomas has been estimated to be 1.9 (95% CI 1.3 to 2.7) 45 to 2.0 (95% CI 1.5 to 2.6).44 The risk of lymphoma was lowest in patients with low inflammatory activity (odds ratio (OR) = 1.0) and increased in patients with medium (OR = 5.4; 95% CI 0.7 to 42.0) and high (OR = 25.8; 95% CI 3.1 to 213.0) 45 in a population based cohort study of 11 683 patients. In a prospective study of 18 572 patients, the SIR for lymphomas in patients treated with etanercept was 3.8 (95% CI 1.9 to 7.5).46 Currently, there are insufficient data to link biological treatments with increased rates of lymphoma in patients with RA. Our data suggest that the use of etanercept does not increase the risk of malignancy in patients with RA, and the malignancy rates are stable over time.
RA,47 PsA,48 and AS49 are associated with increased mortality. In a recent analysis of 13 studies, the most common causes of death in patients with RA and the general United States population were cardiovascular disease, cancer, and infection; however, the life span of the patients with RA was decreased by 5–15 years.50 Based on age adjusted estimates, our results show fewer deaths than would be expected in subjects aged ⩾65.51
Rheumatic diseases are associated with significant comorbidities, including infections, cardiovascular disease, and lymphomas. The role of drug treatment as a contributing factor, separate from the effects of the disease itself, is a complicated construct that is not fully understood at the present time and deserves further study and analysis. The investigators recognise that the elderly subjects enrolled in these clinical trials are not truly representative of elderly patients with rheumatic disease treated in community settings. This is largely owing to fulfilment of the strict inclusion and exclusion criteria required by the clinical trials, which limits the interpretation and generalisability of this safety analysis. Nevertheless, the data summarised here, representing a large and well studied prospective clinical trial database, indicate that etanercept is well tolerated and safe in elderly subjects with RA, PsA, and AS, and that subjects aged ⩾65 years are at no greater risk of AE than younger subjects.
Acknowledgements
We thank all of the individual physicians and their study staff, as well as the subjects of these trials, for their thoughtful participation. We thank Julia R Gage for editorial assistance and Lizhi Xie and Ellen Lin for SAS programming support.
This work was supported by Immunex Corporation, a wholly owned subsidiary of Amgen Inc, and by Wyeth.
Abbreviations
AE - adverse events
AS - ankylosing spondylitis
CHF - congestive heart failure
IE - infectious events
JRA - juvenile rheumatoid arthritis
MII - medically important infections
MTX - methotrexate
OR - odds ratio
PsA - psoriatic arthritis
RA - rheumatoid arthritis
SAE - serious adverse events
SIR - standardised incidence ratio(s)
TNF - tumour necrosis factor
References
- 1.Gabriel S E, Crowson C S, O'Fallon W M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum 199942415–420. [DOI] [PubMed] [Google Scholar]
- 2.Rochon P A, Fortin P R, Dear K B, Minaker K L, Chalmers T C. Reporting of age data in clinical trials of arthritis. Deficiencies and solutions. Arch Intern Med 1993153243–248. [PubMed] [Google Scholar]
- 3.Papadopoulos I A, Katsimbri P, Alamanos Y, Voulgari P V, Drosos A A. Early rheumatoid arthritis patients: relationship of age. Rheumatol Int 20032370–74. [DOI] [PubMed] [Google Scholar]
- 4.Goronzy J J, Matteson E L, Fulbright J W, Warrington K J, Chang‐Miller A, Hunder G G.et al Prognostic markers of radiographic progression in early rheumatoid arthritis. Arthritis Rheum 20045043–54. [DOI] [PubMed] [Google Scholar]
- 5.Young A, Dixey J, Kulinskaya E, Cox N, Davies P, Devlin J.et al Which subjects stop working because of rheumatoid arthritis? Results of five years' follow up in 732 patients from the early RA study (ERAS). Ann Rheum Dis 200261335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivieri I, van Tubergen A, Salvarani C, van der Linden S. Seronegative spondyloarthritides. Best Pract Res Clin Rheumatol 200216723–739. [DOI] [PubMed] [Google Scholar]
- 7.Punzi L, Pianon M, Rossini P, Schiavon F, Gambari P F. Clinical and laboratory manifestations of elderly onset psoriatic arthritis: a comparison with younger onset disease. Ann Rheum Dis 199958226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplanne D, Tubach F, Le Parc J M. Late onset spondylarthropathy: clinical and biological comparison with early onset subjects. Ann Rheum Dis 199756176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goronzy J J, Weyand C M. T‐cell regulation in rheumatoid arthritis. Curr Opin Rheumatol 200416212–217. [DOI] [PubMed] [Google Scholar]
- 10.Aringer M. T lymphocyte activation—an inside overview. Acta Med Austriaca 2002297–13. [DOI] [PubMed] [Google Scholar]
- 11.Bingham CO I I I. The pathogenesis of rheumatoid arthritis: pivotal cytokines involved in bone degradation and inflammation. J Rheumatol Suppl 2002653–9. [PubMed] [Google Scholar]
- 12.Suryaprasad A G, Prindiville T. The biology of TNF blockade. Autoimmun Rev 20032346–357. [DOI] [PubMed] [Google Scholar]
- 13.Moreland L W. Drugs that block tumour necrosis factor: experience in patients with rheumatoid arthritis. Pharmacoeconomics 20042239–53. [DOI] [PubMed] [Google Scholar]
- 14.Enbrel ® (etanercept) Prescribing information. Amgen Inc 2004
- 15.Weinblatt M E, Genovese M C, Moreland L W, Bathon J M, Kremer J M, Fleischmann R M.et al Efficacy and safety of over 7 years of etanercept (Enbrel®) therapy in North American patients with early and long‐standing rheumatoid arthritis [abstract]. Arthritis Rheum 200450(suppl)S184 [Google Scholar]
- 16.Moreland L W, Margolies G, Heck L W, Jr, Saway A, Blosch C, Hanna R.et al Recombinant soluble tumor necrosis factor receptor (p80) fusion protein: toxicity and dose finding trial in refractory rheumatoid arthritis. J Rheumatol 1996231849–1855. [PubMed] [Google Scholar]
- 17.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver Al.et al Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)‐Fc fusion protein. N Engl J Med 1997337141–147. [DOI] [PubMed] [Google Scholar]
- 18.Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I.et al A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999340253–259. [DOI] [PubMed] [Google Scholar]
- 19.Lovell D J, Giannini E H, Reiff A, Cawkwell G D, Silverman E D, Nocton J J.et al Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 2000342763–769. [DOI] [PubMed] [Google Scholar]
- 20.Moreland L W, Schiff M H, Baumgartner S W, Tindall E A, Fleischmann R M, Bulpitt K J.et al Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999130478–486. [DOI] [PubMed] [Google Scholar]
- 21.Bathon J M, Martin R W, Fleischmann R M, Tesser J R, Schiff M H, Keystone E C.et al A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 20003431586–1593. [DOI] [PubMed] [Google Scholar]
- 22.Keystone E C, Schiff M H, Kremer J M, Kafka S, Lovy M, DeVries T.et al Once‐weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double blind, placebo‐controlled trial. Arthritis Rheum 200450353–363. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Kimko H C, Rogge M, Wang D, Nestorov I, Peck C C. Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin Pharmacol Ther 200373348–365. [DOI] [PubMed] [Google Scholar]
- 24.Weinblatt M, Moreland L W, Schiff M H, Baumgartner S W, Tindall E, Fleischmann R M.et al Long‐term and phase III treatment of DMARD failing rheumatoid arthritis subjects with TNF receptor p75 Fc fusion protein (TNFR:Fc; Enbrel™) [abstract]. Arthritis Rheum 199740(suppl)S126 [Google Scholar]
- 25.Moreland L W, Cohen S B, Baumgartner S W, Tindall E A, Bulpitt K, Martin R.et al Longterm safety and efficacy of etanercept in subjects with rheumatoid arthritis. J Rheumatol 2001281238–1244. [PubMed] [Google Scholar]
- 26.Dore R, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A.et al The safety and efficacy profile of etanercept (Enbrel®) liquid administered once weekly in patients with rheumatoid arthritis [abstract]. Arthritis Rheum 200450(suppl)S564–S565. [PubMed] [Google Scholar]
- 27.Mease P J, Goffe B S, Metz J, VanderStoep A, Finck B, Burge D J. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000356385–390. [DOI] [PubMed] [Google Scholar]
- 28.Mease P J, Kivitz A J, Burch F X, Siegel E L, Cohen S B, Ory P.et al Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004502264–2272. [DOI] [PubMed] [Google Scholar]
- 29.Davis J C, Jr, van der Heijde D, Braun J, Dougados M, Cush J, Clegg D O.et al Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003483230–3236. [DOI] [PubMed] [Google Scholar]
- 30.Davis J, Jr, Webb A, Lund S, Sack K. Results from an open‐label extension study of etanercept in ankylosing spondylitis. Arthritis Rheum 200451302–304. [DOI] [PubMed] [Google Scholar]
- 31.Fleischmann R M, Iqbal I, Stern R L. Considerations with the use of biological therapy in the treatment of rheumatoid arthritis. Expert Opin Drug Saf 20043391–403. [DOI] [PubMed] [Google Scholar]
- 32.Humira (adalimumab) Product insert. Abbot Laboratories 2004
- 33.Remicade (infliximab) Prescribing information. Centocor Inc 2004
- 34.Ellerin R, Rubin R H, Weinblatt M E. Infections and anti‐tumor necrosis factor alpha therapy. Arthritis Rheum 2003483013–3022. [DOI] [PubMed] [Google Scholar]
- 35.Goodson N. Coronary artery disease and rheumatoid arthritis. Curr Opin Rheumatol 200214115–120. [DOI] [PubMed] [Google Scholar]
- 36.Maradit‐Kremers H, Crowson C S, Nicola P J, Ballman K V, Roger V L, Jacobsen S J.et al Increased unrecognized coronary heart disease in rheumatoid arthritis: a population‐based cohort study [abstract]. Arthritis Rheum 200450(suppl)S688. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel S E, Crowson C S, O'Fallon W M. Comorbidity in arthritis. J Rheumatol 1999262475–2479. [PubMed] [Google Scholar]
- 38.Jones S M, Harris C P D, Lloyd J, Stirling C A, Reckless J P, McHugh N J. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis 200059904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saricaoglu H, Güllülü S, Bülbül Baskin E, Cordan J, Tunali S. Echocardiographic findings in subjects with psoriatic arthropathy. J Eur Acad Dermatol Venereol 200317414–417. [DOI] [PubMed] [Google Scholar]
- 40.Kwon H J, Coté T R, Cuffe M S, Kramer J M, Braun M M. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 2003138807–811. [DOI] [PubMed] [Google Scholar]
- 41.Khanna D, McMahon M, Furst D E. Anti‐tumor necrosis factor α therapy and heart failure: what have we learned and where do we go from here? Arthritis Rheum 2004501040–1050. [DOI] [PubMed] [Google Scholar]
- 42.Kazi S, Cole J, Bisti A. The incidence of new onset congestive heart failure and heart failure exacerbation in Veteran's Affairs patients receiving tumor necrosis factor alpha antagonists [abstract]. Arthritis Rheum 200450(suppl)S374. [DOI] [PubMed] [Google Scholar]
- 43.Mangano M D, Robinson W H, Genovese M C. Demyelination and inhibition of tumor necrosis factor (TNF). Clin Exp Rheumatol 200422(suppl 35)S134–S140. [PubMed] [Google Scholar]
- 44.Gridley G, McLaughlin J K, Ekbom A, Klareskog L, Adami H O, Hacker D G.et al Incidence of cancer among patients with rhtumatoid arthritis. J Natl Cancer Inst 199385307–311. [DOI] [PubMed] [Google Scholar]
- 45.Baecklund E, Ekbom A, Sparen P, Feltelius N, Klareskog L. Disease activity and risk of lymphoma in patients with rheumatoid arthritis: nested case‐control study. BMJ 1998317180–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti‐tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004501740–1751. [DOI] [PubMed] [Google Scholar]
- 47.Kvien T K. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 200422(suppl)1–12. [DOI] [PubMed] [Google Scholar]
- 48.Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs 2002622447–2457. [DOI] [PubMed] [Google Scholar]
- 49.Braun J, Pincus T. Mortality, course of disease and prognosis of patients with ankylosing spondylitis. Clin Exp Rheumatol 200220(suppl 28)S16–S22. [PubMed] [Google Scholar]
- 50.Pincus T, Kavanaugh A, Sokka T. Benefit/risk of therapies for rheumatoid arthritis: underestimation of the “side effects” or risks of RA leads to underestimation of the benefit/risk therapies. Clin Exp Rheumatol 200422(suppl 35)S2–11. [PubMed] [Google Scholar]
- 51.Kochanek K D, Smith B L, Anderson R N. Deaths: preliminary data for 1999. Nat Vital Stat Rep 2001491–48. [PubMed] [Google Scholar]