Abstract
Background
Cardiovascular mortality is increased in rheumatoid arthritis. Possible reasons include an increased incidence of ischaemic heart disease or worse outcome after acute coronary syndrome (ACS).
Objectives
To assess the outcome of ACS in rheumatoid arthritis compared with case matched controls in the context of underlying cardiac risk factors, clinical presentation, and subsequent management.
Methods
40 patients with rheumatoid arthritis and ACS identified from coronary care admission registers between 1990 and 2000 were case matched as closely as possible for age, sex, classical cardiovascular risk factors, type and severity of ACS, and admission date (±3 months) with 40 controls. A standardised proforma was used for detailed case note review.
Results
Age, sex, other cardiovascular risk factors, and type and severity of presenting ACS were not significantly different between cases and controls. Recurrent cardiac events were commoner in rheumatoid arthritis (23/40, 57.5%) than controls (12/40, 30%) (p = 0.013); there were 16/40 deaths in rheumatoid arthritis (40%) v 6/40 (15%) in controls (p = 0.012). Recurrent events occurred earlier in rheumatoid arthritis (log rank survival, p = 0.05). Presentation with chest pain occurred in all controls compared with 33/40 rheumatoid patients (82%) (p = 0.006); collapse occurred in one control (2.5%) v 7/40 rheumatoid patients (17.5%) (p = 0.025). Treatment during the ACS was not significantly different in the two groups.
Conclusions
Recurrent ischaemic events and death occur more often after ACS in rheumatoid arthritis. Atypical presentation is commoner in rheumatoid arthritis. There is an urgent need to develop identification and intervention strategies for ACS specific to this high risk group.
Keywords: rheumatoid arthritis, cardiovascular disease, myocardial infarction
Rheumatoid arthritis is associated with increased overall mortality and reduced life expectancy. A major reason is increased cardiovascular mortality compared with the general population.1,2 Rheumatoid heart disease rarely has haemodynamic consequences and is an unlikely cause for this. The most probable cause of cardiac death in rheumatoid arthritis, as in the general population, is atherosclerotic coronary artery disease leading to ischaemic heart disease.3
Several studies have shown a higher incidence or prevalence of ischaemic cardiac pathologies such as myocardial infarction, congestive heart failure, and coronary deaths in patients with rheumatoid arthritis than in the general population,4,5,6,7,8 and objective testing with myocardial perfusion imaging9 has detected an increased background burden of stable ischaemic heart disease in rheumatoid arthritis.
In the general population, unstable angina and myocardial infarction, collectively referred to as acute coronary syndromes (ACS), are associated with an increased risk of further cardiac events (death, recurrent ACS, left ventricular failure, arrhythmia) and poor overall outcome.10 ACS are therefore an obvious target for intervention aiming to reduce mortality. There has been no systematic assessment of whether ACS in patients with rheumatoid arthritis have a worse prognosis than in controls, and if so, what the potential reasons for this might be.
We hypothesised that patients with rheumatoid arthritis sustaining an ACS have a worse outcome than patients without rheumatoid arthritis who have a similar ACS. We assessed this in an observational, retrospective, case–control study in which we compared the outcome of a definite ACS in patients with rheumatoid arthritis with age and sex matched non‐rheumatoid controls. In order to explore possible causes of a difference in outcome, we investigated this in the context of cardiac risk factors, clinical presentation, and subsequent management.
Methods
All Dudley Group of Hospitals (DGoH) patients who had had an ICD code for rheumatoid arthritis (ever) plus an ICD code for an ACS (within the period 1 January 1990 to 31 December 1999) were identified from discharge diagnosis coding using the hospital information system and cross referenced with a separate coronary care unit (CCU) admissions register. The DGoH at the time consisted of four hospitals with a catchment population of 380 000, of mixed socioeconomic status in a mixed rural and urban setting, with an about average cardiovascular disease prevalence for England and Wales. There were two CCUs with 14 acute and 24 postacute beds and an excellent cardiac rehabilitation service (“Action Heart”). Patients' notes were reviewed by a cardiologist and a rheumatologist to confirm the diagnoses of ACS and rheumatoid arthritis (see below). Only patients with first ever ACS were included in the study; those with a confirmed previous ACS were excluded. This process identified 40 rheumatoid patients for further study. Each case was matched for sex and as closely as possible for age and type of ACS to a non‐rheumatoid control who also had a first ever ACS within three months of the respective index patient (to allow for variations in CCU management practices over time). Diagnosis of rheumatoid arthritis required fulfilment of the 1987 ACR criteria applied retrospectively.11 First ACS (index event, occurring in the absence of any known previous acute event) was defined as follows:
Non‐ST‐elevation acute coronary syndrome: this could be either unstable angina (>20 minutes of typical cardiac pain at rest with or without typical ECG changes of ischaemic heart disease but without creatine kinase (MB fraction, CK‐MB) cardiac enzyme rise), or non‐Q‐wave myocardial infarction (NQWMI) (>20 minutes of typical cardiac pain, dyspnoea, or collapse, with or without ECG change but with a more than twofold transient increase in cardiac enzymes on serial testing).
ST elevation acute coronary syndrome (STEACS): as for non‐Q‐wave myocardial infarction, plus ST segment elevation in more than two contiguous leads (limb leads 1 mm, chest leads 2 mm) with subsequent Q wave formation or new left bundle branch block.
Troponin was not used in our definitions as most admissions predated its introduction into routine clinical practice. The definitions used in the present study largely correspond to the following current ACS definitions12: unstable angina in this study included cases of ACS–unstable angina and ACS–minimal myocardial injury; non‐Q‐wave myocardial infarction (NQWMI) in this study included current ACS–NQWMI (there is a possibility that some such events may have been classified as unstable angina owing to the reduced sensitivity of CK‐MB compared with troponin); STEACS in this study corresponded to current definition of Q wave myocardial infarction.
Discharge diagnosis was defined as the final diagnosis used for coding, made on the basis of the above with the benefit of serial ECGs and cardiac enzymes as stated in the hospital discharge summary and confirmed with CCU records and by the cardiologist (MJB) as above (there were no cases of disagreement); however, the discharge diagnosis was not necessarily the “working diagnosis” made by the admitting doctor.
Killip class is a clinical and prognostic marker of haemodynamic upset resulting from ACS: Killip I, no clinical signs of cardiac decompensation; Killip II, heart failure with rales or S3 gallop or venous hypertension; Killip III, frank pulmonary oedema; Killip IV, hypotension (systolic blood pressure ⩽90 mm Hg) cyanosis, oliguria, diaphoresis, pulmonary oedema.13
Outcome
Outcomes were determined from review of hospital notes and information from death certificates. In the absence of definite evidence of death, direct contact was made with the patient, family, or general practitioner to verify whether the patient was still alive at the end of the study. For the purposes of the study, outcomes should have occurred up to the 31 December 2001. They included: Death of any cause (as per part I of death certificate); Cardiovascular death (if cardiovascular cause stated on part I of death certificate); Recurrent ACS (the first ACS occurring after the index event); and Recurrent cardiac events (cardiovascular death or recurrent ACS). A confirmed recurrent ACS occurring during the index admission was counted as a separate event. To avoid double counting, any further events (for example, a third ACS) were not included in the analysis.
Data collection
Data were collected using a standardised proforma to interrogate the notes, and were entered into a specifically designed spreadsheet. Patients were deemed to have entered the study at first presentation (index event) to CCU with ACS, and the date and time were recorded. The following information was collected: demographic variables and classical cardiovascular risk factors (family history, smoking, past history of hypertension, diabetes mellitus or hypercholesterolaemia, systolic and diastolic blood pressure, body mass index, serum total and high density lipoprotein cholesterol), clinical presentation of index event, clinical severity of presentation (Killip class), treatment of index event in the first 24 hours, serological and ECG findings, discharge drug treatment, risk assessment (exercise test, echocardiogram, myocardial perfusion imaging), and cardiac rehabilitation). Recurrent events were recorded and their time and date noted.
Statistical analysis
Statistical analysis was carried out using SPSS statistical software version 9.0 (SPSS Inc, Chicago, Illinois, USA). Results are presented as frequencies (%) or means with standard deviations (SD). Frequencies were compared using the χ2 test, or Fisher's exact test if n was less than 5 in one or more cells. Means were compared using Student's t test or the Mann–Whitney U test as appropriate, after tests for normality. Kaplan–Meier plots of probability of event‐free survival were made for each group and compared using a log rank survival comparison.
Results
Discharge diagnosis at index event
Twenty of 40 rheumatoid patients (60%) and 22/40 controls (55%) had a non‐ST elevation ACS. The remaining patients in each group (rheumatoid arthritis: 16/40 (40%); controls: 18/40 (45%)) had an ST elevation ACS (p = 0.65). The total observation period (mean (SD) from index event to study closure on 31 December 2001) was 2498 (900) days for the rheumatoid arthritis group compared with 2297 (741) days for the controls (p = 0.28).
Classical cardiac risk factors
As intended, at the index event the rheumatoid patients were matched to the controls for age (years, mean (SD): rheumatoid, 65.2 (15.1); controls, 68.1 (11.0) (p = 0.28)) and sex (15/40 male (37.5%) in both groups). There were no significant differences between the rheumatoid patients and the controls in the presence of any other classical cardiac risk factors, including smoking, diabetes, family history, hypertension, hypercholesterolaemia, and clinical obesity (body mass index ⩾30) (table 1). All patients were white (this was not an inclusion criterion; however, the local population has a particularly low proportion of other ethnic groups (<6%)).
Table 1 “Classical” cardiovascular risk factors in the rheumatoid and control populations.
| Variable | RA (n = 40) | Controls (n = 40) | p Value | |||
|---|---|---|---|---|---|---|
| Age (years) | 65.2 (15.1) | 68.5 (11) | NS | |||
| Male sex | 15 (37.5%) | 15 (37.5%) | NS | |||
| Diabetes | 4 (10%) | 8 (20%) | NS | |||
| Family history | 11 (27.5%) | 19 (47.5%) | NS | |||
| Hypercholesterolaemia | 4 (10%) | 7 (17.5%) | NS | |||
| Smoking (current) | 11 (27.5%) | 9 (22.5%) | NS | |||
| Hypertension | 14 (35%) | 19 (47.5%) | NS | |||
| BMI >30 kg/m2 | 3 (7.5%) | 5 (12.5%) | NS |
Values are mean (SD) or n (%).
BMI, body mass index; RA, rheumatoid arthritis.
Clinical presentation
All 40 controls (100%) had chest pain on presentation, compared with 33/40 (82.5%) of patients with rheumatoid arthritis (p = 0.003). Seven rheumatoid patients (17.5%) presented with collapse, compared with only one control (2.5%) (p = 0.025). Dyspnoea was present in 17 rheumatoid patients (42.5%) compared with 14 controls (35%) (p = 0.49). Serious arrhythmia occurred in two rheumatoid patients (5%) compared with one control (2.5%). Overall, the number of patients in each Killip class of clinical severity was similar in the two groups (table 2).
Table 2 Clinical presentation of index event for the rheumatoid arthritis and control populations studied.
| Variable | RA (n = 40) | Controls (n = 40) | p Value | |||
|---|---|---|---|---|---|---|
| Presenting symptoms | ||||||
| Chest pain | 33 (82.5%) | 40 (100%) | 0.003 | |||
| Dyspnoea | 17 (42.5%) | 14 (35%) | NS | |||
| Collapse | 7 (17.5%) | 1 (2.5%) | 0.025 | |||
| Arrhythmia | 2 (5%) | 1 (2.5%) | NS | |||
| Killip class (severity) | ||||||
| I | 21 (52.5%) | 26 (65%) | NS | |||
| II | 18 (45%) | 14 (35%) | NS | |||
| III | 0 | 0 | NS | |||
| IV | 1 (2.5%) | 0 | NS |
Values are n (%).
Results were analysed using the χ2 test or Fisher's exact test where n<5 in any cell.
RA, rheumatoid arthritis.
Management
There were no significant differences between rheumatoid patients and controls in the frequency of patients receiving oxygen, diamorphine, aspirin, heparin, or β blockers in the first 24 hours after the index event. Of the patients who had ST elevation ACS, thrombolysis was used in 8/16 (50%) in the rheumatoid group, compared with 13/18 (72%) in the control group (p = 0.18). Complete data on administration times for thrombolysis were available on 6/8 rheumatoid patients and 7/13 controls. The mean (SD) time from onset of symptoms to thrombolysis was 26 (40) hours in the rheumatoid group, compared with 6.2 (12) hours in the controls (p = 0.09); time from admission to thrombolysis was 5.8 (10) hours in the rheumatoid group, and 3.3 (9.0) hours in the controls (p = 0.12). Clinically silent ST elevation ACS (Q wave myocardial infarction) occurred in 2/16 rheumatoid patients but in none of the 18 controls (p = 0.25); these two rheumatoid patients did not receive thrombolysis.
The frequency of use of angiotensin converting enzyme (ACE) inhibitors (12/40 rheumatoid patients v 8/40 controls), β blockers (13/40 rheumatoid patients v 15/40 controls), or statins (6/40 rheumatoid patients v 8/40 controls) on discharge after the index event was not significantly different between the rheumatoid patients and the controls.
Pre‐discharge risk assessment with exercise testing occurred in 14/40 rheumatoid patients (35%) compared with 22/40 controls (55%) (p = 0.06). There were no significant differences in the frequency of use of echocardiography (19/40 rheumatoid patients (47.5%) v 12/40 controls (30%)), myocardial perfusion single photon emission computed tomography (10/40 rheumatoid patients (25%) v 8/40 controls (20%)), or coronary angiography (2/40 rheumatoid patients (5%) v 6/40 controls (15%)) between the two groups. No percutaneous coronary angioplasty was undertaken in either group; only one coronary artery bypass was carried out in the control group and none in the rheumatoid group.
Outcomes
Nineteen of 40 (47.5%) of the rheumatoid group died, compared with 10 of 40 (25%) of controls (p = 0.036) (table 3); 16/40 rheumatoid patients (40%) died from a cardiovascular cause, compared with 6/40 controls (15%) (p = 0.012). Cardiovascular deaths represented 84% of the all cause mortality in rheumatoid arthritis and 60% in the controls (p = 0.14).
Table 3 Outcomes in rheumatoid and control patients.
| Variable | RA (n = 40) | Controls (n = 40) | p Value | |||
|---|---|---|---|---|---|---|
| Recurrent cardiac events | 23 (57.5%) | 12 (30%) | 0.013 | |||
| Recurrent ACS | 18 (45%) | 10 (25%) | 0.06 | |||
| Cardiovascular death | 16 (40%) | 6 (15%) | 0.012 | |||
| Death within 30 days of initial event | 5 | 2 | 0.162 | |||
| Death from any cause | 19 (47.5%) | 10 (25%) | 0.036 |
Results were analysed using the χ2test.
ACS, acute coronary syndrome; RA, rheumatoid arthritis.
Non‐cardiovascular deaths in the rheumatoid group resulted from pneumonia (n = 2) and perforated duodenal ulcer (n = 1), and in the control group from pneumonia (n = 2), ampullary tumour (n = 1), and old age, cause unspecified (n = 1).
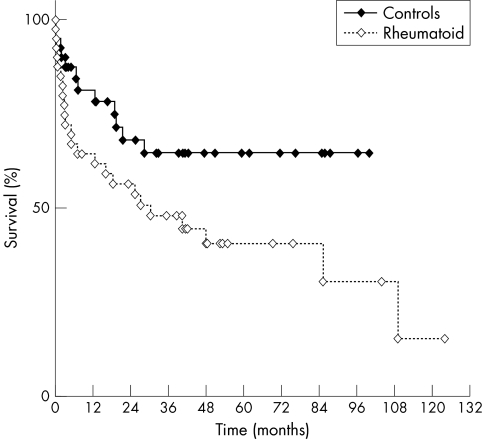
Recurrent ACS occurred in 18/40 rheumatoid patients (45%) compared with 10/40 controls (25%) (p = 0.011). Recurrent cardiac events (recurrent ACS or cardiovascular death) occurred in 23/40 rheumatoid patients (57.5%) compared with 12/40 controls (30%) (p = 0.013) (table 4). The median time (with interquartile range) from the index event to the recurrent cardiac event was 149 (56 to 797) days in the rheumatoid patients, compared with 203 (47 to 577) days in the controls (p = 0.85). The probability of recurrent cardiac event‐free survival was significantly lower in the rheumatoid group than in the controls (log rank survival, p = 0.05) (fig 1).
Table 4 Classical cardiac risk factors, presentation, severity, and treatment in rheumatoid arthritis and controls for those who suffered recurrent cardiac events compared with those who did not.
| Variable | RA (n = 40) | Control (n = 40) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent event (n = 23) | No recurrent event (n = 17) | p Value | Recurrent event (n = 12) | No recurrent event (n = 28) | p Value | |||||||
| Traditional risk factors | ||||||||||||
| Age | 65.2 (15.1) | 65.8 (11) | NS | 74.7 (10.3) | 65.8 (10.7) | 0.016 | ||||||
| Male sex | 9 (39%) | 6 (35%) | NS | 4 (33%) | 11 (39%) | NS | ||||||
| Diabetes | 3 (13%) | 1 (6%) | NS | 3 (25%) | 5 (18%) | NS | ||||||
| Family history | 6 (26%) | 5 (29%) | NS | 5 (42%) | 14 (50%) | NS | ||||||
| Hypercholesterolaemia | 2 (9%) | 2 (12%) | NS | 2 (17%) | 5 (18%) | NS | ||||||
| Smoking (current) | 6 (26%) | 5 (29%) | NS | 2 (17%) | 7 (25%) | NS | ||||||
| Hypertension | 10 (44%) | 4 (24%) | NS | 8 (67%) | 11 (39%) | NS | ||||||
| Obesity (BMI > 30) | 2 (9%) | 1 (6%) | NS | 1 (8%) | 4 (15%) | NS | ||||||
| Index event | ||||||||||||
| Non‐ST‐elevation ACS | 14 (61%) | 10 (59%) | NS | 9 (75%) | 13 (47%) | NS | ||||||
| ST elevation ACS | 9 (39%) | 7 (41%) | NS | 3 (25%) | 15 (53%) | NS | ||||||
| Symptoms | ||||||||||||
| Chest pain | 18 (77%) | 15 (88%) | NS | 12 (100%) | 28 (100%) | NS | ||||||
| Dyspnoea | 10 (44%) | 7 (41%) | NS | 5 (42%) | 9 (32%) | NS | ||||||
| Collapse | 5 (22%) | 2 (12%) | NS | 0 | 1 | NS | ||||||
| Arrhythmia | 1 (4%) | 1 (6%) | NS | 0 | 1 | NS | ||||||
| Killip class | ||||||||||||
| I | 8 (35%) | 13 (76%) | 0.009 | 5 (42%) | 21 (75%) | 0.04 | ||||||
| II | 14 (61%) | 4 (24%) | 0.017 | 7 (58%) | 7 (25%) | 0.04 | ||||||
| III | 0 | 0 | NS | 0 | 0 | NS | ||||||
| IV | 1 (4%) | 0 | NS | 0 | 0 | NS | ||||||
| Discharge drugs | ||||||||||||
| ACE inhibition | 5 (22%) | 7 (41%) | NS | 2 (17%) | 6 (21%) | NS | ||||||
| β Blockade | 4 (17%) | 9 (53%) | 0.02 | 4 (33%) | 11 (39%) | NS | ||||||
| Statin | 3 (13%) | 3 (18%) | NS | 3 (25%) | 5 (18%) | NS | ||||||
| NSAIDs | 18 (78%) | 10 (58%) | NS | |||||||||
| DMARDs (any) | 23 (100%) | 17 (74%) | NS | |||||||||
| Methotrexate | 14 (60%) | 11 (64%) | NS | |||||||||
| Steroids* | 13 (56%) | 11 (64%) | NS | |||||||||
| Exercise rehabilitation | 7 (30%) | 9 (53%) | NS | 2 (16%) | 13 (46%) | NS | ||||||
| Risk assessment | ||||||||||||
| Echocardiography | 12 (52%) | 7 (41%) | NS | 3 (25%) | 9 (28%) | NS | ||||||
| Exercise test | 6 (26%) | 8 (47%) | NS | 4 (33%) | 18 (64%) | NS | ||||||
| SPECT | 7 (30%) | 3 (18%) | NS | 2 (17%) | 6 (21%) | NS | ||||||
Values are mean (SD) or n (%). Results were analysed using the χ2 test or Fisher's exact test (if n<5 in any cell). Age was analysed by t test. Significant p values in bold.
*Steroids defined as prednisolone 7.5 mg or more for more than six months ever. Data are not available on controls for NSAID and steroid use.
ACE, angiotensin converting enzyme; ACS, acute coronary syndrome; DMARD, disease modifying antirheumatic drug; NSAID, non‐steroidal anti‐inflammatory drug; SPECT, single photon emission computed tomography.
Figure 1 Kaplan–Meier plots of the probability of event‐free survival for the rheumatoid arthritis and control populations (log rank survival, p = 0.05).
Predictors of recurrent cardiac events
The baseline characteristics at the index event were compared between patients who subsequently experienced recurrent cardiac events and those who did not. In the control group, patients who experienced recurrent events were significantly older (74 (10.3) years) than those who did not (65 (10.7) years) (p = 0.016) (table 4). There were no significant differences in the frequency of any of the classical cardiovascular risk factors between the rheumatoid arthritis subgroups. The exact nature of the index ACS did not differ between patients who had a recurrent cardiac event and those who had not, within either the rheumatoid group or the control group. Significantly more patients with recurrent cardiac events presented in Killip class II in both groups: rheumatoid group, 14/23 (61%) with recurrent cardiac events v 4/17 (24%) without (p = 0.02); control group, 7/12 (58%) v 7/28 (25%) (p = 0.04). The frequency of use of various drugs (including thrombolysis in patients with ST elevation ACS) did not differ between the groups, with the exception of β blockers: within the rheumatoid group (but not the control group) those prescribed β blockers were significantly less likely to suffer from a recurrent event. Risk assessment or exercise rehabilitation did not differ between the groups.
Discussion
Previous studies have documented an increase in cardiovascular mortality in rheumatoid arthritis.2,14,15,16 This could either be because cardiovascular disease is commoner in rheumatoid arthritis, or because it has a worse outcome in rheumatoid arthritis than in the general population. ACS account for most cardiovascular mortality both in the general population and in rheumatoid arthritis.6,8 The present study addressed specifically whether ACS has a worse outcome in rheumatoid arthritis, and is the first to document that ACS is associated with an increase in recurrent ischaemic events and death in this disease. This was a retrospective study in a relatively small sample, rendering it prone to type II errors. The multiple significance tests may also have led to type I errors, so the results should be interpreted with caution. However, the great similarity between cases and controls for age, sex, classical cardiovascular risk factors, severity of presentation, type of ACS, and period of treatment was a significant strength. The morbidity and mortality patterns seen in the control population in the present study are comparable with those reported in similarly treated contemporary populations,10 suggesting that our population was representative. Unrecognised myocardial infarction and sudden deaths are more common in patients with rheumatoid arthritis than in the general population, so more patients with rheumatoid arthritis may have died before admission to a CCU16 or have been treated outside a CCU. However, this study did not set out to investigate the incidence of ACS in rheumatoid arthritis but rather the outcome in similarly managed patients with ACS; thus only patients admitted to a CCU were included. Interestingly, the very low rates of post‐ACS coronary angiography and revascularisation in this study, which reflected routine practice (resulting from the low availability of these services) in UK district general hospitals in the 1990s,10 provided a unique opportunity to study the “natural course” of events both in rheumatoid arthritis and controls.
Rheumatoid patients may have worse “background atherosclerosis” than even subjects matched for classical cardiovascular risk factors. Continuous exposure to high grade systemic inflammation may be linked to accelerated atherosclerosis.17 Evidence for this is available from studies assessing in vivo endothelial function,18 carotid intima–media thickness,19,20 and arterial elasticity21 and compressibility,22 which show earlier and more severe changes in rheumatoid arthritis than in age and sex matched controls. However, arterial stenoses from advanced lesions may be “benign”, remaining clinically silent or causing stable symptoms (for example, effort angina). Lesion size has no relation to thrombosis development; in fact most ACS develop at sites where atheroma causes less than 50% stenosis,23 and rupture is more likely at less stenotic lesions where the wall tension is greater.24 It is the rupture of the fibrous cap caused by plaque instability—exposing the highly thrombogenic core material and leading to the sudden development of local thrombosis—that leads to ACS. Plaque instability rather than accelerated plaque formation may be especially important in the context of ACS (and its worse outcome) in rheumatoid arthritis.
There is currently no direct evidence that atheromatous plaques may be more unstable in patients with rheumatoid arthritis, but several mechanisms may contribute to this. Key inflammatory cytokines such as tumour necrosis factor α (TNFα), interleukin (IL) 1β, and interferon γ (IFNγ) involved in plaque rupture may be present at much higher levels in the “high grade” inflammatory state of rheumatoid arthritis.17 The resulting inhibition of vascular smooth muscle cell proliferation and collagen synthesis by T cell derived IFNγ,25,26 and collagen degradation from enhanced metalloproteinase14 and free radical release by TNFα activated macrophages, may make plaques particularly vulnerable in rheumatoid patients. CD25+ (IL2 receptor expressing, activated) T cells in atherectomy lesions relate to the severity of ACS27 and are particularly prevalent in the peripheral blood of patients with rheumatoid arthritis.28 Patients with ACS also have an expanded population of CD4+CD28null cells, present in culprit lesions but not in stable plaques.29 These appear to represent senescent T cells with cytotoxic potential implicated in plaque rupture.30 These cells were first noticed in rheumatoid vasculitis. Their presence in large numbers has been linked to more advanced atherosclerotic changes and possibly to plaque instability in rheumatoid arthritis,31 as in the presence of raised CRP they can be cytotoxic to endothelial cells.32 Levels of CRP are strongly linked to ACS outcome in the general population33 for reasons that remain unclear. It may play a pathogenic role locally in the plaque as an endogenous activator of complement, foam cell formation from uptake of CRP‐opsonised LDL by macrophages,34 endothelial activation with enhanced expression of cellular adhesion molecules,35 MCP‐1 induction,36 and sensitisation to damage from cytotoxic T cells.32 Acute stress leads to a rapid increase in C reactive protein, particularly in patients with active rheumatoid arthritis; combined with haemostatic, rheological, and haemodynamic reactions over and above the already high baseline levels, this could underlie the increased risk for reinfarction in this vulnerable patient group.37 An inflammation induced dysregulated prothrombotic state38,39 may also explain the higher recurrence rate observed in rheumatoid patients in this study.
Differences in pre‐existing comorbidity, clinical presentation, and the subsequent management of ACS in the two populations may be equally important reasons for the worse outcomes observed in rheumatoid arthritis. It should be noted that the method of identifying controls resulted in a (non‐significantly) longer total observation period in the rheumatoid group compared with the controls; this may have allowed additional events to emerge. The presence of multiple physical and psychosocial comorbidities is common in rheumatoid arthritis,7 and they are bad prognostic factors in ACS. Depression, for example, which is itself a predictor of adverse outcome in ACS, is both commoner and more severe in patients suffering from a combination of rheumatoid arthritis and cardiovascular disease.40 Clinical presentation is also important, as it may lead to misdiagnosis and delayed treatment. One fifth of rheumatoid patients with ACS (compared with none in the control group) presented without chest pain, leading to later and reduced use of thrombolytic treatment. It appears that the previously noted silent ischaemic heart disease in rheumatoid arthritis9,39 may occur even in the context of ACS, whereas presentation with primary symptoms other than chest pain (for example collapse) is known to associate with poorer prognosis in the general population.41 It is possible that steroid, analgesic, or NSAID use may have modified symptoms. Subsequent pharmacological management was not particularly different in the two groups. It is, however, interesting that, specifically in rheumatoid arthritis, the lack of use of β blockers may be associated with recurrent events. Long term β blockade reduces post‐myocardial infarction morbidity and mortality in unselected patients.42 Rheumatoid patients have increased sympathetic activity,43 so β blockers may be more beneficial in this group. Finally, few rheumatoid patients appear to receive appropriate post‐ACS risk assessment. We have previously suggested that physical disability may be an important referral barrier for risk assessment with exercise testing or coronary angiography in patients with rheumatoid arthritis.44
Diabetes mellitus is a recognised coronary heart disease risk factor, but over the past decade, aggressive treatment has translated into significantly improved ACS outcomes. As with diabetes,44 we suggest that rheumatoid patients presenting with suspected ACS should be stratified as high risk. In this study, Killip class ⩾II at presentation was associated with a poor outcome and may be a useful tool to identify patients at the highest risk. Admitting physicians need to have an increased awareness of unusual presentations without chest pain. Post‐ACS risk assessment strategies need to be more aggressive, and pharmacologically stressed myocardial perfusion imaging9 may be a good initial option for physically disabled patients unable to have exercise testing, as it is a highly sensitive and specific tool for predicting ACS recurrence in the general population.45 Rheumatoid patients should not be discriminated against from early intervention with coronary angioplasty and stenting. Strategies involving traditional antirheumatic drugs aimed at effectively controlling systemic inflammation and thus improving plaque stabilisation may already be reducing risk,46 but additional cardiovascular drugs—for example statins47—need to be investigated prospectively in this high risk group.
Acknowledgements
We would like to thank the staff of the Coronary Care Units at Russells Hall and Wordsley Hospitals for their help and support in conducting this study. The Dudley Group of Hospitals R&D Directorate supported this work. The Department of Rheumatology was in receipt of an Arthritis Research Campaign integrated clinical arthritis centre grant.
Abbreviations
ACS - acute coronary syndrome
CCU - coronary care unit
NQWMI - non‐Q‐wave myocardial infarction
NSAID - non‐steroidal anti‐inflammatory drug
STEACS - ST elevation acute coronary syndrome
References
- 1.Cobb S, Anderson F, Bauer W. Length of life and cause of death in rheumatoid arthritis. N Engl J Med 1953249553–556. [DOI] [PubMed] [Google Scholar]
- 2.Goodson N. Coronary artery disease in rheumatoid arthritis. Curr Opin Rheumatol 200214115–120. [DOI] [PubMed] [Google Scholar]
- 3.Kitas G D, Banks M, Bacon P A. Cardiac involvement in rheumatoid disease. Clin Med 2001118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. BMJ 19852901797–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myllykangas‐Luosujarvi R, Isomaki H, Koota K. Cardiovascular mortality in women with rheumatoid arthritis. J Rheumatol 1995221065–1067. [PubMed] [Google Scholar]
- 6.Wållberg‐Jonsson S, Ohman M L, Rantapaa‐Dahlqvist S. Cardiovascular morbidity and mortality in patients with seropositive RA in northern Sweden. J Rheumatol 199724445–451. [PubMed] [Google Scholar]
- 7.Gabriel S E, Crowson C S, O'Fallon W M. Comorbidity in arthritis. J Rheumatol 1999262475–2479. [PubMed] [Google Scholar]
- 8.del Rincon I, Williams K, Stern M P, Freeman G L, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by classical risk factors. Arthritis Rheum 2001442737–2745. [DOI] [PubMed] [Google Scholar]
- 9.Banks M, Flint J, Bacon P A, Kitas G D. Rheumatoid arthritis is an independent risk factor for ischaemic heart disease [abstract]. Arthritis Rheum 200043(suppl 9)S385 [Google Scholar]
- 10.Herlitz J, Karlson B W, Sjolin M, Lindqvist J. Ten year mortality in subsets of patients with an acute coronary syndrome. Heart 200186391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett F C, Edwarthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 12.Fox K A A, Birkhead J, Wilcox R, Knight C, Barth J. British Cardiac Society Working Group on the definition of myocardial infarction. Heart 200490603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killip T, Kimball J. Treatment of myocardial infarction in a coronary care unit: a two year experience with 250 patients. Am J Cardiol 196720457–465. [DOI] [PubMed] [Google Scholar]
- 14.Kitas G D, Erb N. Tackling ischaemic heart disease in rheumatoid arthritis. Rheumatology 200342607–613. [DOI] [PubMed] [Google Scholar]
- 15.Maradit‐Kremers H, Nicola P J, Crowson C S, Ballman K V, Gabriel S E. Cardiovascular death in rheumatoid arthritis: a population‐based study. Arthritis Rheum 200552722–732. [DOI] [PubMed] [Google Scholar]
- 16.Maradit‐Kremers H, Crowson C S, Nicola P J, Ballman K V, Roger V L, Jacobsen S J.et al Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheum 200552402–411. [DOI] [PubMed] [Google Scholar]
- 17.Sattar N, McCarey D W, Capell H, McInnes I B. Explaining how “high‐grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 20031082957–2963. [DOI] [PubMed] [Google Scholar]
- 18.Bacon P A, Raza K, Banks M J, Townend J, Kitas G D. The role of endothelial cell dysfunction in the cardiovascular mortality of RA. Int Rev Immunol 2002211–17. [DOI] [PubMed] [Google Scholar]
- 19.Park Y B, Ahn C W, Choi H K, Lee S H, In B H, Lee H C. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum 2002461714–1719. [DOI] [PubMed] [Google Scholar]
- 20.Wållberg‐Jonsson S, Backman C, Johnson O, Karp K, Lundstrom E, Sundqvist K G.et al Increased prevalence of atherosclerosis in patients with medium term rheumatoid arthritis. J Rheumatol 2001282597–2602. [PubMed] [Google Scholar]
- 21.Klocke R, Cockcroft J R, Taylor G J, Hall I R, Blake D R. Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Ann Rheum Dis 200362414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Rincon I, Haas R W, Pogosian S, Escalante A. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis 200564425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith S C. Risk‐reduction therapy: the challenge to change. Presented at the 68th Scientific Sessions of the American Heart Association November 13, 1995 Anaheim, California. Circulation 1996932205–2211. [DOI] [PubMed] [Google Scholar]
- 24.Lee R T, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol 1997171859–1867. [DOI] [PubMed] [Google Scholar]
- 25.Hansson G K, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation‐specific alpha‐smooth muscle actin in arterial smooth muscle cells. J Exp Med 19891701595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amento E P, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1991111223–1230. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal A C, Piek J J, de Boer O J, Koch K T, Teeling P, van der Loos C M.et al Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. Heart 19988014–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitas G D, Salmon M, Farr M, Gaston J S, Bacon P A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol 198873242–249. [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzo G, Goronzy J J, Yang H, Kopecky S L, Holmes D R, Frye R L.et al Monoclonal T‐cell proliferation and plaque instability in acute coronary syndromes. Circulation 20001012883–2888. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima T, Goek O, Zhang X, Kopecky S L, Frye R L, Goronzy J J.et al De novo expression of killer immunoglobulin‐like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ Res 200393106–113. [DOI] [PubMed] [Google Scholar]
- 31.Gerli R, Schillaci G, Giordano A, Bocci E B, Bistoni O, Vaudo G.et al CD4+CD28‐ T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 20041092744–2748. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima T, Schulte S, Warrington K J, Kopecky S L, Frye R L, Goronzy J J.et al T‐Cell‐mediated lysis of endothelial cells in acute coronary syndromes. Circulation 2002105570–575. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfield G M, Pepys M B. C‐reactive protein and cardiovascular disease: new insights from an old molecule. QJM 200396793–807. [DOI] [PubMed] [Google Scholar]
- 34.Zwaka T P, Hombach V, Torzewski J. C‐reactive protein‐mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation 20011031194–1197. [DOI] [PubMed] [Google Scholar]
- 35.Pasceri V, Willerson J T, Yeh E T H. Direct proinflammatory effect of C‐reactive protein on human endothelial cells. Circulation 20001022165–2168. [DOI] [PubMed] [Google Scholar]
- 36.Torzewski M, Rist C, Mortensen R F, Zwaka T P, Bienek M, Waltenberger J.et al C‐reactive protein in the arterial intima: role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000202094–2099. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuijzen van Zanten J J, Ring C, Carroll D, Kitas G D. Increased C reactive protein in response to acute stress in patients with rheumatoid arthritis. Ann Rheum Dis 2005641299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEntegart A, Capell H A, Creran D, Rumley A, Woodward M, Lowe G D O. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology 200140640–644. [DOI] [PubMed] [Google Scholar]
- 39.Wållberg‐Jonsson S, Cederfelt M, Rantapaa‐Dahlqvist S. Hemostatic factors and cardiovascular disease in active rheumatoid arthritis: an 8 year follow up study. J Rheumatol 20002771–75. [PubMed] [Google Scholar]
- 40.Treharne G J, Hale E D, Lyons A C, Booth D A, Banks M J, Erb N.et al Cardiovascular disease and psychological morbidity among rheumatoid arthritis patients. Rheumatology 200544241–246. [DOI] [PubMed] [Google Scholar]
- 41.Dorsch M F, Lawrance R A, Sapsford R J, Durham N, Oldham J, Greenwood D C.et al Poor prognosis of patients presenting with symptomatic myocardial infarction but without chest pain. Heart 200186494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 19993181730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dekkers J C, Geenen R, Godaert G L, Bijlsma J W, van Doornen L J. Elevated sympathetic nervous system activity in patients with recently diagnosed rheumatoid arthritis with active disease. Clin Exp Rheumatol 20042263–70. [PubMed] [Google Scholar]
- 44.Banks M, Kitas G. Patients' physical disability may influence doctors' perceptions of suitability for risk assessment of CHD. BMJ 19993191266a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu S, Senior R, Dore C, Lahiri A. Value of thallium‐201 imaging in detecting adverse cardiac events after myocardial infarction and thrombolysis: a follow up of 100 consecutive patients. BMJ 1996313844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnan E, Lingala V B, Singh G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation 20041101774–1779. [DOI] [PubMed] [Google Scholar]
- 47.McCarey D W, McInnes I B, Madhok R, Hampson R, Scherbakov O, Ford I.et al Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double‐blind, randomised placebo‐controlled trial. Lancet 20043632015–2021. [DOI] [PubMed] [Google Scholar]