Abstract
Objectives
To evaluate the prevalence and incidence of antimalarial myopathy in patients with rheumatic diseases treated with antimalarial drugs.
Methods
Over a three year period, all patients with rheumatic diseases who were taking antimalarial drugs were studied. Serum muscle enzymes were assessed at the time of inclusion and every six months thereafter. Muscle strength, electromyography (EMG), and muscle biopsy were assessed in patients with a persistent muscle enzyme disturbances.
Results
119 patients were included (111 chloroquine, eight hydroxychloroquine). Of these, 22 (18.5%) had a persistent muscle enzyme disturbance: lactate dehydrogenase 19/22 (86%); creatine kinase 7/22 (32%), and aldolase 3/22 (14%). Findings of antimalarial myopathy were detected in 3/15 biopsied patients (20%) by light microscopy and in all 15 by electron microscopy. Eleven patients had myopathy at the time of inclusion (prevalence 9.2%) and four patients developed muscle injury during follow up (annual incidence 1.2%). Muscle weakness was observed in 8 of 15 patients with biopsy proven myopathy, giving a prevalence of clinical antimalarial myopathy of 6.7%. All these patients also had a myopathic pattern on electromyography.
Conclusions
The prevalence of antimalarial myopathy is higher than previously recognised when muscle enzyme determination is used as a screening method. When a persistent muscle enzyme disturbance is observed, clinical and electromyographic studies should be undertaken periodically to detect the development of clinical myopathy. In cases of clinical myopathy, an anatomical‐pathological tissue study, including an ultrastructural study, is mandatory to confirm the diagnosis.
Keywords: antimalarial drugs, chloroquine, hydroxychloroquine, myopathy
Antimalarial drugs have proved to be beneficial in the treatment of various rheumatic diseases. It is thought that hydroxychloroquine sulphate is less toxic and has the same efficacy as chloroquine.1 Thus, currently, hydroxychloroquine is more often used in most countries, although in some, including Spain, chloroquine is still frequently prescribed.
Antimalarial drugs have significant lisosomal affinity and induce the prominent development of autophagic vacuoles in several tissues.2,3,4 Long term administration of these drugs may result in accumulation of intracellular deposits, mainly in retina and muscle. Although myopathy is one of the most recognised toxic adverse effects, its prevalence in patients chronically treated with antimalarial drugs remains unclear. Several cases of antimalarial induced myopathy, most of them isolated cases, have been reported.5,6,7,8,9,10,11,12,13,14,15,16,17
Initial symptoms of muscular injury are characteristically mild. However, painless proximal weakness in both upper and lower extremities may become more severe with time. In many cases this clinical feature is masked by the musculoskeletal manifestations of the underlying disease, which could explain why the diagnosis of antimalarial myopathy is usually difficult and often delayed.
To determine the incidence and prevalence of antimalarial myopathy and its clinical consequences in patients with rheumatic diseases, we designed a longitudinal study using serum muscle enzyme analysis as a single sensitive screening method.
Methods
A three year prospective longitudinal study was carried out in a rheumatology unit. All consecutive patients who had been taking antimalarial drugs at the time of inclusion for a period of at least six months were recruited. This minimum period of time was chosen because no cases of antimalarial myopathy have been reported within the first six months of treatment. One hundred and nineteen white patients were included in the study (84 female, 35 male; mean (SD) age 57.5 (13.9) years). The underlying rheumatic disease was rheumatoid arthritis in 69 patients, palindromic rheumatism in 14, Sjögren's syndrome in 11, systemic lupus erythematosus in nine, undifferentiated connective tissue disease in seven, psoriatic arthritis in four, and other rheumatic conditions in five. In all, 111 patients were being treated with chloroquine and eight with hydroxychloroquine. In no case did the daily prescribed amount of antimalarial drug exceed the recommended dose (3.5 mg/kg/day for chloroquine and 6.5 mg/kg/day for hydroxychloroquine). Mean duration of the treatment was 40.4 months (range 6 to 192).
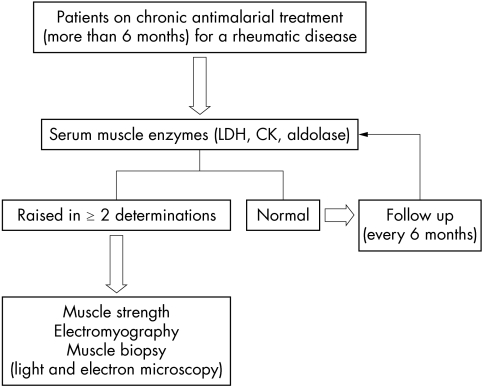
Serum muscle enzyme determinations served as the initial screening test in all patients, regardless of their clinical symptoms. The enzymes studied included lactate dehydrogenase (LDH; normal value 0–480 IU/l), creatine kinase (CK; normal value 0–195 IU), and aldolase (normal value 0–7.6 IU/l). Determinations were carried out at the time of inclusion and at six monthly intervals during the follow up period in all patients, regardless of whether they had symptoms or not. The diagnostic algorithm used in the study is shown in fig 1. Ethics approval was given for the study.
Figure 1 Diagnostic algorithm followed in the study.
Myopathy was suspected in all patients with persistent muscle enzyme disturbance, defined as a rise in any one of the muscle enzymes measured in the serum and confirmed in a second determination two or three weeks later. Other causes of myopathy or muscle enzyme increase—such as haemolysis, myocardial and renal infarction, low grade infections, chronic liver and pulmonary diseases, and malignancy—were excluded by clinical and laboratory assessment. After informed consent, all patients with persistent muscle enzyme disturbances underwent a muscle strength assessment, electromyographic study, and muscle biopsy to make a definitive diagnosis. A muscle biopsy was also done in any patient with clinical weakness, even if muscle enzyme determinations were normal.
A complete neurological examination, including tendon reflexes, sensory and motor assessment, was carried out in all cases by the same physician (JMM) immediately after a persistent muscle enzyme disturbance was detected. Muscle strength was assessed in proximal and distal muscles of upper and lower limbs and in neck flexor muscles, and was graded according to the standard 0–5 on the Medical Research Council scale. We considered muscle weakness as mild when muscle strength was 4+, moderate when it was between 3+ and 4, and severe when it was 3 or lesser in at least one muscle group.
Electromyography and nerve conduction studies of the proximal muscles of the upper and lower limbs were carried out employing surface electrodes by standard methods, using the Viking IV electromyograph system (Nicolet, Biomedical, Madison, Wisconsin, USA) in all patients with persistent muscle enzyme disturbances.
An open biopsy was obtained from an electromyographically involved muscle group from each patient who had given consent. If no significant electromyographic findings were present, a biopsy sample from either the deltoid or the quadriceps femoris muscle was obtained. Standard light and electron microscopic studies were carried out by the same pathologists (IO, AA) in each case. Briefly, tissue samples were frozen at −156°C in isopentane, cooled by liquid nitrogen. Transverse cryostat 10–20 µm thick sections were stained with haematoxylin‐eosin and Gomori's modified trichrome for light microscopic study. Thin sections were subsequently stained with uranyl acetate and lead citrate for electron microscopic study. Antimalarial muscle toxicity was diagnosed only if the electron microscopic study showed evidence of curvilinear bodies, with or without myeloid bodies. Non‐specific alterations such as vacuolar myopathy on light microscopy or free glycogen on electron microscopy were considered insufficient for diagnosis.
Definitions
We defined antimalarial myopathy as the presence of the specific ultrastructural microscopic findings associated with persistent muscle enzyme disturbances, regardless of the clinical symptoms of the patients.
We defined clinical antimalarial myopathy as the presence of antimalarial myopathy associated with an objective muscle weakness, through direct examination of proximal and distal muscles of upper and lower limbs and neck flexor muscles.
Statistical analysis
Statistical analysis was undertaken using an SPSS 11 database. Data are presented as mean (SD) or range, and percentages of total with 95% confidence intervals (CI). Student's t tests and Levene tests were used as appropriate to determine statistically significant differences between groups. Differences were considered significant at p<0.05.
Results
Antimalarial myopathy, as defined above, was demonstrated in 12.6% of the patients included in the study. Eleven had myopathy at the inclusion point (prevalence 9.2%) and four patients developed it during the follow up period (annual incidence 1.2%). The presence of symptoms of muscle weakness was observed in 6.7% of the patients included in the study, and in most cases (75%) these symptoms were mild to moderate. When the antimalarial treatment was withdrawn the clinical myopathy tended to disappear in all cases.
Twenty two of the 119 patients included in the study (18.5%) had a persistent muscle enzyme disturbance. Raised LDH (mean value 646.3 IU/l, range 498 to 1282 IU/l) was the most frequent muscle enzyme disturbance (86%), and was the only muscle enzyme increased in 14 patients (64%). Seven patients (32%) showed raised CK serum levels (mean value 460 IU/l, range 201 to 1479 IU/l); however, an isolated increase in CK was observed in only three patients (14%), and no patient had an isolated increase in aldolase.
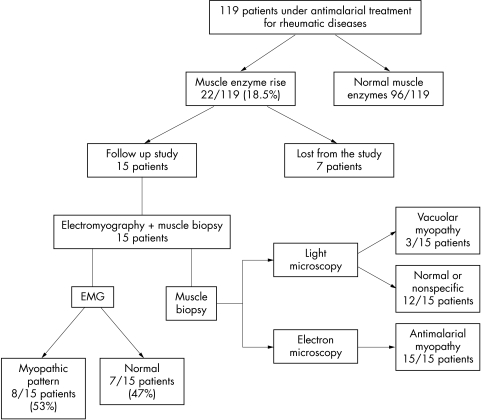
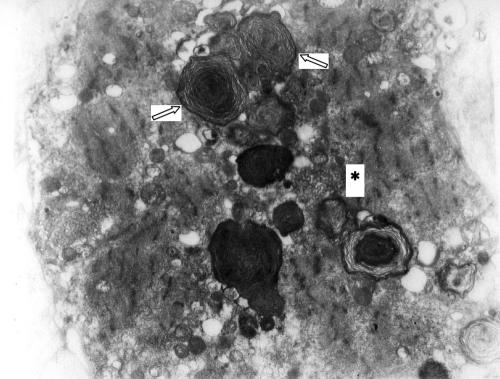
Muscle biopsy was carried out in 15 of 22 patients with persistent muscle enzyme disturbances. Seven with persistent muscle enzyme disturbances dropped out of the study for the following reasons: neoplasm detected (1), change of residence (3), and consent denial (3) (fig 2). All the patients with persistent muscle enzyme disturbances who had biopsies had specific findings of antimalarial muscle toxicity (lipid deposits as curvilinear or myeloid bodies or both), so they were diagnosed as having antimalarial myopathy (fig 3). In contrast, vacuolar myopathy suggestive of antimalarial toxic myopathy was seen in only three of the patients by light microscopy (20%).
Figure 2 Flow of patients in the study.
Figure 3 Sarcoplasm of a muscle fibre showing the characteristic findings of antimalarial myopathy: Myeloid bodies (arrow) and curvilinear bodies (asterisk). (ME, ×13 000.)
Eleven patients had myopathy at the time of inclusion, giving a prevalence of 9.2% (95% CI, 4.0% to 14.4%). A further four patients developed this complication during follow up, representing an annual incidence of 1.2% (0.03% to 2.4%). Thus the cumulative prevalence of antimalarial myopathy in the present study was 12.6% (6.6% to 18.5%).
Eight of the 15 patients with antimalarial myopathy (53%) also had muscle weakness on physical examination: mild to moderate in six cases (75%) and severe in two. Thus eight of the 119 patients included in the study proved to have clinical antimalarial myopathy, which represents a prevalence of 6.7% (95% CI, 4.8% to 8.6%).
Electromyographic study showed a myopathic pattern in eight of 15 patients with antimalarial myopathy (53%). A muscle strength assessment and an electromyogram were also undertaken in three of the seven patients who had dropped out of the study, all of which were normal.
Thirteen patients with antimalarial myopathy were being treated with chloroquine and two with hydroxychloroquine, although one of the latter had being receiving chloroquine at the onset of his rheumatic condition. Ten patients also received a maximum daily dose of 7.5 mg of prednisone or equivalent for their rheumatic disease. No other potential myotoxic drugs were prescribed, and other causes of metabolic myopathy were ruled out. The main characteristics of patients with antimalarial myopathy are summarised in table 1.
Table 1 Clinical, analytical, and histological characteristics of patients with biopsy proven antimalarial myopathy.
| Case | Sex | Age (years) | Underlying disease | Drug | Duration of treatment | Daily dose | Laboratory* mean value | Muscle strength† | EMG | Light microscopy | Electronmicroscopy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | RA | CQ | 31 months | 250 mg | LDH 501.5 | 2/5 | Myopathic | Vacuolar myopathy | CB, MB |
| 2 | F | 73 | RA | CQ | 23 months | 250 mg | LDH 1152; CK 1497 | 4/5 | Myopathic | Vacuolar myopathy | MB |
| 3 | F | 77 | RA | CQ | 26 months | 250 mg | LDH 587 | 4‐/5 | Myopathic | Vacuolar myopathy | CB, MB |
| 4 | M | 77 | Psoriatic arthritis | HCQ | 74 months | 400 mg | LDH 1282; aldolase 23 | 4/5 | Myopathic | Normal | CB, MB |
| 5 | F | 78 | RA | CQ | 28 months | 250 mg | LDH 661 | 1/5 | Myopathic | Normal | CB, MB |
| 6 | F | 28 | SLE | HCQ | 12 months | 400 mg | CK 201.7 | 5/5 | Normal | Fibres atrophy | CB |
| 7 | F | 74 | RA | CQ | 62 months | 250 mg | LDH 546 | 4/5 | Myopathic and neuropathic | Normal | CB |
| 8 | M | 57 | RA | CQ | 76 months | 250 mg | CK 276.3 | 5/5 | Normal | Fibre atrophy | CB, MB |
| 9 | F | 65 | RA | CQ | 26 months | 250 mg | LDH 506 | 5/5 | Normal | Normal | MB |
| 10 | F | 61 | RA | CQ | 104 months | 250 mg | LDH 610 | 5/5 | Normal | Normal | CB, MB |
| 11 | F | 59 | RA | CQ | 70 months | 250 mg | LDH 572; CK 255.5 | 4/5 | Myopathic | Fibre atrophy | CB, MB |
| 12 | M | 39 | Pal R | CQ | 11 months | 250 mg | CK 213 | 5/5 | Normal | Normal | CB, MB |
| 13 | F | 53 | SS | CQ | 6 months | 250 mg | LDH 498 | 5/5 | Normal | Normal | CB, MB |
| 14 | F | 64 | SS | CQ | 68 months | 250 mg | LDH 532.3; CK 319.7 | 5/5 | Normal | Fibre atrophy | CB |
| 15 | F | 62 | RA | CQ | 30 months | 250 mg | LDH 554.7 | 4+/5 | Myopathic | Fibre atrophy | CB |
*Normal laboratory values: lactic dehydrogenase (LDH), 0 to 480 IU/l; creatine kinase (CK), 0 to 195 IU/l; aldolase, 0 to 7.6 IU/l.
†Muscle strength according the standard 0–5 MRC scale.
CB, curvilinear bodies; CQ, chloroquine; F, female; HCQ, hydroxychloroquine; M, male; MB, myeloid bodies; Pal R, palindromic rheumatism; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome.
It is noteworthy that no patient with normal muscle enzymes suffered from clinical weakness during the period of the study.
The antimalarial treatment was withdrawn from the seven patients with moderate to severe muscle weakness (muscle strength ⩽4). The signs and symptoms of clinical myopathy diminished or disappeared in all of these (table 2). One patient (case 7) had both neuropathic and myopathic findings on electromyography, although only the latter resolved when the antimalarial drug was discontinued. Three patients died during follow up. One had a myocardial infarct two years after complete recovery of the muscle enzyme disturbance; a second had a diverticulitis complicated by peritonitis and sepsis and died two months after the diagnosis of the antimalarial myopathy; the third, who had previous diabetes and coronary artery disease, died from heart failure three years after drug withdrawal and muscle enzyme normalisation (table 2).
Table 2 Evolution of serum muscle enzyme levels, muscle weakness, and electromyographic findings in patients with moderate and severe clinical antimalarial myopathy after drug withdrawal.
| Case | Age/sex* | Drug | Laboratory* (before/after†) | Muscle weakness‡ (before/after†) | Electromyography (before/after†) | Observations |
|---|---|---|---|---|---|---|
| 1 | 68M | CQ | LDH 501/389 | +++/+ | Myop/normal | |
| 2 | 73F | CQ | LDH 1152/436 | ++/− | Myop/normal | Death (myocardial infarction) |
| CK 1497/38 | ||||||
| 3 | 77F | CQ | LDH 587/459 | ++/− | Myop/normal | |
| 4 | 77M | HCQ | LDH 1282/NA | ++/NA | Myop/NA | Death (sepsis) |
| Aldolase 23/NA | ||||||
| 5 | 78F | CQ | LDH 661/466 | +++/− | Myop/normal | Death (heart failure) |
| 7 | 74F | CQ | LDH 546/403 | ++/− | Myoneurop/neurop | |
| 11 | 59F | CQ | LDH 572/449 | ++/− | Myop/normal | |
| CK 255/91 |
*Normal laboratory values: lactic dehydrogenase (LDH), 0 to 480 IU/l; creatine kinase (CK), 0 to 195 IU/l; aldolase, 0 to 7.6 IU/l.
†Before/after, before and after antimalarial withdrawal.
‡Muscle weakness: − absent; + mild; ++ moderate; +++ severe, as defined in Methods.
CQ, chloroquine; F, female; HCQ, hydroxychloroquine; M, male; Myoneurop, myoneuropathic pattern; Myop, myopathic pattern; NA, not available; Neurop, neuropathic pattern.
Discussion
The spectrum of muscle toxicity caused by antimalarial drugs is extensive and in some cases controversial. The presence of the specific ultrastructural findings of antimalarial toxicity in muscle tissue may not always imply a muscle disease but could be a muscular deposit of these drugs or their metabolites. Thus Kumamoto et al18 observed by electron microscopy that experimental chloroquine treated rats developed dense membranous structures (curvilinear bodies) in soleus muscle fibres after the eighth day of daily intraperitoneal injections of chloroquine. In our study we considered that an antimalarial myopathy was present only if the patient had both these specific histological findings and a persistent muscle enzyme disturbance, regardless of their clinical symptoms. On this basis we found a prevalence of antimalarial myopathy of 9.2% and an annual incidence of 1.2%, because four new patients developed the myopathy during the follow up period. This represents an accumulated prevalence of 12.6%. Though 11 patients had antimalarial myopathy at the start of the study, we do not know exactly when these patients developed the myopathy. Most had been taking antimalarial drugs for more than two years, but this complication can be present at a subclinical stage.
Our results seem to suggest a much higher incidence of myopathy than was found in previous small retrospective uncontrolled studies.5,10,11 No report with prospective data on the incidence of antimalarial myopathy has been published to date. Avina‐Zubieta et al11 reported an incidence of 1 in 100 patient‐years of treatment, but their study was retrospective and showed the frequency of clinical myopathy related to the time of treatment and not the true incidence of this complication. Furthermore, the differences between our study and other series5,10,11 could be explained by the screening method used to reach a diagnosis of antimalarial myopathy. While earlier reports based their diagnosis on the patient's clinical symptoms, a persistent disturbance of serum muscle enzymes was used as a starting point in the present study. We chose these biochemical tests because they are simple to perform and sensitive enough to detect muscle injury.
The serum muscle enzyme disturbance observed in most patients with myopathy in our study was mild, and LDH was by far the most sensitive enzyme for detecting muscle damage, as has previously been suggested.12 However, it is well known that LDH is not specific for muscle disease and raised concentrations can be found in other conditions, for example myocardial infarction, chronic liver and pulmonary diseases, haemolysis, renal and intestinal infarction, stroke, pulmonary emboli, pancreatitis, low grade infections, neoplasias, and fractures. Nevertheless, in our patients we were able to exclude these conditions (for example, a patient with a hepatic carcinoma was excluded from the study). That all patients with abnormal LDH from whom antimalarial drugs were withdrawn subsequently showed normalisation of their levels (table 2) reinforces the muscular origin of the LDH in those cases. We have no definite explanation for the poor sensitivity of CK as a screening tool, but low serum CK levels in some rheumatic diseases are a reflection of the inflammatory activity.19,20,21
We did not use clinical manifestations as a starting point in our study as muscle weakness can be difficult to detect clinically. Furthermore, the underlying chronic rheumatism may mask muscle symptoms and can delay the diagnosis. However, we also determined the prevalence of clinical myopathy, investigating how many of the 15 patients with proven antimalarial myopathy had signs or symptoms of muscle weakness. Some degree of weakness was observed in 53% of these patients, which represent a prevalence of clinical myopathy of 6.7% in our study—quite a high figure compared with previous series.5,10,11 These differences can be explained by the high sensitivity of the screening method which we used, which was different from those used in previous studies, which were specifically based on the patient's symptoms. In addition, it is important to emphasise that only two of these patients developed severe clinical disease as a result of muscle weakness. We do not have muscle strength assessments for all 119 patients treated with antimalarial drugs, but in all patients with clinical antimalarial myopathy for whom we had data, muscle strength improved after drug withdrawal (table 2), suggesting that the muscle weakness was caused by the antimalarial agents and not primarily by the underlying rheumatic disease.
It is known that electromyography is useful in the evaluation of any myopathy. However, our study showed that its sensitivity was low for the diagnosis of antimalarial myopathy (53%), and therefore it seems inadvisable to use it as the sole diagnostic screening tool in this context. However, all patients with clinical myopathy also had abnormal electromyography, so the technique may be useful in monitoring patients with antimalarial myopathy to detect the evolution of the disease from a subclinical to a clinical stage.
Confirmation of a suspicious diagnosis of antimalarial myopathy should be made through a histological study of the tissue samples. In our series, a muscle biopsy was carried out in all patients with a persistent muscle enzyme disturbance, regardless of their clinical manifestations or electromyography. In these cases an ultrastructural examination is absolutely mandatory to detect the characteristic tissue deposits that confirm the diagnosis of an antimalarial myopathy, since light microscopy has numerous false negatives (80% in our series). The three patients with vacuolar myopathy in the light microscopy had clinical involvement, with muscle strength impairment and electromyographic changes, which could mean that this technique may only detect the most advanced cases. Cytoplasmic complex lipid bodies (myeloid and curvilinear bodies) constitute the characteristic features of antimalarial myopathy. These findings have not been detected in any other muscle disease except ceroid lipofuccinosis, a rare lipid storage disease.22 Whether these specific findings are seen in patients on antimalarial drugs but with no myopathy is unknown, but all our biopsied patients had muscle impairment as reflected by raised muscle enzymes.
During the follow up, after the discontinuation of the antimalarial treatment in all patients with moderate to severe clinical myopathy, the muscle weakness, muscle enzyme disturbances, and electromyographic changes tended to normalise, as previously reported,9 reinforcing the view that the antimalarial agents caused the myopathy in our patients.
It is thought that hydroxychloroquine has less neuromuscular toxicity than chloroquine. Nevertheless, in the present study, a proven antimalarial myopathy was found in two of the eight patients taking this drug. Although the patient sample is insufficient to draw any firm conclusions about this, a toxic myopathy with hydroxychloroquine may not be as rare as previously thought. More studies are required to establish the prevalence of myopathy during treatment with hydroxychloroquine, using sensitive screening tools.
What clinical importance should be attached to the diagnosing of antimalarial myopathy in asymptomatic patients? Should the antimalarial drug be withdrawn when a subclinical myopathy is detected in well controlled patients? The high prevalence of this adverse effect may, in the future, make it advisable to recommend regular determinations of serum muscle enzymes in patients chronically treated with antimalarials. We opted for discontinuation of antimalarial treatment only in patients with clinical myopathy, while monitoring the remaining patients (with a complete muscle strength test and an electromyographic study). A prospective controlled study to determine the likelihood of progression of a subclinical to a clinical myopathy is needed before definitive recommendations can be made.
Conclusions
Our study suggests that the prevalence of antimalarial myopathy is higher than previously recognised. Regular determination of serum muscle enzymes, mainly LDH, seems to be a good screening tool for myopathy. When a persistent muscle enzyme disturbance is detected, a clinical and electromyographic study should be carried out periodically to establish the development of a clinical myopathy as soon as possible. In cases of clinical myopathy, an anatomical‐pathological tissue study, including an ultrastructural study, is mandatory to confirm the diagnosis, and the withdrawal of antimalarial drugs should be considered.
Acknowledgements
We wish to thank Mrs Elena Fernández, nurse in rheumatology, as without her collaboration this study would not have been possible, and Miss Christine O'Hara and Mr Neil McLeod for helping with the English version of the manuscript.
Abbreviations
CK - creatine kinase
LDH - lactate dehydrogenase
References
- 1.Maksymowych W, Russell A S. Antimalarials in rheumatology: efficacy and safety. Semin Arthritis Rheum 198716206–221. [DOI] [PubMed] [Google Scholar]
- 2.Abraham R, Hendy R. Effects of chronic chloroquine treatment of lysosomes of rat liver cell. Exp Mol Pathol 197012148–159. [DOI] [PubMed] [Google Scholar]
- 3.Fedorko M. Effect of chloroquine on morphology of leukocytes and pancreatic exocrine cells from the rat. Lab Invest 19681827–37. [PubMed] [Google Scholar]
- 4.Kumamoto T, Ikebe N, Araki S, Fukuhara N. Ultrastructural studies on the autophagic vacuoles in the skeletal muscles of rats with chloroquine myopathy and cases with distal myopathy. J Clin Electron Microsc 198417946–947. [Google Scholar]
- 5.Whisnant J P, Espinosa R E, Kierland R R, Lambert E H. Chloroquine neuromyopathy. Mayo Clin Proc 1963381–13. [PubMed] [Google Scholar]
- 6.Chapman R S, Ewen S W. Chloroquine‐induced myopathy. Br J Dermatol 196981217–219. [DOI] [PubMed] [Google Scholar]
- 7.Ebringer A. Chloroquine myopathy. BMJ 197126770–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes J T, Esiri M, Oxbury J M, Whitty C W. Chloroquine myopathy. Q J Med 19714085–93. [PubMed] [Google Scholar]
- 9.Itabashi H H, Kökmen E. Chloroquine neuromyopathy, a reversible granulovacuolar myopathy. Arch Pathol 197293209–218. [PubMed] [Google Scholar]
- 10.Estes M L, Ewing‐Wilson D, Chou S M, Mitsumoto H, Hanson M, Shirey E.et al Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med 198782447–455. [DOI] [PubMed] [Google Scholar]
- 11.Avina‐Zubieta J A, Johnson E S, Suárez‐Almazor M E, Russell A S. Incidence of myopathy in patients treated with antimalarials. A report of three cases and a review of the literature. Br J Rheumatol 199534166–170. [DOI] [PubMed] [Google Scholar]
- 12.Rabunal R, Sánchez‐Andrade A, Navarro C, Brañas F. Myotoxicity by chloroquine: report of a case (letter). Med Clin (Bar) 1997109277–278. [PubMed] [Google Scholar]
- 13.Richards A J. Hydroxychloroquine myopathy. J Rheumatol 1998251642–1643. [PubMed] [Google Scholar]
- 14.Wasay M, Wolfe G I, Herrold J M, Burns D K, Barohn R J. Chloroquine myopathy and neuropathy with elevated CSF protein (letter). Neurology 1998511226–1227. [DOI] [PubMed] [Google Scholar]
- 15.Stein M, Bell M J, Ang L C. Hydroxychloroquine neuromyotoxicity. J Rheumatol 2000272927–2931. [PubMed] [Google Scholar]
- 16.Finsterer J, Jarius C. Increased CSF protein in chloroquine‐induced axonal polyneuropathy and myopathy. Clin Neurol Neurosurg 2003105231–236. [DOI] [PubMed] [Google Scholar]
- 17.Becerra‐Cuñat J L, Coll‐Cantí J, Gelpí‐Mantius E, Ferrer‐Avellí X, Lozano‐Sánchez M, Millán‐Torné M.et al Chloroquine‐induced myopathy and neuropathy: progressive tetraparesis with areflexia that simulates a polyradiculoneuropathy. Two case reports. Rev Neurol 200336523–526. [PubMed] [Google Scholar]
- 18.Kumamoto T, Araki S, Watanabe S, Ikebe N, Fukuhara N. Experimental chloroquine myopathy: morphological and biochemical studies. Eur Neurol 198929202–207. [DOI] [PubMed] [Google Scholar]
- 19.Wei N, Pavlidis N, Tsokos G, Elin R J, Plotz P H. Clinical significance of low creatine phosphokinase values in patients with connective tissue disease. JAMA 19812461921–1923. [PubMed] [Google Scholar]
- 20.Sanmartí R, Collado A, Gratacós J, Herrera B E, Font J, Cañete J D.et al Reduced serum creatine kinase activity in inflammatory rheumatic diseases. J Rheumatol 199623310–312. [PubMed] [Google Scholar]
- 21.Sanmartí R, Collado A, Gratacós J, Bedini J L, Pañella D, Filella X.et al Reduced activity of serum creatine kinase in rheumatoid arthritis: a phenomenon linked to the inflammatory response. Br J Rheumatol 199433231–234. [DOI] [PubMed] [Google Scholar]
- 22.Neville H E, Maunder‐Sewry C A, McDougall J, Sewell J R, Dobowitz V. Chloroquine‐induced cytosomes with curvilinear profiles in muscle. Muscle Nerve 19792376–381. [DOI] [PubMed] [Google Scholar]