Abstract
Objectives
To investigate whether tumour necrosis factor α (TNFα) is expressed in subacute cutaneous lupus erythematosus (SCLE) skin lesions.
Methods
The in situ expression of TNFα in refractory lesional and non‐lesional skin biopsy specimens from patients with SCLE was analysed using an immunohistochemical approach. At the time of biopsy these patients were receiving treatment with systemic medications such as antimalarial agents, immunosuppressive drugs, and thalidomide. Expression of TNFα was also evaluated in cutaneous lesions of patients with other inflammatory and neoplastic skin diseases as controls.
Results
The data showed that refractory lesional skin tissue from patients with SCLE displays a strongly positive distribution of TNFα, particularly within the epidermis. No prominent staining was seen in non‐lesional skin from the same group of patients or in cutaneous lesions from the control group.
Conclusions
These findings suggest that TNFα is localised and produced by epidermal cells within SCLE skin lesions and support its potential role in the pathogenesis of SCLE. The tissue localisation of TNFα may represent a potential therapeutic target providing a new perspective in the treatment of refractory skin lesions in patients with SCLE.
Keywords: tumour necrosis factor, subacute cutaneous lupus erythematosus, refractory skin lesions, immunohistochemistry, epidermis
Many cytokines, including tumour necrosis factor α (TNFα), seem to have a significant role in the inflammatory process, as well as in the regulation of the autoimmune response in systemic lupus erythematosus (SLE).
High serum levels of this cytokine and receptors (sTNFRI and sTNFRII) have been detected in patients with SLE in correlation with disease activity,1,2 even during pregnancy.3
It is worth noting that the increased TNFα found in SLE sera is bioactive,4 supporting the hypothesis that TNFα contributes to the pathogenesis of some lupus manifestations.4 In addition, TNFα has been specifically immunolocalised in kidney sections from patients with SLE and glomerulonephritis5 in correlation with histological activity.
TNFα is produced by a variety of cells, including those of the epidermis; in particular, keratinocytes are a potent source of cytokines.6
Subacute cutaneous lupus erythematosus (SCLE) is one of the LE‐specific skin lesions consisting of non‐scarring papulosquamous or annular skin lesions that occur in characteristic photodistribution.7 About 75% of SCLE can be managed with conventional topical and systemic treatment; in the remaining 25% of cases skin lesions can be particularly severe and refractory to conventional treatment.
Our study aimed at investigating the in situ expression of TNFα in cutaneous lesions in patients with SCLE.
Patients and methods
Patients and skin biopsies
Upon informed consent, excisional biopsy specimens of lesional and non‐lesional sun protected skin were obtained from four patients with SLE (American Rheumatism Association criteria) with specific and diagnostic cutaneous manifestations of SCLE according to Gilliam and Sontheimer's classification.7
Detection of autoantibodies
Antinuclear antibodies and anti‐double stranded DNA antibodies were detected by indirect immunofluorescence technique, on HEp‐2 cells and Crithidia luciliae, respectively.
Antibodies against extractable nuclear antigens were tested by immunoblotting technique on cytoplasmic and nuclear cell extracts from Raji cultured cells.
Antibodies directed against SSA/Ro and SSB/La antigens were also tested by enzyme linked immunosorbent assay (ELISA) (INOVA Diagnostics, Inc), following the manufacturer's instructions.
TNFα serum levels
TNFα was measured in the serum of patients with SCLE by chemiluminescence immunoassay using an automated analyser (Immulite, DPC) and anti‐TNFα monoclonal antibody (DPC, Biermann GmbH).
Direct immunofluorescence
Cryosections of 4 μm thickness from lesional and non‐lesional skin biopsy specimens were mounted on Polysine glass slides and air dried for 30 minutes. The sections were then stained for 30 minutes in a moist chamber at room temperature, using fluorescein isothiocyanate labelled, rabbit antibodies against human IgG, IgA, IgM, C3, C1q, and C4 (Dako, Glostrup, Denmark). Conjugate dilutions were made in 0.01 M phosphate buffered saline (PBS), pH 7.3, supplemented with 1% bovine serum albumin. After washing in PBS for 30 minutes, the sections were coverslipped under fresh PBS/glycerol (50% vol/vol).
Immunohistochemistry
Detection of TNFα was performed on frozen sections from four samples of active lesions of SCLE, four of normal human skin obtained from sun protected areas of the same patients, three samples of mycosis fungoides, three of cutaneous T cell pseudolymphoma, and two of parapsoriasis.
Samples were stained with the following monoclonal antibodies (mAbs): anti‐TNFα (clone 68B6A3L1; BioSource Europe, Nivelles, Belgium),8 anti‐CD3 (clone UCHT1; Dako), anti‐CD1a (clone NA1/34; Dako), and anti‐CD19 (clone HD37; Dako).
Air dried, acetone fixed, frozen sections were incubated overnight with the mAbs and, after washing, processed with a standard alkaline phosphatase anti‐alkaline phosphatase (APAAP) technique. Rabbit antimouse immunoglobulin (Dako) was applied for 30 minutes and, after washing, the sections were incubated with APAAP complex (Dako) for 30 minutes. Naphthol‐AS‐MX phosphate together with fast red TR salt were used for the development of alkaline phosphatase. The endogenous alkaline phosphatase was blocked by adding levamisole to the substrate. Sections were counterstained for 5 minutes with Mayer's haematoxylin. Negative controls were performed by omitting the primary mAb on samples or consisted of replacement of the primary antibody with another irrelevant mAb of identical isotype.
Results
Table 1 summarises the demographic, clinical, and immunological data of the patients at the time of the biopsy.
Table 1 Prominent features at the time of biopsy in our patients affected with SCLE.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Sex/age (years) | F/28 | F/37 | F/33 | F/49 |
| Disease duration (years) | 6 | 14 | 14 | 11 |
| Skin lesion duration (months) | 60 | 170 | 12 | 134 |
| Type of skin lesion | Papulosquamous | Papulosquamous | Annular/polycyclic | Annular/polycyclic |
| Biopsy site: | ||||
| Lesional skin | Back | Shoulder | Back | Chest |
| Non‐lesional skin | Gluteus | Gluteus | Gluteus | Gluteus |
| TNFα staining | Moderate | Moderate | Moderate | Strong |
| TNFα serum levels (ng/l)* | 18.6 | 13.0 | 7.3 | 9.9 |
| ECLAM | 6 | 4 | 1 | 2 |
| Clinical manifestations: | ||||
| Arthritis | + | – | – | – |
| Pericarditis | – | – | – | – |
| Pleuritis | – | – | – | – |
| Renal disease | – | – | – | – |
| Haematological disorders | + | + | – | – |
| Neurological disorders | – | + | – | – |
| Autoantibodies: | ||||
| ANA | + | + | + | + |
| Anti‐dsDNA | – | + | – | – |
| Anti‐La/SSB | – | – | + | – |
| Anti‐Ro/SSA 60 kDa | – | – | + | + |
| Anti‐Ro/SSA 52 kDa | – | – | + | – |
| Anti‐ribosomal P proteins | + | + | – | – |
| Anti‐Sm | – | + | – | – |
| Prednisone (mg/day) | 25 | 10 | 5 | 5 |
| Other treatment | MMF | MMF, HCQ | HCQ, AZA | HCQ |
| Thalidomide (mg/day) | – | – | – | 25 |
*Reference level 8.1 ng/l.
F, female; ECLAM, European Consensus Lupus Activity Measure; ANA, antinuclear antibodies; MMF, mycophenolate mofetil; HCQ, hydroxychloroquine; AZA, azathioprine.
All patients were treated initially with topical corticosteroids. Because topical treatment did not provide adequate improvement of the skin lesions, patients were treated with conventional systemic treatment, and three of the four also with thalidomide. At the time of biopsy only one patient was still receiving treatment with thalidomide, because the other two patients had stoped treatment owing to the occurrence of peripheral neuropathy. Moreover, at the time of biopsy one patient was not taking antimalarial drugs because they had been withdrawn owing to side effects (itch).
Two independent observers, blindly identified moderate to strong staining of TNFα in the lesional epidermis of the four samples of SCLE (table 1, figs 1 and 2). Positive staining was observed on keratinocytes of all layers of the epidermal compartment. In particular, no correlation between TNFα tissue localisation and duration of skin lesions or systemic treatment was seen in any of the patients (table 1). Samples of both the sun protected normal skin from patients with SCLE and the pathological skin conditions investigated were negative for TNFα immunostaining.
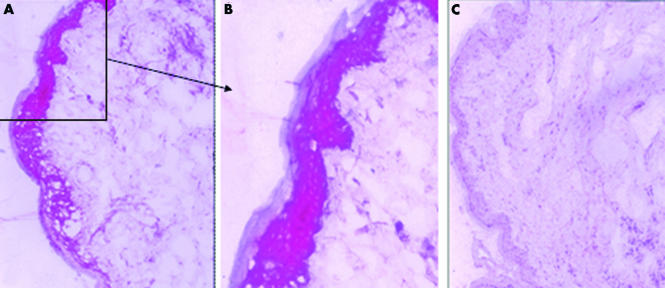
Figure 1 Representative image of strong immunohistochemical staining of TNFα in a case of SCLE (patient No 4). TNFα is clearly expressed in the epidermal compartment of lesional skin (A and B), whereas it is absent in the epidermis of non‐sun‐exposed skin from the same patient (C). A high power magnification of the positive epidermal areas is shown (B).
Figure 2 Representative image of moderate staining of TNFα in a case of SCLE (patient No 1). In the lesional skin, TNFα is expressed within the epidermis (A) and positive dermal lymphocyte infiltrate can be identified as well (A). The sun protected normal skin from the same patient is negative (B). Representative image of negative TNFα staining in a control case of parapsoriasis (C).
TNFα serum levels were higher than the reference level in patients Nos 1 and 2, and borderline in patient No 4 (table 1) and the TNFα level seemed to parallel the ECLAM score. No relationship between the TNFα serum levels and their expression in lesional skin tissue was found.
A mild (cases 1 and 2) to moderate (cases 3 and 4) dermal lymphocyte infiltrate was seen in lesional skin of all cases of SCLE. The predominant cells at the dermal‐epidermal interface were identified as CD3+ T lymphocytes, whereas CD19+ B cells were generally absent. CD1a expression was generally reduced in the epidermal compartment of affected skin of SCLE, in comparison with sun protected normal skin from the same patient.
Direct immunofluorescence of lesional skin showed deposition along the dermal‐epidermal junction of IgG, IgM, C3, C1q in cases 1 and 2, IgM in case 3, IgG, IgM, and C3 in case 4.
Discussion
It has been demonstrated that there is a strong association of the polymorphism of –308A promoter of TNFα linked to HLA‐DR3 with SCLE.9 Recently, similar findings have been reported also in children affected with cutaneous neonatal lupus, a type of skin lesion which resembles SCLE.10 In addition, in the same study it was shown that TNFα is expressed within the epidermis of lesional skin biopsy specimens from affected children.10 These findings support the hypothesis of a pathogenetic role of this proinflammatory cytokine in the pathogenesis of cutaneous neonatal lupus.
In our study, we observed that lesional skin of patients with SCLE was characterised by an increased expression of TNFα in comparison with normal skin obtained from the same patient and with a small group of inflammatory and neoplastic skin conditions. TNFα immunostaining was mainly localised in the epidermal compartment, suggesting that keratinocytes are the major source of this cytokine during the cutaneous inflammatory process.
A moderate dermal lymphocyte infiltrate was generally detected in lesional skin biopsies investigated, and the characterisation of cell infiltrates showed the presence of CD3+ cells, whereas CD19+ B cells were almost absent. No correlation was found between TNFα immunostaining or CD3+ dermal infiltrates and skin lesions or the duration of systemic treatment.
In addition, CD1a+ cells were reduced in lesional skin biopsy specimens compared with non‐lesional skin from the same patient. CD1a is expressed in differentiated antigen presenting cells and monocytes and is used as a marker for the presence of Langerhans' cells in the skin.11 Skin antigen presenting cells (Langerhans' cells) constitute a subset of dendritic cells which appear to have an important role in the initiating events that lead to the T cell mediated immune responses in skin. Our findings indicate that these cells may not have a pivotal role in the immunopathological mechanism involved in the development of cutaneous lesions in SCLE.
We found that TNFα serum levels were associated with disease activity, but we did not find a relation between the level of TNFα in the sera and the intensity of the immunostaining on skin tissue. These findings support the hypothesis that TNFα is locally produced by keratinocytes and could have not leaked from the patient's circulation into inflammatory lesions, binding to TNF receptors locally.
There is strong evidence that thalidomide, a TNFα blocking agent, can be very effective in the treatment of some refractory SCLE. However, the relevant side effects of this drug have to be carefully considered. In fact, thalidomide is highly teratogenic and can induce irreversible peripheral neuropathy.12
Recently, an open label study on patients treated with a chimeric anti‐TNFα antibody in addition to conventional treatment, showed a clinical improvement in the inflammatory manifestations of the disease, although the development of autoantibodies in those patients has been reported.13 The possibility of inducing cutaneous LE during anti‐TNFα treatment has to be considered as a possible side effect.14
The results of our study indicating the presence of TNFα in the epidermal compartment of active lesions of SCLE are consistent with the hypothesis of localised and site‐specific contribution of TNFα to the pathogenesis of the skin disease.
Successful treatment with TNFα blockers in a patient with SCLE15 and our findings suggest that anti‐TNFα might be useful in the treatment of selected cases of refractory SCLE.
Acknowledgements
This work was supported by “Ministero dell'Istruzione, dell'Università e della Ricerca”, project 2004062238‐004.
Abbreviations
APAAP - alkaline phosphatase anti‐alkaline phosphatase
mAbs - monoclonal antibodies
PBS - phosphate buffered saline
SCLE - subacute cutaneous lupus erythematosus
SLE - systemic lupus erythematosus
TNFα - tumour necrosis factor α
Footnotes
Competing interest: None.
Ethics approval: This study was approved by the local ethics committee.
References
- 1.Gabay C, Cakir N, Moral F, Roux‐Lombard P, Meyer O, Dayer J M.et al Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol 199724303–308. [PubMed] [Google Scholar]
- 2.Aderka D, Wysenbeek A, Engelmann H, Cope A P, Brennan F, Molad Y.et al Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum 1993361111–1120. [DOI] [PubMed] [Google Scholar]
- 3.Doria A, Ghirardello A, Iaccarino L, Zampieri S, Punzi L, Tarricone E.et al Pregnancy, cytokines, and disease activity in systemic lupus erythematosus. Arthritis Rheum 200451989–995. [DOI] [PubMed] [Google Scholar]
- 4.Aringer M, Feierl E, Steiner G, Stummvoll G H, Hofler E, Steiner C W.et al Increased bioactive TNF in human systemic lupus erythematosus: associations with cell death. Lupus 200211102–108. [DOI] [PubMed] [Google Scholar]
- 5.Malide D, Russo P, Bendayan M. Presence of tumor necrosis factor alpha and interleukin‐6 in renal mesangial cells of lupus nephritis patients. Hum Pathol 199526558–564. [DOI] [PubMed] [Google Scholar]
- 6.Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel J C.et al Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 19901721609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sontheimer R D, Thomas J R, Gilliam J N. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol 19791151409–1415. [PubMed] [Google Scholar]
- 8.Shields D C, Avgeropoulos N G, Banik N L, Tyor W R. Acute multiple sclerosis characterized by extensive mononuclear phagocyte infiltration. Neurochem Res 2000251517–1520. [DOI] [PubMed] [Google Scholar]
- 9.Werth V P, Zhang W, Dortzbach K, Sullivan K. Association of a promoter polymorphism of tumor necrosis factor‐alpha with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol 2000115726–730. [DOI] [PubMed] [Google Scholar]
- 10.Clancy R M, Baccker C B, Yin X, Chang M W, Cohen S R, Lee L A.et al Genetic association of cutaneous neonatal lupus with HLA Class II and tumor necrosis factor α. Implication for pathogenesis. Arthitis Rheum 2004502598–2603. [DOI] [PubMed] [Google Scholar]
- 11.Romani N, Holzmann S, Tripp C H, Koch F, Stoitzner P. Langerhans cells‐dendritic cells of the epidermis. APMIS 2003111725–740. [DOI] [PubMed] [Google Scholar]
- 12.Briani C, Zara G, Rondinone R, Della Libera S, Ermani M, Ruggero S.et al Thalidomide neurotoxicity. Prospective study in patients with lupus erythematosus. Neurology 2004622288–2290. [DOI] [PubMed] [Google Scholar]
- 13.Aringer M, Graninger W B, Steiner G, Smolen J S. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open‐label study. Arthritis Rheum 2004503161–3169. [DOI] [PubMed] [Google Scholar]
- 14.Bleumink G S, Ter Borg E J, Ramselaar C G, Ch Stricker B H. Etanercept‐induced subacute cutaneous lupus erythematosus. Rheumatology (Oxford) 2001401317–1319. [DOI] [PubMed] [Google Scholar]
- 15.Hiepe F, Bruns A, Feist E, Burmester G‐R. Successful treatment of a patient suffering from a refractory subacute cutaneous lupus erythematosus (SCLE) with blockers of tumor necrosis factor A [abstract]. Arthritis Rheum 200450(suppl 9)S413 [Google Scholar]