Abstract
Objective
To develop evidence based recommendations for the management of ankylosing spondylitis (AS) as a combined effort of the ‘ASsessment in AS' international working group and the European League Against Rheumatism.
Methods
Each of the 22 participants was asked to contribute up to 15 propositions describing key clinical aspects of AS management. A Delphi process was used to select 10 final propositions. A systematic literature search was then performed to obtain scientific evidence for each proposition. Outcome data for efficacy, adverse effects, and cost effectiveness were abstracted. The effect size, relative risk, number needed to treat, and incremental cost effectiveness ratio were calculated. On the basis of the search results, 10 major recommendations for the management of AS were constructed. The strength of recommendation was assessed based on the strength of the literature evidence, risk‐benefit trade‐off, and clinical expertise.
Results
The final recommendations considered the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) (conventional NSAIDs, coxibs, and co‐prescription of gastroprotective agents), disease modifying antirheumatic drugs, treatments with biological agents, simple analgesics, local and systemic steroids, non‐pharmacological treatment (including education, exercise, and physiotherapy), and surgical interventions. Three general recommendations were also included. Research evidence (categories I–IV) supported 11 interventions in the treatment of AS. Strength of recommendation varied, depending on the category of evidence and expert opinion.
Conclusion
Ten key recommendations for the treatment of AS were developed and assessed using a combination of research based evidence and expert consensus. Regular updating will be carried out to keep abreast of new developments in the management of AS.
Keywords: ankylosing spondylitis, management, recommendations, evidence based medicine, spondyloarthropathies, ASAS, EULAR
Ankylosing spondylitis (AS) is a chronic, inflammatory rheumatic disease characterised by inflammatory back pain due to sacroiliitis and spondylitis, the formation of syndesmophytes leading to ankylosis, and frequently associated with peripheral arthritis, enthesitis, and acute anterior uveitis. Symptoms commonly begin in late adolescence and early adulthood.1,2 With an estimated prevalence of 0.9% in northern European white populations3 AS is a significant health burden to the community.
AS has long been a therapeutic challenge for the clinician. Exercise and non‐steroidal anti‐inflammatory drugs (NSAIDs) have been the mainstay of symptom control for decades, but there has until recently been a dearth of disease modifying treatments. The advent of biological treatment is currently revolutionising the management of AS, but too little is known yet about the long term benefits and risks of such treatment. Clearly, these treatments necessitate socioeconomic cost calculations. Moreover, there is a need for rational, evidence based recommendations to guide the physician in the management of AS. The ASsessment in AS (ASAS) international working group has made important contributions to the evaluation and standardisation of assessments in AS in the past decade.4,5
This project is a collaborative effort of ASAS and EULAR with the ultimate objective to contribute to the improvement of outcomes in patients with AS by constructing evidence based management recommendations. To obtain and maintain a high level of intrinsic quality and comparability of this approach, the EULAR standard operating procedures6 were followed.
Methods
Participants
A multidisciplinary guideline development committee was formed from within the ASAS working group, with participants selected on the basis of publication history in AS, personal knowledge, and approval by EULAR. Twenty two experts in the field of AS (20 rheumatologists, one also a patient with AS, and two orthopaedic surgeons) representing 14 countries took part in the study. Each participant was asked to contribute independently up to 15 key propositions relevant to the management of AS, to create a comprehensive list of potential topics of interest. A Delphi technique was then used to reduce these to a predefined final 10 propositions over three rounds. Questions were accepted automatically if selected by 80% or more of the participants in any round, whereas questions receiving less than 20% of the votes were removed.
Systematic literature search
A general search of Medline, Embase, CINHAL, PEDro, and the Cochrane Library was conducted summarising the current available AS treatments from the literature, and the results reported to the committee before the Delphi exercise. After the 10 propositions had been generated, an intervention‐specific literature search was undertaken to identify evidence for each specified intervention.7 The “online first” sections of rheumatology journals as well as the abstracts of rheumatology scientific meetings from the years 2003 and 2004 were hand searched for additional relevant studies. Only studies with clinical outcomes for AS were included. Animal studies, narrative review articles, commentaries, and guidelines were excluded.
Categorising evidence
Evidence was categorised according to study design using a traditional hierarchy8 (table 1). Questions on efficacy were answered using the “best available” evidence. The highest available category of evidence for each intervention was reviewed in depth, and the next highest category considered when few high level studies were retrieved. Whereas efficacy was assessed specifically for AS, side effects were evaluated specifically for the intervention, and so studies in other musculoskeletal diseases were also reviewed.
Table 1 Evidence hierarchy and traditional strength of recommendation.
| Category of evidence | Strength of recommendation |
|---|---|
| Ia Meta‐analysis of randomised controlled trials | A Category I evidence |
| Ib Randomised controlled trial | |
| IIa Controlled study without randomisation | B Category II evidence or extrapolated from category I evidence |
| IIb Quasi‐experimental study | |
| III Non‐experimental descriptive studies, such as comparative, correlation, and case‐control studies | C Category III evidence or extrapolated from category I or II evidence |
| IV Expert committee reports or opinion or clinical experience of respected authorities, or both | D Category IV evidence or extrapolated from category II or III evidence |
Estimation of effectiveness and cost effectiveness
Effect size (ES) and 95% confidence interval (95% CI) for each intervention were calculated for two predefined continuous outcomes: pain relief and improvement in function.9 ES is the standardised mean difference (mean change divided by standard deviation of the change), and therefore has no units and is comparable across interventions in similar populations. Clinically, an ES of 0.2 is considered small, 0.5 is moderate, and >0.8 is large.10 Statistical pooling was undertaken as appropriate.11 The percentage of patients responding to treatment (an ASAS20 response, pain relief of more than 50%, or functional improvement of more than 20%) was calculated where possible, and the number needed to treat (the number of patients who need to be treated to prevent one additional poor outcome) was estimated.12 Relative risk was calculated for adverse effects.
For economic evaluations, the incremental cost effectiveness ratio was calculated. Data were extracted by one investigator (JZ). A customised form was used for the data extraction.
Strength of recommendation
The strength of each recommendation was graded A–D based on the category of efficacy evidence (table 1)8 by two members of the committee (JZ, JB). A numerical rating scale (NRS) was used to quantify “expert opinion” for each intervention identified. Each member of the committee was asked to rate their strength of recommendation for each intervention on a 0–10 NRS, according to both the research evidence presented (efficacy, safety, and cost effectiveness) and their own clinical expertise (logistics, patient perceived acceptance, and tolerability). The mean (SEM) for the strength of the recommendation was calculated for each intervention.
When the final 10 recommendations had been agreed, a flow chart was constructed by the expert group to summarise the most important aspects of the management of AS, based on clinical expertise and research evidence.
Results
Details of the literature search results and the specific studies discussed during the elucidation of each recommendation have been published elsewhere.7 Table 2 gives the final 10 recommendations.
Table 2 Experts' propositions developed through three Delphi rounds—order according to topic (general, non‐pharmacological, pharmacological, invasive, and surgical).
| No | Proposition |
|---|---|
| 1 | Treatment of AS should be tailored according to: |
| • Current manifestations of the disease (axial, peripheral, entheseal, extra‐articular symptoms and signs) | |
| • Level of current symptoms, clinical findings, and prognostic indicators | |
| ‐ Disease activity/inflammation | |
| ‐ Pain | |
| ‐ Function, disability, handicap | |
| ‐ Structural damage, hip involvement, spinal deformities | |
| • General clinical status (age, sex, comorbidity, concomitant drugs) | |
| • Wishes and expectations of the patient | |
| 2 | Disease monitoring of patients with AS should include: patient history (for example, questionnaires), clinical parameters, laboratory tests, and imaging, all according to the clinical presentation, as well as the ASAS core set. The frequency of monitoring should be decided on an individual basis depending on symptoms, severity, and drug treatment |
| 3 | Optimal management of AS requires a combination of non‐pharmacological and pharmacological treatments |
| 4 | Non‐pharmacological treatment of AS should include patient education and regular exercise. Individual and group physical therapy should be considered. Patient associations and self help groups may be useful |
| 5 | NSAIDs are recommended as first line drug treatment for patients with AS with pain and stiffness. In those with increased GI risk, non‐selective NSAIDs plus a gastroprotective agent, or a selective COX‐2 inhibitor could be used |
| 6 | Analgesics, such as paracetamol and opioids, might be considered for pain control in patients in whom NSAIDs are insufficient, contraindicated, and/or poorly tolerated |
| 7 | Corticosteroid injections directed to the local site of musculoskeletal inflammation may be considered. The use of systemic corticosteroids for axial disease is not supported by evidence |
| 8 | There is no evidence for the efficacy of DMARDs, including sulfasalazine and methotrexate, for the treatment of axial disease. Sulfasalazine may be considered in patients with peripheral arthritis |
| 9 | Anti‐TNF treatment should be given to patients with persistently high disease activity despite conventional treatments according to the ASAS recommendations. There is no evidence to support the obligatory use of DMARDs before, or concomitant with, anti‐TNF treatment in patients with axial disease |
| 10 | Total hip arthroplasty should be considered in patients with refractory pain or disability and radiographic evidence of structural damage, independent of age. Spinal surgery—for example, corrective osteotomy and stabilisation procedures, may be of value in selected patients |
AS, ankylosing spondylitis; GI, gastrointestinal; NSAIDs, non‐steroidal anti‐inflammatory drugs; DMARDs, disease modifying antirheumatic drugs; TNF, tumour necrosis factor.
The first three recommendations deal with general concepts in the management of AS, and the remaining seven describe specific treatments in use for AS. Tables 3‐5 summarise the evidence for efficacy, toxicity, and cost effectiveness for each intervention. Table 6 gives the strength of each recommendation as assigned by the expert group.
Table 3 Evidence of efficacy—pooled effect size (ES) and number needed to treat (NNT).
| Intervention | Studies | ESpain, spinal (95% CI) | ESpain, peripheral (95% CI) | ESfunction (95% CI) | NNT (95% CI) (ASAS 20) | ||
|---|---|---|---|---|---|---|---|
| Category* | No | Duration (weeks) | |||||
| Physiotherapy | Ib | 1 | 16 | NS | – | 1.14 (0.55 to 1.73) | – |
| Home exercise | IIa | 1 | 8 | 1.99 (1.30 to 2.67) | – | 0.80 (0.23 to 1.38) | – |
| NSAIDs | Ib | 4 | 6 | 1.11 (0.96 to 1.26) | 0.62 (0.26 to 0.97) | 0.62 (0.47 to 0.76) | – |
| Coxibs | Ib | 3 | 6 | 1.05 (0.88 to 1.22) | – | 0.63 (0.47 to 0.80) | – |
| Sulfasalazine | Ia | 6 | 26–52 | NS | NS | NS | – |
| Methotrexate | Ib | 2 | 26–52 | NS | – | NS | – |
| Leflunomide | Ib | 1 | 24 | NS | – | NS | NS |
| Etanercept | Ib | 4 | 6–24 | 2.25 (1.92 to 2.59) | 0.56 (0.07 to 1.04)† | 2.11 (1.81 to 2.41) | 2.7 (2.2 to 3.4) |
| Infliximab | Ib | 2 | 12–24 | 0.90 (0.66 to 1.14) | 0.66 (0.17 to 1.14)† | 0.93 (0.69 to 1.17) | 2.3 (1.8 to 2.4) |
| TNFα inhibitors | Ib | 6 | 6–24 | 1.36 (1.16 to 1.55) | 0.61 (0.27 to 0.95)† | 1.39 (1.20 to 1.57) | 2.6 (2.2 to 3.0) |
*See table 1 for definitions; †peripheral joint pain was reported in two etanercept studies and one infliximab study, n (total) = 3
No, number of studies included in pooling data; ES, effect size of treatment compared with placebo unless otherwise stated; NNT, number needed to treat to obtain ASAS20 response; –, not available; NS, not significant; ASAS20, ankylosing spondylitis assessment definition of clinical response to treatment.
Table 4 Evidence of side effects—pooled relative risk (RR) and 95% confidence interval (CI) from randomised controlled trials (RCTs).
| Intervention* | RR (95% CI) |
|---|---|
| NSAIDs | 5.36 (1.79 to 16.10) GI (serious) |
| 0.86 (0.75 to 0.99) CV | |
| Coxibs v NSAIDs | 0.18 (0.14 to 0.23) GI (serious) |
| 0.79 (0.40 to 1.55) CV | |
| Misoprostol | 0.26 (0.17 to 0.39) GI (serious) |
| 1.81 (1.52 to 2.61) diarrhoea | |
| H2 blockers (double doses) | 0.44 (0.03 to 0.74) GI (serious) |
| PPIs | 0.40 (0.32 to 0.51) GI (serious) |
| Sulfasalazine | 2.37 (1.58 to 3.55) any |
| 1.79 (1.36 to 2.34) GI (any) | |
| 1.82 (1.13 to 2.93) mucocutaneous | |
| 4.01 (2.12 to 7.59) haematological | |
| 1.90 (0.75 to 4.82) hepatic | |
| Methotrexate | 2.12 (1.50 to 2.98) nausea |
| 4.12 (2.22 to 7.63) hepatic | |
| 2.62 (0.71 to 9.68) haematological | |
| 1.31 (0.57 to 3.01) mucocutaneous | |
| 1.54 (0.64 to 3.70) alopecia | |
| 2.03 (0.55 to 7.50) rash | |
| Folate+MTX v MTX | 0.56 (0.38 to 0.80) |
| Paracetamol | 0.80 (0.27 to 2.37) GI |
| TNF inhibitors | 1.07 (0.92 to 1.24) any infection |
| 3.12 (2.50 to 3.90) injection site | |
| 2.38 (1.61 to 3.53) ANA | |
| 0.81 (0.62 to 1.06) serious AEs |
*:Compared with placebo/non‐exposure unless otherwise stated. Results were pooled from RCTs, or obtained from the latest systematic review which contains the most RCTs.
H2 blockers, histamine type 2 receptor antagonists; PPIs, proton pump inhibitors; MTX, methotrexate; TNF, tumour necrosis factor; GI, gastrointestinal; CV, cardiovascular; ANA, antinuclear antibodies; AEs, adverse effects.
Table 5 Evidence of cost effectiveness for the proposed interventions.
| Intervention | Comparator | Perspective | Duration | Discounting | Effectiveness | C1–C2 | E1–E2 | ICER |
|---|---|---|---|---|---|---|---|---|
| Group physiotherapy | Home exercise | Institutional/payer | 1 year | No | Patients global health (VAS) cm | $409–0 | 1.7–0.3 | 292 |
| Spa exercise therapy | Conventional treatment | Institutional/payer | 40 weeks | No | QALYs | €3129–1754 | – | 12869 |
| Education programme | Conventional treatment | Societal | 1 year | No | Work days lost | Saving | ||
| Coxib (patients with OA/RA) | Naproxen | 3rd party payer | Life | 3% | QALYs | $16620–5037 | – | 395324 |
| Coxib (patients with OA/RA) (previous GI haemorrhage) | Naproxen | 3rd party payer | Life | 5% | QALYs | $19015–14294 | – | 55803 |
| NSAIDs+misoprostol (all patients with OA) | NSAIDs | Canadian Health services | 3 months | No | GI events averted | $32396–25622 | 96–86 | 684 |
| NSAIDs+misoprostol (patients with OA aged ⩾65 years) | NSAIDs | Canadian Health services | 3 months | No | GI events averted | $28971–25622 | 91–86 | 644 |
| Infliximab | Conventional treatment | Societal | 2 years | 3% | QALYs | £25126–17240 | 0.17–0 | 6624 |
C1, total costs with intervention; C2, total costs with comparator; E1, effects with intervention; E2, effects with comparator; ICER, incremental cost effectiveness ratio, base case scenario; QALYs, quality adjusted life years.
Table 6 Strength of recommendations.
| Intervention | Research evidence* | SOR based on efficacy (A–D) | SOR based on all evidence and clinical expertise NRS, mean (SEM) | ||
|---|---|---|---|---|---|
| Efficacy | Side effects | Cost effectiveness | |||
| Physiotherapy | Ib + | – | – | A | 7.95 (0.37) |
| Exercise | IIa + | – | – | B | 8.86 (0.27) |
| Education | – | – | – | N/A | 8.18 (0.38) |
| Cognitive therapy | – | – | – | N/A | 4.77 (0.58) |
| NSAIDs | Ib + | Ia + (GI) | N/A | A | 9.14 (0.24) |
| Coxibs | Ib + | Ia − (GI) | Higher GI risk population | A | 7.82 (0.38) |
| Misoprostol | Ia + (GI protection) | Ia + (diarrhoea) | Higher GI risk population | A | 5.55 (0.55) |
| H2 Blockers (double dose) | Ia + (GI protection) | – | High GI risk population | A | 5.73 (0.46) |
| Proton pump inhibitors | Ia + (GI protection) | – | – | A | 7.14 (0.39) |
| Sulfasalazine | Ia ± | Ib + (haematological, mucocutaneous, GI) | – | A | 6.11 (0.72) |
| Methotrexate | Ib − | Ib + (nausea, hepatic) | – | A | 3.14 (0.46) |
| Ciclosporin | IV + | – | – | D | 1.48 (0.38) |
| Azathioprine | IV + | – | – | D | 1.33 (0.33) |
| Hydroxychloroquine | – | – | – | N/A | 0.86 (0.30) |
| Auranofin | III − | – | – | C | 0.57 (0.24) |
| Cyclophosphamide | IV + | – | – | D | 0.86 (0.26) |
| Leflunomide | Ib − | – | – | A | 2.19 (0.39) |
| d‐Penicillamine | Ib − | – | – | A | 0.90 (0.33) |
| Pamidronate | III + | III + (acute phase reaction) | – | C | 4.29 (0.38) |
| Thalidomide | III + | III + (neurological) | – | C | 3.48 (0.39) |
| Methylprednisolone (IV) | IV + | – | – | D | 3.90 (0.45) |
| Infliximab | Ib + | Ib + (ANA formation) | Cost effective | A | 9.48 (0.20) |
| Etanercept | Ib + | Ib + (injection site reactions) | Cost effective | A | 9.48 (0.20) |
| Adalimumab | III + | – | – | C | 7.24 (0.56) |
| Anakinra | III ± | – | – | C | 3.14 (0.53) |
| Total hip replacement | IV + | – | – | D | 9.05 (0.28) |
| Spinal surgery | IV + | – | – | D | 7.23 (0.35) |
*Evidence was categorised according to the hierarchy in table 1. –, not supportive; +, supportive. ±, uncertain. Example, Ia + (GI) means there is category Ia evidence to support the statement that the treatment causes GI side effects.
SOR, strength of recommendation; NRS, numeric rating scale (0–10, 0 = not recommended at all, 10 = fully recommended); GI, gastrointestinal; ANA, antinuclear antibodies; –, not available; N/A, not applicable.
Propositions
1. Treatment of AS should be tailored according to:
Current manifestations of the disease (axial, peripheral, entheseal, extra‐articular symptoms and signs)
-
Level of current symptoms, clinical findings, and prognostic indicators
-
-
Disease activity/inflammation
-
-
Pain
-
-
Function, disability, handicap
-
-
Structural damage, hip involvement, spinal deformities
-
-
General clinical status (age, sex, comorbidity, concomitant drugs)
Wishes and expectations of the patient.
This statement represents ideal practice and includes clinical markers that are often used to guide clinical decisions. However, although it has considerable face validity, there is little experimental evidence to support it. Trials to evaluate treatment efficacy frequently include a highly selected population. Few studies are therefore designed to differentiate therapeutic effects according to patient characteristics. General information on these factors can better be obtained from observational studies. For example, in observational cohorts, hip involvement is the most important and consistent factor predisposing to severe disease.13 However, there is no international consensus on disease severity of patients with AS. Other clinical prognostic indicators include age, sex, number of peripheral joints affected, smoking, raised erythrocyte sedimentation rate, poor response to NSAIDs,13,14,15,16 and radiological changes at baseline.17,18
In a recent review, in which the results of two randomised trials of biological treatment in AS were analysed according to patient factors,19 the response to anti‐tumour necrosis factor (TNF) treatment was better in younger patients with shorter disease duration and less functional disability. However, because both studies were carried out in patients with high disease activity at baseline, it is not possible to generalise these conclusions to a wider population of patients with AS.
2. Disease monitoring of patients with AS should include: patient history (for example, questionnaires), clinical parameters, laboratory tests, and imaging, all according to the clinical presentation as well as the ASAS core set. The frequency of monitoring should be decided on an individual basis depending on symptoms, severity, and drug treatment
Clinical presentation includes all aspects of disease expression in AS, including axial disease, peripheral disease, enthesitis, and extra‐articular manifestations (such as acute uveitis, conjunctivitis, carditis).
The ASAS working group has developed a core set for use in clinical record keeping.20,21 This core set includes domains on axial, peripheral, and enthesopathological manifestations, and for each domain, one or more specific instruments are recommended (table 7).
Table 7 ASAS core sets.
| Domain | Core set | Instruments | ||
|---|---|---|---|---|
| CR | SMARD/PT | DC‐ART | ||
| Physical function | x | x | x | Bath Ankylosing Spondylitis Functional Index (BASFI) or Dougados Functional Index |
| Pain | x | x | x | VAS in the past week, spine at night, due to AS and VAS in the past week, spine due to AS |
| Spinal mobility | x | x | x | Chest expansion and modified Schober and occiput to wall distance and (BASMI or lateral side flexion) |
| Patient's global assessment | x | x | x | VAS in the past week |
| Stiffness | x | x | x | Morning stiffness |
| Peripheral joints and entheses | x | x | Number of swollen joints and assessment of painful entheses | |
| Acute phase reactants | x | x | ESR | |
| Fatigue | x | VAS question on fatigue from BASDAI | ||
| Imaging | x | AP and lateral x ray examination of the lumbar spine, lateral cervical spine, AP pelvis (SI and hip joints) | ||
CR, clinical record keeping; SMARD, symptom modifying antirheumatic drug; PT, physical therapy; DC‐ART, disease controlling antirheumatic treatment; VAS, visual analogue scale; BASMI, Bath Ankylosing Spondylitis metrology Index; ESR, erythrocyte sedimentation rate; AP, anteroposterior; SI, sacroiliac.
Imaging is an evolving science in the evaluation of AS. The expert group discussed at length what might be the optimal frequency for radiological evaluation of AS, and it was concluded that based on the recent modified Stokes AS Spinal Score data,22 and clinical experience, radiographic monitoring may not be needed more often than once every 2 years. However, exceptions are possible because syndesmophytes may have developed already within 6 months in some patients—this is considered to be the smallest interval between two x ray examinations. Films of the lateral cervical and lumbar spine are recommended for assessing change over time. Assessments of the thoracic spine may also be useful in individual patients, especially where fractures are suspected. Similarly, an additional anteroposterior (AP) lumbar spine film may give further information for assessing disease status in some patients. Once diagnostic changes have been detected in the sacroiliac joints, sacroiliac x ray examinations add little information, but periodic radiographic assessment of the hips may be of value. Recommendations on this item have not been finalised as yet.
Magnetic resonance imaging (MRI) of the sacroiliac joints and the spine is increasingly used to assess disease activity in AS. Although it has not been incorporated in the ASAS core set to date, it seems likely on the basis of recent data that MRI will have a role both in clinical trials and in daily care of the patients, because it is advantageous to have some objective evidence of spinal inflammation.23,24,25
3. Optimal management of AS requires a combination of non‐pharmacological and pharmacological treatments
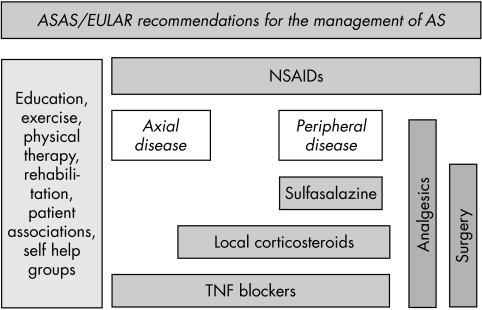
Over 15% of the studies retrieved from the broad literature search reported the effects of non‐pharmacological treatments in AS. Again, no specific head to head studies have been performed to compare the effect of pharmacological and non‐pharmacological treatments. The group consensus was that non‐pharmacological and pharmacological treatments are complementary and both are of value in the initial and continuing treatment of patients with AS. Whether this combined approach applies equally to early and advanced disease or to very active and inactive disease states has not yet been resolved. Figure 1 shows the recommended management strategies for AS based on clinical expertise and research evidence. The disease progression with time moves vertically from top to bottom. This figure emphasises the importance of non‐pharmacological treatments throughout the course of the disease, early introduction of NSAID treatment and options for refractory disease, and alternatives for concomitant peripheral disease.
Figure 1 Flow chart summary of the recommended management of AS, based on clinical expertise and research evidence. The disease progression with time moves vertically from top to bottom.
4. Non‐pharmacological treatment of AS should include patient education and regular exercise. Individual and group physical therapy should be considered. Patient associations and self help groups may be useful
The most recent systematic review of physiotherapy for AS reviewed six randomised controlled trials (RCTs),26 showing that home exercise improved function in the short term compared with no intervention. Supervised exercise programmes failed to show improvements in pain or function compared with home exercise, but patient global assessment was significantly better in patients who underwent group therapy. Specific physical modalities have not been well studied. Level Ib evidence supports spa therapy for physical functioning in patients with AS over 3 months but not longer, and was shown to be cost effective.27,28
Patient education has been shown to have short term benefit for function in AS29 in one controlled trial. There are no studies examining the effect of education on pain. Education and behavioural therapy have, however, been shown to be beneficial for other outcomes such as motivation and anxiety,30 and are cost effective over 12 months.31 Patient associations and self help groups have not been studied for their effect on pain or functional outcomes.
5. NSAIDs are recommended as first line drug treatment for patients with AS with pain and stiffness. In those with increased gastrointestinal (GI) risk, non‐selective NSAIDs plus a gastroprotective agent, or a selective COX‐2 inhibitor could be used
There is convincing evidence (level Ib) that NSAIDs improve spinal pain, peripheral joint pain, and function over a short time period (6 weeks). Coxibs are equally effective, showing large improvements in spinal pain and function in patients with AS, but peripheral joint pain has not been specifically examined. Comparative studies of different NSAIDs have not shown one preparation to be clearly better than the others.
A recent RCT comparing the efficacy of continuous celecoxib treatment for AS with intermittent “on demand” use suggests that continuous treatment retards radiographic disease progression at 2 years.32 This is the first study to show a possible disease modifying effect of continuous treatment, and warrants further investigation.
The GI toxicity of NSAIDs and coxibs has been elegantly presented in the recent EULAR recommendations for the management of hip osteoarthritis.33 In summary, NSAIDs cause an increased risk of GI bleeding, which is dose dependent, and can be reduced with the use of gastroprotective agents. Table 4 shows the relative risks. Coxibs have a lower risk of serious GI events34 than NSAIDs. The GI toxicity of NSAIDs in AS may be accounted for by the recognised risks for NSAID gastropathy (age, concomitant corticosteroids, etc), suggesting that there is no specific impact of the disease on GI toxicity from NSAIDs.
The evidence for cardiovascular toxicity related to anti‐inflammatory drugs is rapidly evolving. What was initially seen as a cardiovascular toxicity signal with rofecoxib35 has now also been described in large trials of other coxib preparations in various settings.36,37,38 Emerging evidence suggests that non‐coxib NSAIDs may possibly share some of this effect (unpublished data). In general, the choice of NSAID or coxib should be based on the GI risk profile of the patient, and should take into account concomitant risk factors for cardiovascular disease.
6. Analgesics, such as paracetamol and opioids, might be considered for pain control in patients in whom NSAIDs are insufficient, contraindicated, and/or poorly tolerated
Paracetamol and other simple analgesics have not been prospectively studied in AS. GI toxicity with paracetamol has been shown to be not significantly higher than placebo in level 1a studies in other musculoskeletal diseases.39,40
7. Corticosteroid injections directed to the local site of musculoskeletal inflammation may be considered. The use of systemic corticosteroids for axial disease is not supported by evidence
Local inflammation is a key feature of AS, and can occur at many different musculoskeletal sites, including the axial joints (most commonly the sacroiliac joints, but can also occur at the costovertebral and manubriosternal joints), peripheral joints (usually an asymmetric oligoarthritis, with predominance of the lower limbs), and enthesitis (plantar fasciitis, Achilles enthesitis, patellar tendon insertional enthesitis, involvement near the tibial tuberosity). Intra‐ or periarticular corticosteroid injections have been shown to be effective for the pain of sacroiliitis in small RCTs (level Ib evidence).41,42 There are no clinical studies evaluating the efficacy of intra‐articular corticosteroid on peripheral arthritis in AS, or on the use of local corticosteroid injections for the enthesitis of AS, although the expert group feels that these can be helpful in selected cases. Potential toxicity including tendon rupture must be considered.
8. There is no evidence for the efficacy of disease modifying antirheumatic drugs (DMARDs), including sulfasalazine and methotrexate, for the treatment of axial disease. Sulfasalazine may be considered in patients with peripheral arthritis
The available level Ia evidence for the efficacy of sulfasalazine in AS is inconclusive. The most recent meta‐analysis43 identified a possible differential response to treatment for spinal symptoms and for peripheral joint disease. When data from the individual trials was pooled, there was no significantly greater effect seen on back pain (ES −2.38, 95% CI −5.78 to 1.03) or physical function (ES 0.20, 95% CI −0.77 to 1.18) with sulfasalazine than with placebo. Long term trials of sulfasalazine in spondyloarthritis in general support an effect on peripheral joints but not spinal inflammation—especially not in patients with longer disease duration. There is a need for a study of the efficacy of sulfasalazine and other DMARDs for axial disease in patients with short disease duration.
In the only extended trial of AS retrieved,44 patients treated with sulfasalazine over 3 years had significantly fewer episodes of peripheral joint symptoms than the placebo group (p<0.05). One observational study failed to show any effect of sulfasalazine on peripheral enthesitis (level IV evidence).45 The expert group felt that sulfasalazine was more relevant for peripheral joint symptoms (mean (SEM) strength of recommendation 6.53 (0.48)) than for axial disease (2.80 (0.60)). One RCT was retrieved showing that sulfasalazine decreases the occurrence of recurrent acute uveitis in patients with AS.46
Toxicity with sulfasalazine is common but usually mild: GI symptoms, mucocutaneous manifestations, hepatic enzyme abnormalities, and haematological abnormalities have been described (table 4).
There has been no meta‐analysis of methotrexate for AS, with the only systematic review retrieved unable to combine results.47 Therefore the best evidence from the literature is level Ib, with three RCTs identified.48,49,50 Pooling results where possible did not show a significant effect of methotrexate on spinal pain or function. The only study to report separate outcomes for peripheral joint disease49 did not identify significant benefit with treatment.
The most commonly reported side effects occurring with methotrexate treatment include nausea and hepatic abnormalities (table 4). Folate is effective in preventing GI symptoms and mucocutaneous adverse events.51
There is little evidence to support the use of other DMARDs commonly used in other inflammatory arthritides in AS (table 3). There is level III evidence for a beneficial effect of intravenous pamidronate on both axial pain and function,52 but the study was not powered to assess an effect on peripheral joint disease. Further RCTs are needed to answer this question. Side effects include transient post‐infusional arthralgias and myalgias52,53,54 and an acute phase response with lymphopenia and raised C reactive protein.55 Open trials suggest a beneficial effect for thalidomide on spinal disease,56,57 but toxicity is substantial, and when combined with the well recognised association of thalidomide with severe birth defects and potentially irreversible peripheral neuropathies, the expert opinion of the group was that the toxicity profile of thalidomide outweighed any potential therapeutic benefit.
9. Anti‐TNF treatment should be given to patients with persistently high disease activity despite conventional treatments according to the ASAS recommendations. There is no evidence to support the obligatory use of DMARDs before, or concomitant with, anti‐TNF treatment in patients with axial disease
RCTs (level Ib evidence) support the use of the TNF inhibitors etanercept58,59,60,61 and infliximab62,63 for spinal pain, function, and peripheral joint disease. Effect sizes are large (table 3), and the number needed to treat to achieve an ASAS20 response is low. Adalimumab, the most recent TNF antagonist to become available for treatment in rheumatic diseases, has also been shown to be effective in a level III study.64
The onset of clinical effect with TNF blockers is rapid,59 and therapeutic effect persists for up to 3 years with continuing treatment.65,66,67,68 Stopping treatment results in a high proportion of patients with clinical relapse.69 Although adding methotrexate to infliximab treatment in rheumatoid arthritis improves clinical outcome70 and reduces side effects,71 there has been no evidence to support any additional benefit with concomitant methotrexate use in AS.72 The ASAS group has published a comprehensive, evidence based consensus statement for the initiation of anti‐TNF treatment in AS73,74 (table 8) to identify appropriate therapeutic candidates.
Table 8 ASAS consensus for anti‐TNF treatment.
| Patient selection | Specification (definition of the terms) |
|---|---|
| Diagnosis | (a) Patients normally fulfilling modified New York Criteria for definitive AS |
| (b) Modified New York criteria 1984 (van der Linden et al) | |
| Radiological criterion | |
| ‐ Sacroiliitis, grade ⩾II bilaterally or grade III to IV unilaterally | |
| Clinical criteria (two out of the following three) | |
| ‐ Low back pain and stiffness for >3 months that improves with exercise but is not relieved by rest | |
| ‐ Limitation of motion of the lumbar spine in both the sagittal and frontal planes | |
| ‐ Limitation of chest expansion relative to normal values correlated for age and sex | |
| Active disease | (a) Active disease for ⩾4 weeks |
| (b) BASDAI ⩾4 (0–10) and an expert* opinion† | |
| Treatment failure | (a) All patients must have had adequate therapeutic trials of at least two NSAIDs. An adequate therapeutic trial is defined as: |
| ‐ Treatment for at least 3 months at maximal recommended or tolerated anti‐inflammatory dose unless contraindicated | |
| ‐ Treatment for <3 months where treatment was withdrawn because of intolerance, toxicity, or contraindications | |
| (b) Patients with pure axial manifestations do not have to take DMARDs before anti‐TNF treatment can be started | |
| (c) Patients with symptomatic peripheral arthritis should have an insufficient response to at least one local corticosteroid injection, if appropriate | |
| (d) Patients with persistent peripheral arthritis must have had a therapeutic trial of sulfasalazine‡ | |
| (e) Patients with symptomatic enthesitis for whom appropriate local treatment failed | |
| Contraindication | (a) Women who are pregnant or breast feeding; effective contraception must be practised |
| (b) Active infection | |
| (c) Patients at high risk of infection including: | |
| ‐ Chronic leg ulcer | |
| ‐ Previous tuberculosis (note: please follow local recommendations for prevention or treatment) | |
| ‐ Septic arthritis of a native joint within the past 12 months | |
| ‐ Sepsis of a prosthetic joint within the past 12 months, or indefinitely if the prosthesis remains in situ | |
| ‐ Persistent or recurrent chest infections | |
| ‐ Indwelling urinary catheter | |
| (d) History of lupus or multiple sclerosis | |
| (e) Malignancy or premalignancy states excluding: | |
| ‐ Basal cell carcinoma | |
| ‐ Malignancies diagnosed and treated more than 10 years previously (where the probability of total cure is very high) | |
| Assessment of disease | |
| ASAS core set for daily practice | (a) Physical function (BASFI or Dougados functional index) |
| (b) Pain (VAS, past week, spine at night, due to AS and VAS, past week, spine due to AS) | |
| (c) Spinal mobility (chest expansion and modified Schober and occiput to wall distance and lateral lumbar flexion) | |
| (d) Patient's global assessment (VAS, past week) | |
| (e) Stiffness (duration of morning stiffness, spine, past week) | |
| (f) Peripheral joints and entheses (number of swollen joints (44 joint count), enthesitis score such as developed in Maastricht, Berlin, or San Francisco) | |
| (g) Acute phase reactants (ESR or CRP) | |
| (h) Fatigue (VAS) | |
| BASDAI | (a) VAS overall level of fatigue/tiredness past week |
| (b) VAS overall level of AS neck, back, or hip pain past week | |
| (c) VAS overall level of pain/swelling in joints other than neck, back, or hips past week | |
| (d) VAS overall discomfort from any areas tender to touch or pressure past week | |
| (e) VAS overall level of morning stiffness from time of awakening past week | |
| (f) Duration and intensity (VAS) of morning stiffness from time of awakening (up to 120 minutes) | |
| Assessment of response | |
| Responder criteria | BASDAI: 50% relative change or absolute change of 20 mm (on a scale of 0–100) and expert opinion in favour of continuation |
| Time of evaluation | Between 6 and 12 weeks |
*The expert is a physician, usually a rheumatologist, with expertise in inflammatory back pain and the use of biological agents. Expert should be locally defined; †an expert opinion comprises both clinical features (history and examination) and serum acute phase reactant levels and/or imaging results, such as radiographs demonstrating rapid progression or MRI scans indicating continuing inflammation; ‡sulfasalazine: treatment for at least 4 months at standard target dose or maximally tolerated dose unless contraindicated or not tolerated. Treatment for <4 months, where treatment was withdrawn because of intolerance or toxicity or contraindicated.
VAS, visual analogue scale; all VAS can be replaced by a numerical rating scale (NRS).
Toxicity with anti‐TNF treatment includes injection site reactions with subcutaneous injections (etanercept and adalimumab) and uncommon infusion reactions with intravenous infliximab. Open trials have shown treatment to be associated with increased risk of infection75,76—in particular, tuberculosis.77,78 Screening for Mycobacterium tuberculosis is now a standard prerequisite for anti‐TNF treatment. Demyelinating disease,79 lupus‐like syndromes,80,81,82 and worsening of pre‐existing congestive heart failure83,84,85 have also been reported in case series, although precise incidences are not known.
Although significantly more expensive than traditional AS treatments, the large improvements in pain and function with TNF blocker treatment may well outweigh the high costs in a formal cost‐benefit analysis,86,87 projecting over 30 years that such treatments are even more cost effective when function and therefore productivity are preserved. Further economic evaluation is required to confirm this projection.
There is insufficient evidence available at present to make a definite statement on the role of interleukin 1 antagonists in AS.
10. Total hip arthroplasty should be considered in patients with refractory pain or disability and radiographic evidence of structural damage, independent of age. Spinal surgery—for example, corrective osteotomy and stabilisation procedures, may be of value in selected patients
The best evidence for total hip arthroplasty (THA) is level IV, with prospective cohort studies in patients with AS showing good pain relief and functional improvement with surgery.88 Although age and sex predict revision rate in THA,89 revision rates in AS are not unduly high. Rates of heterotopic bone formation and re‐ankylosis after THA are not increased in patients with AS.90,91,92,93 Collective clinical experience is that heterotopic ossification is no more common in the AS population, dependent on surgical technique, and this is likely to be affected by NSAID use in AS, and the routine practice of preoperative NSAID prophylaxis. The administration of NSAIDs on the evening before surgery (conventional practice) does not have an effect on perioperative bleeding or complication rate. The expert group agreed that NSAID treatment does not need to be discontinued for THA surgery. The difference in long term durability or complications between cemented and non‐cemented hip prostheses is not large, but THA in younger patients generally uses non‐cemented prostheses, as later revisions are technically easier (related to loss of bone structure). There are no comparative studies on this item in patients with AS.
Spinal surgery is performed for a number of indications in AS, including disabling kyphosis, loss of horizontal vision without compensation, painful spinal pseudarthrosis or Andersson lesion, pain and/or segmental instability of spinal fractures, and less commonly, neurological complications such as spinal stenosis, myelopathy, and rarely, cauda equina syndrome. Closing wedge lumbar osteotomy for fixed kyphotic deformity causing major disability can give excellent functional results by restoring balance and horizontal vision.94 Instrumentation failure has been a problem with polysegmental wedge osteotomies,95 but permanent neurological complications are rare. Fusion procedures should be considered in patients with segmental instability as a result of spinal pseudarthrosis or Andersson lesion, and in cases of instability or intractable pain due to spinal fracture. Corrective surgery of the cervical spine should be reserved for those patients with AS with specific indications, and considered for patients individually.
Discussion
This is the first time that international recommendations for the management of AS have been developed, and it is also the first official collaboration of ASAS and EULAR. Recently, ASAS has successfully collaborated with the FDA and the North American patient organisation “Spondylitis Association of America” to produce recommendations for clinical trials.96
The major driving forces for this current project were (a) the rapid developments seen in available treatments for AS over recent years; (b) the dedicated aim of ASAS to provide valuable assessment tools for AS; and (c) the standardised approach by EULAR to producing recommendations for common rheumatic diseases. Given the almost dramatic history of successful approvals of anti‐TNF agents in the past 3 years—which is still continuing—it is becoming increasingly important to assess relative benefits of the different treatments and to distinguish which are the most efficacious in particular patient settings. For this reason the ASAS and EULAR took on the challenge of developing evidence based recommendations for the management of AS.
The methods used to develop the recommendations were based on the standardised operating procedures published by EULAR,6 created to assist the promotion of high quality and comparability between studies of the management of musculoskeletal diseases. Recommendations for the management of knee osteoarthritis (OA)97 and for hip OA33 have already been developed using these methods. Evidence hierarchy was used as the primary means of describing how strong or convincing the available evidence might be. This method considers the problem that poorly conducted or reported trials may be included, falsely increasing the level of evidence. In those cases where a particular study was felt to be of poor quality, this was disclosed to the expert group and included in the discussion of the literature.
The literature supported 10 of the treatments identified for AS with level III evidence or higher, and supported negative evidence for four modalities. Six modalities had limited level IV evidence. It is important to emphasise that this assessment focused on an effect on pain or function, not on other outcomes which might be important and relevant to particular treatments, but were not used as primary outcomes in this exercise.
The group decided at the outset of this project to concentrate on the effect of treatment on pain and function, two cardinal clinical features of AS which are commonly impaired and impart significant disability and distress to patients. The recently described ASAS20 composite measure of treatment response, comprising pain, function, spinal stiffness, and patient global assessment,98,99 was also included as a primary outcome measure where available. This is a relatively new outcome measure, with only the anti‐TNF studies and the more recent NSAID trials reporting this. The expert committee emphasises that this in no way suggests that pain and function are the only important outcomes in AS; often the most important measure of therapeutic effect is very treatment‐specific—for example, patient satisfaction, compliance, confidence, and coping. Thus, a broader approach is usually required in individual patient care.
The use of an evidence hierarchy to reflect the strength of support for a treatment is intrinsically flawed when considering such interventions as surgery. There can only be level III evidence to support the use of THA in AS owing to the technical, as well as ethical, impossibilities of performing a randomised, double blind controlled trial for a surgical procedure. In this case expert opinion is a valuable addition to the available literature. Similarly, in instances where no studies have been conducted to answer a specific question, the absence of clinical trials means that expert opinion (level IV evidence) is the best available evidence.
A new method for measuring the strength of a recommendation was recently proposed in the EULAR recommendations for hip OA,100 combining available research evidence with expert opinion to give a single index, measured by a 100 mm visual analogue scale (VAS) or an ordinal scale. The measure reflects a combination of literature evidence, clinical experience, and patient perceived acceptance and tolerability, as rated by the experts. We have used this approach to quantify the overall support the experts gave each of the treatment modalities, with an NRS in place of the VAS for the final strength of recommendation for each modality. Zhang et al did not show good correlation between expert opinion and the research evidence.100 This was not surprising, as in many situations with low level evidence there is still strong expert opinion owing to clinical experience. Therefore the two approaches should complement each other, but may not necessarily agree.
The GRADE group has developed a multidimensional system to assess the grade of evidence,101,102,103 which is slightly more extensive than the concept of level of evidence used for the AS recommendations. It incorporates the study design, methodological quality, consistency of results between studies, applicability of evidence to the population under consideration, and missing data, in order to assess the overall quality of the result, and the confidence that the result of the research evidence is correct. Although a very comprehensive method of assessing treatment modalities, the GRADE system is complicated and difficult to implement in extensive therapeutic reviews such as that carried out here. The additional problems examined by GRADE were incorporated into the expert group discussions on each recommendation point where applicable.
Taken together, the expert group agreed on 10 major recommendations for the management of AS on the basis of the best available evidence. This is considered an important starting point to provide guidance for monitoring and treatment of patients with AS. It is important to realise that these are recommendations not guidelines; the lengthy discussions of the expert group for each of the 10 final points indicate that the final proposals were a synthesis of quite marked variations in opinion. The recommendations reflect expert opinion based on the current research evidence. They will be updated regularly, to keep abreast of new developments in the treatment of AS.
Acknowledgements
We acknowledge the conceptual and financial assistance of EULAR in the development of these recommendations.
Abbreviations
AS - ankylosing spondylitis
ASAS - ASsessment in AS
CI - confidence interval
DMARDs - disease modifying antirheumatic drugs
ES - effect size
MRI - magnetic resonance imaging
NRS - numerical rating scale
NSAIDs - non‐steroidal anti‐inflammatory drugs
OA - osteoarthritis
RCT - randomised controlled trial
THA - total hip arthroplasty
TNF - tumour necrosis factor
VAS - visual analogue scale
References
- 1.Khan M A. Update on spondyloarthropathies. Ann Intern Med 2002135896–907. [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis 200261(suppl 3)iii8–NaN18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A.et al Prevalence of spondylarthropathies in HLA‐B27 positive and negative blood donors. Arthritis Rheum 19984158–67. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D, van der Linden S, Bellamy N, Calin A, Dougados M, Khan M A. Which domains should be included in a core set for endpoints in ankylosing spondylitis? Introduction to the ankylosing spondylitis module of OMERACT IV. J Rheumatol 199926945–947. [PubMed] [Google Scholar]
- 5.van der Heijde D, Calin A, Dougados M, Khan M A, van der Linden S, Bellamy N. Selection of instruments in the core set for DC‐ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol 199926951–954. [PubMed] [Google Scholar]
- 6.Dougados M, Betteridge N, Burmester G R, Euller‐Ziegler L, Guillemin F, Hirvonen J.et al EULAR standardised operating procedures for the elaboration, evaluation, dissemination, and implementation of recommendations endorsed by the EULAR standing committees. Ann Rheum Dis 2004631172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zochling J, van der Heijde D, Dougados M, Braun J. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis 200665423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekelle P G, Woolf S H, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ 1999318593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedges L V. Fitting continuous models to effect size data. J Educat Stat 19827245–270. [Google Scholar]
- 10.Cohen J.Statistical power analysis for the behavioural sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates, 1988
- 11.Whitehead A, Whitehead J. A general parametric approach to the meta‐analysis of randomized clinical trials. Stat Med 1991101665–1677. [DOI] [PubMed] [Google Scholar]
- 12.Cook R J, Sackett D L. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995310452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amor B, Santos R S, Nahal R, Listrat V, Dougados M. Predictive factors for the longterm outcome of spondyloarthropathies. J Rheumatol 1994211883–1887. [PubMed] [Google Scholar]
- 14.Guillemin F, Briancon S, Pourel J, Gaucher A. Long‐term disability and prolonged sick leaves as outcome measurements in ankylosing spondylitis. Possible predictive factors. Arthritis Rheum 1990331001–1006. [DOI] [PubMed] [Google Scholar]
- 15.Ward M M. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol 2002291420–1425. [PubMed] [Google Scholar]
- 16.Doran M F, Brophy S, MacKay K, Taylor G, Calin A. Predictors of longterm outcome in ankylosing spondylitis. J Rheumatol 200330316–320. [PubMed] [Google Scholar]
- 17.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Sieper J, Braun J. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor α antibody infliximab. Ann Rheum Dis 2005641462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijde D, Wanders A, Mielants H, Dougados M, Landewe R. Prediction of progression of radiographic damage over 4 years in patients with ankylosing spondylitis [abstract]. Ann Rheum Dis 200463(suppl 1)98 [Google Scholar]
- 19.Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor a blockers in ankylosing spondylitis. Ann Rheum Dis 200463665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Heijde D, Bellamy N, Calin A, Dougados M, Khan M A, van der Linden S. Preliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working Group. J Rheumatol 1997242225–2229. [PubMed] [Google Scholar]
- 21.van der Heijde D, van der Linden S, Dougados M, Bellamy N, Russell A S, Edmonds J. Ankylosing spondylitis: plenary discussion and results of voting on selection of domains and some specific instruments. J Rheumatol 1999261003–1005. [PubMed] [Google Scholar]
- 22.Creemers M C W, Franssen M J A M, van 't Hof M A, Gribnau F W J, van de Putte L B A, van Riel P L C M. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 200564127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun J, Baraliakos X, Golder W, Brandt J, Rudwaleit M, Listing J.et al Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003481126–1136. [DOI] [PubMed] [Google Scholar]
- 24.Baraliakos X, Landewe R, Hermann K G, Listing J, Golder W, Brandt J.et al Inflammation in ankylosing spondylitis: a systematic description of the extent and frequency of acute spinal changes using magnetic resonance imaging. Ann Rheum Dis 200564730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baraliakos X, Davis J, Tsuji W, Braun J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept. Arthritis Rheum 2005521216–1223. [DOI] [PubMed] [Google Scholar]
- 26.Dagfinrud H, Kvien T K, Hagen K. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev 2004(4)CD002822. [DOI] [PubMed]
- 27.van Tubergen A, Landewe R, van der Heijde D, Hidding A, Wolter N, Asscher M.et al Combined spa‐exercise therapy is effective in patients with ankylosing spondylitis: a randomized controlled trial. Arthritis Rheum 200145430–438. [DOI] [PubMed] [Google Scholar]
- 28.van Tubergen A, Boonen A, Landewe R, Rutten‐van Molken M, van der Heijde D, Hidding A.et al Cost effectiveness of combined spa‐exercise therapy in ankylosing spondylitis: a randomized controlled trial. Arthritis Rheum 200247459–467. [DOI] [PubMed] [Google Scholar]
- 29.Barlow J H, Barefoot J. Group education for people with arthritis. Pt Educat Counsel 199627257–267. [DOI] [PubMed] [Google Scholar]
- 30.Basler H D, Rehfisch H P. Cognitive‐behavioral therapy in patients with ankylosing spondylitis in a German self‐help organization. J Psychosom Res 199135345–354. [DOI] [PubMed] [Google Scholar]
- 31.Krauth C, Rieger J, Bonisch A, Ehlebracht‐Konig I. Costs and benefits of an education program for patients with ankylosing spondylitis as part of an inpatient rehabilitation programs‐study design and first results. Z Rheumatol 200362(suppl 2)II14–II16. [DOI] [PubMed] [Google Scholar]
- 32.Wanders A, van der Heijde D, Landewé R, Béhier J ‐ M, Calin A, Olivieri I.et al Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis. Arthritis Rheum 2005521756–1765. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther K P.et al EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 200564669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeks J J, Smith L A, Bradley M D. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002325619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe P A, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta‐analysis. Lancet 20043642021–2029. [DOI] [PubMed] [Google Scholar]
- 36.Bresalier R S, Sandler R S, Quan H, Bolognese J A, Oxenius B, Horgan K.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 37.Solomon S D, McMurray J J V, Pfeffer M A, Wittes J, Fowler R, Finn P.et al Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 38.Nussmeier N A, Whelton A A, Brown M T, Langford R M, Hoeft A, Parlow J L.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 39.Lewis S C, Langman M J, Laporte J R, Matthews J N, Rawlins M D, Wiholm B E. Dose‐response relationships between individual nonaspirin nonsteroidal anti‐inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta‐analysis based on individual patient data. Br J Clin Pharmacol 200254320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? : a meta‐analysis of randomised controlled trials, Ann Rheum Dis 200463901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maugars Y, Mathis C, Vilon P, Prost A. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondylarthropathy. Arthritis Rheum 199235564–568. [DOI] [PubMed] [Google Scholar]
- 42.Luukkainen R, Nissila M, Asikainen E, Sanila M, Lehtinen K, Alanaatu A.et al Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol 19991788–90. [PubMed] [Google Scholar]
- 43.Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2005(2)CD004800. [DOI] [PubMed]
- 44.Kirwan J, Edwards A, Huitfeldt B, Thompson P, Currey H. The course of established ankylosing spondylitis and the effects of sulphasalazine over 3 years. Br J Rheumatol 199332729–733. [DOI] [PubMed] [Google Scholar]
- 45.Lehtinen A, Leirisalo‐Repo M, Taavitsainen M. Persistence of enthesopathic changes in patients with spondyloarthropathy during a 6‐month follow‐up. Clin Exp Rheumatol 199513733–736. [PubMed] [Google Scholar]
- 46.Benitez‐Del‐Castillo J M, Garcia‐Sanchez J, Iradier T, Banares A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye 200014(Pt 3A)340–343. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Liu C. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2004(3)CD004524. [DOI] [PubMed]
- 48.Altan L, Bingol U, Karakoc Y, Aydiner S, Yurtkuran M, Yurtkuran M. Clinical investigation of methotrexate in the treatment of ankylosing spondylitis. Scand J Rheumatol 200130255–259. [DOI] [PubMed] [Google Scholar]
- 49.Roychowdhury B, Bintley‐Bagot S, Bulgen D Y, Thompson R N, Tunn E J, Moots R J. Is methotrexate effective in ankylosing spondylitis? Rheumatology (Oxford) 2002411330–1332. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez‐Lopez L, Garcia‐Gonzalez A, Vazquez‐Del‐Mercado M, Munoz‐Valle J F, Gamez‐Nava J I. Efficacy of methotrexate in ankylosing spondylitis: arandomized, double blind, placebo controlled trial. J Rheumatol 2004311568–1574. [PubMed] [Google Scholar]
- 51.Ortiz Z. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev 1999(4)CD000951. [DOI] [PubMed]
- 52.Maksymowych W P, Jhangri G S, Fitzgerald A A, LeClercq S, Chiu P, Yan A.et al A six‐month randomized, controlled, double‐blind, dose‐response comparison of intravenous pamidronate (60 mg versus 10 mg) in the treatment of nonsteroidal antiinflammatory drug‐refractory ankylosing spondylitis. Arthritis Rheum 200246766–773. [DOI] [PubMed] [Google Scholar]
- 53.Maksymowych W P, Jhangri G S, LeClercq S, Skeith K, Yan A, Russell A S. An open study of pamidronate in the treatment of refractory ankylosing spondylitis. J Rheumatol 199825714–717. [PubMed] [Google Scholar]
- 54.Maksymowych W P, Lambert R, Jhangri G S, LeClercq S, Chiu P, Wong B.et al Clinical and radiological amelioration of refractory peripheral spondyloarthritis by pulse intravenous pamidronate therapy. J Rheumatol 200128144–155. [PubMed] [Google Scholar]
- 55.Adami S, Bhalla A K, Dorizzi R, Montesanti F, Rosini S, Salvagno G.et al The acute‐phase response after bisphosphonate administration. Calcif Tiss Int 198741326–331. [DOI] [PubMed] [Google Scholar]
- 56.Wei J C, Chan T W, Lin H, Huang F, Chou C. Thalidomide for severe refractory ankylosing spondylitis: a 6‐month open‐label trial. J Rheumatol 2003302627–2631. [PubMed] [Google Scholar]
- 57.Huang F, Gu J, Zhao W, Zhu J, Zhang J, Yu D T Y. One‐year open‐label trial of thalidomide in ankylosing spondylitis. Arthritis Care Res 20024715. [DOI] [PubMed] [Google Scholar]
- 58.Gorman J D, Sack K E, Davis J C., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 20023461349–1356. [DOI] [PubMed] [Google Scholar]
- 59.Davis J C, Jr, van der Heijde D, Braun J, Dougados M, Cush J, Clegg D O.et al Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003483230–3236. [DOI] [PubMed] [Google Scholar]
- 60.Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Grassnickel L.et al Six‐month results of a double‐blind, placebo‐controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003481667–1675. [DOI] [PubMed] [Google Scholar]
- 61.Calin A, Dijkmans B A, Emery P, Hakala M, Kalden J, Leirisalo‐Repo M.et al Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004631594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W.et al Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 20023591187–1193. [DOI] [PubMed] [Google Scholar]
- 63.van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P.et al Efficacy and safety of infliximab in patients with ankylosing spondylitis. Results of a randomized controlled trial (ASSERT). Arthritis Rheum 200552582–591. [DOI] [PubMed] [Google Scholar]
- 64.Haibel H, Brandt H C, Rudwaleit M, Listing J, Braun J, Kupper H.et al Efficacy and safety of adalimumab in the treatment of active ankylosing spondylitis: peliminary results of an open‐label, 20‐week trial [abstract] Rheumatology (Oxford) 200450(suppl)S217 [Google Scholar]
- 65.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G.et al Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 200564229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nikas S N, Alamanos Y, Voulgari P V, Pliakou X I, Papadopoulos C G, Drosos A A. Infliximab treatment in ankylosing spondylitis: an observational study. Ann Rheum Dis 200564940–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van den Bosch F, Devinck M, Kruithof E, Baeten D, Verbruggen G, de Keyser F.et al A prospective long‐term study of the efficacy and safety of infliximab in 107 patients with spondyloarthropathy [abstract]. Rheumatology (Oxford) 200450(suppl)S611 [Google Scholar]
- 68.Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R.et al Persistent clinical response to the anti‐TNF‐{alpha} antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford) 200544670–676. [DOI] [PubMed] [Google Scholar]
- 69.Baraliakos X, Listing J, Brandt J, Rudwaleit M, Sieper J, Braun J. Clinical response to discontinuation of anti‐TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 20057R439–R444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. [DOI] [PubMed] [Google Scholar]
- 71.Elliott M J, Maini R N, Feldmann M, Long‐Fox A, Charles P, Bijl H.et al Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 19943441125–1127. [DOI] [PubMed] [Google Scholar]
- 72.Marzo‐Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O'Connor P.et al Infliximab in combination with methotrexate in active ankylosing spondylitis. A clinical and imaging study. Ann Rheum Dis 2005641568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braun J, Pham T, Sieper J, Davis J, van der Linden S, Dougados M.et al International ASAS consensus statement for the use of anti‐tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis 200362817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braun J, Davis J, Dougados M, Sieper J, van der Linden S, van der Heijde D.et al First update of the international ASAS consensus statement for the use of anti‐TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis 200665316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J ‐ H, Slifman N R, Gershon S K, Edwards E T, Schwieterman W D, Siegel J N.et al Life‐threatening histoplasmosis complicating immunotherapy with tumor necrosis factor a antagonists infliximab and etanercept. Arthritis Rheum 2002462565–2570. [DOI] [PubMed] [Google Scholar]
- 76.Slifman N R, Gershon S K, Lee J ‐ H, Edwards E T, Braun M M. Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor a‐neutralizing agents. Arthritis Rheum 200348319–324. [DOI] [PubMed] [Google Scholar]
- 77.Keystone E C. Safety issues related to emerging therapies for rheumatoid arthritis. Clin Exp Rheumatol 200422(suppl 35)S148–S150. [PubMed] [Google Scholar]
- 78.Baeten D, Kruithof E, Van den Bosch F, Van den Bossche N, Herssens A, Mielants H.et al Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 200362829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohan N, Edwards E T, Cupps T R, Oliverio P J, Sandberg G, Crayton H.et al Demyelination occurring during anti‐tumor necrosis factor a therapy for inflammatory arthritides. Arthritis Rheum 2001442862–2869. [DOI] [PubMed] [Google Scholar]
- 80.Ferraccioli G F, Assaloni R, Perin A, Shakoor N, Block J A, Mohan A K.et al Drug‐induced systemic lupus erythematosus and TNF‐a blockers (multiple letters). Lancet 2002360645–646. [DOI] [PubMed] [Google Scholar]
- 81.Shakoor N, Michalska M, Harris C A, Block J A. Drug‐induced systemic lupus erythematosus associated with etanercept therapy. Lancet 2002359579–580. [DOI] [PubMed] [Google Scholar]
- 82.Cairns A P, Duncan M K J, Hinder A E, Taggart A J. New onset systemic lupus erythematosus in a patient receiving etanercept for rheumatoid arthritis. Ann Rheum Dis 2002611031–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung E S, Packer M, Lo K H, Fasanmade A A, Willerson J T, Anti‐TNF therapy against congestive heart failure investigators Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐alpha, in patients with moderate‐to‐severe heart failure: results of the anti‐TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 20031073133–3140. [DOI] [PubMed] [Google Scholar]
- 84.Coletta A P, Clark A L, Banarjee P, Cleland J G. Clinical trials update: RENEWAL (RENAISSANCE and RECOVER) and ATTACH. Eur J Heart Failure 20024559–561. [DOI] [PubMed] [Google Scholar]
- 85.Wolfe F, Michaud M S. Congestive heart failure in rheumatoid arthritis: rates, predictors and the effect of anti‐TNF therapy. Am J Med 2004116311. [DOI] [PubMed] [Google Scholar]
- 86.Kobelt G, Andlin‐Sobocki P, Brophy S, Jonsson L, Calin A, Braun J. The burden of ankylosing spondylitis and the cost‐effectiveness of treatment with infliximab (Remicade). Rheumatology (Oxford) 2004431158–1166. [DOI] [PubMed] [Google Scholar]
- 87.Singh G, Tandon N, Bala M. A cost efficacy analysis on anti‐TNF therapy in ankylosing spondylitis [abstract]. Arthritis Rheum 200450(suppl)S613 [Google Scholar]
- 88.Sweeney S, Gupta R, Taylor G, Calin A. Total hip arthroplasty in ankylosing spondylitis: outcome in 340 patients. J Rheumatol 2001281862–1866. [PubMed] [Google Scholar]
- 89.Furnes O, Lie S A, Espehaug B, Vollset S E, Engesaeter L B, Havelin L I. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987–99. J Bone Joint Surg Br 200183579–586. [DOI] [PubMed] [Google Scholar]
- 90.Brinker M R, Rosenberg A G, Kull L, Cox D D. Primary noncemented total hip arthroplasty in patients with ankylosing spondylitis: clinical and radiographic results at an average follow‐up period of 6 years. J Arthroplasty 199611802–812. [DOI] [PubMed] [Google Scholar]
- 91.Bhan S, Malhotra R. Bipolar hip arthroplasty in ankylosing spondylitis. Arch Orthop Trauma Surg 199611594–99. [DOI] [PubMed] [Google Scholar]
- 92.Diaz de Rada P, Barroso‐Diaz J L, Valenti J R. Follow‐up of the outcome of hip arthroplasty in patients with ankylosing spondylitis. Rev Ortop Traumatol 200448340–344. [Google Scholar]
- 93.Sochart D H, Porter M L. Long‐term results of total hip replacement in young patients who had ankylosing spondylitis. Eighteen to thirty‐year results with survivorship analysis. J Bone Joint Surg Am 1997791181–1189. [DOI] [PubMed] [Google Scholar]
- 94.Van Royen B J, De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. A structured review of three methods of treatment. Ann Rheum Dis 199958399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Royen B J, de Kleuver M, Slot G H. Polysegmental lumbar posterior wedge osteotomies for correction of kyphosis in ankylosing spondylitis. Eur Spine J 19987104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Heijde D, Dougados M, Davis J, Weisman M H, Maksymowych W, Braun J.et al ASsessment in Ankylosing Spondylitis International Working Group/Spondylitis Association of America recommendations for conducting clinical trials in ankylosing spondylitis. Arthritis Rheum 200552386–394. [DOI] [PubMed] [Google Scholar]
- 97.Jordan K M, Arden N K, Doherty M, Bannwarth B, Bijlsma J W J, Dieppe P.et al EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003621145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson J J, Baron G, van der Heijde D, Felson D T, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short‐term improvement in ankylosing spondylitis. Arthritis Rheum 2001441876–1886. [DOI] [PubMed] [Google Scholar]
- 99.Brandt J, Listing J, Sieper J, Rudwaleit M, van der Heijde D, Braun J. Development and preselection of criteria for short term improvement after anti‐TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis 2004631438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther K.et al Alternative method of measuring the strength of recommendation for evidence based guidelines. Ann Rheum Dis. (in press)
- 101.Atkins D, Best D, Briss P A, Eccles M, Falck‐Ytter Y, Flottorp S.et al Grading quality of evidence and strength of recommendations. BMJ 20043281490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Atkins D, Eccles M, Flottorp S, Guyatt G H, Henry D, Hill S.et al Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schunemann H J, Best D, Vist G, Oxman A D, for The GRADE Working Group Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ 2003169677–680. [PMC free article] [PubMed] [Google Scholar]