Abstract
Objective
To compare digital x ray radiogrammetry (DXR) with manual radiography for assessing bone loss in RA and examine the relationship of the scores obtained with other disease indices.
Methods
225 consecutive consenting subjects attending the RA clinic were enrolled. An x ray examination was carried out; demographic details recorded; a self assessment questionnaire completed; blood taken for ESR measurement; and an assessment made by a trained nurse. All x ray films were scored manually using the modified Sharp technique by a single observer; 20 films were rescored by three readers. Films were assessed with the Pronosco X‐Posure system, version 2.0. Analysis included χ2 tests, independent t tests, multiple linear regression, and partial correlations, as appropriate. The smallest detectable difference (SDD), coefficient of variation (CV), and coefficient of repeatability (CR) were determined from Bland and Altman plots.
Results
The DXR precision varied: SDD = 0.002–0.9; CV = 0.09–5.9%; CR = 0.002–0.792, but was better than that of the intra‐ and interobserver Sharp scores: SDD = 73.9; CV = 27.8%; CR = 33.0–47.6. The DXR measurements, bone mineral density (R2 = 0.210), metacarpal index (R2 = 0.222), and cortical thickness (R2 = 0.215), significantly predicted Sharp scores. In women, DXR measurements significantly correlated with modified HAQ scores but with no other disease indices. Sharp scores significantly correlated with assessor's global assessment, swollen and tender joint counts, pain, HAQ, and DAS28.
Conclusion
DXR measurements are more precise than Sharp scores; both are related to long term disease activity in RA. DXR is simple to use, does not require intensive training, and may identify subjects not responding to standard treatment.
Keywords: rheumatoid arthritis, joint erosions, hand bone density, radiogrammetry
Many advances have been made in uncovering the pathogenesis of rheumatoid arthritis (RA) and this has led to the introduction of new treatments. Cytokines produced by macrophages, in particular tumour necrosis factor α, have been found in higher concentrations in rheumatoid joints.1,2 This has led to the development of treatments directed against tumour necrosis factor α and its receptors, which have been shown to slow radiographic joint damage, but these are expensive and can have serious side effects.3,4,5,6 It would be useful if these and other disease modifying drugs could be specifically targeted at an early stage of disease to those subjects who are unresponsive to safer and cheaper drugs and have continuing joint damage.
Current techniques of assessing long term disease progression in RA, although useful in comparing drug treatments in clinically controlled trials, are not very effective in assessing RA in individual subjects. Accordingly, they are rarely used as part of standard day to day clinical practice by rheumatologists.7
It is necessary to use various imaging techniques to assess structural damage. Although there is interest in using magnetic resonance imaging8 and ultrasound imaging 9,10 to assess joint damage, including cartilage loss and erosion counts, the most widely used methods are a variety of plain film x ray scoring techniques. The Sharp scoring method11 is such a technique and in its modified forms, such as that suggested by van der Heijde,12 is commonly used in clinical trials to assess erosions and joint space narrowing for joints of both the hands and feet. Because erosive damage of the periarticular bones is believed to be largely irreversible, the use of such an end point clinically might be considered to have limited benefit, with treatment always lagging behind disease progression. However, a measurement technique that showed a strong relationship with joint damage, which is in itself related to long term functional status, might have real clinical value.
Periarticular bone loss is the earliest radiological feature of RA. If quantitative assessment of hand bone mass is used as a surrogate, bone loss can be seen to occur early in the disease and predate erosive damage.13,14 Peripheral bone mass can be quantified by a number of techniques in RA including dual energy x ray absorptiometry (DXA),13,14,15 quantitative ultrasound,9,10,16 and possibly, magnetic resonance imaging.8 DXA is most widely used for estimating in vivo total hand bone mineral density (BMD) but may also be used to assess BMD specifically at the metacarpal joints.14 In the past few years, an improved method of radiogrammetry has been introduced using digitised plain hand radiographs17 to measure bone density, metacarpal index, cortical thickness, and porosity with high precision.18
In this study we examined the ability of digital x ray radiogrammetry (DXR) to assess bone loss in RA, compared the results with a manual plain radiograph scoring technique—the modified Sharp score19—and examined the relationship of both methods with other indices of disease activity and progression in RA.
Methods
The study was carried out at the Osteoporosis Research Unit, Aberdeen. The Grampian research ethics committee granted ethical approval.
Subjects attending the RA clinic were provided with details of the study, which included an information leaflet. Consecutive, consenting subjects were enrolled in the study. Subjects also underwent an x ray examination of their hands if one had not been carried out in the previous 2 years. JS collected the data between May 2001 and July 2002.
Subjects' demographic details were recorded and a self assessment questionnaire was completed, which included data on the duration of early morning stiffness (EMS); pain, as assessed on a 10 cm horizontal visual analogue scale (VAS); and a patient global assessment (PGA) of disease activity, as assessed on a 10 cm horizontal VAS. A modified Health Assessment Questionnaire (HAQ) was also administered.20 Blood was taken from the subjects and the erythrocyte sedimentation rate (ESR) measured in mm after a 1 hour sample frame. Subject case notes were audited and current medications and dosages, rheumatoid factor positivity, duration of disease, and the presence or absence of erosions noted on the database. Assessments were also carried out by JS, a fully trained research nurse and included an assessor global assessment (AGA) of disease activity on a 10 cm horizontal VAS, and a 28 point tender and/or swollen joint count. If appropriate, the subjects then had a plain film x ray examination of both hands. Only those subjects who had a relevant x ray examination were assessed as part of this study.
Author WBJ scored all the available hand radiographs using the modified Sharp scoring method and the results were recorded. Twenty randomly selected subjects were rescored by WBJ a week after the initial assessment to allow calculation of short term intraobserver variation. Interobserver variation was calculated by examining 20 radiographs randomly selected for rescoring by three observers (DC, DMR, and WBJ).
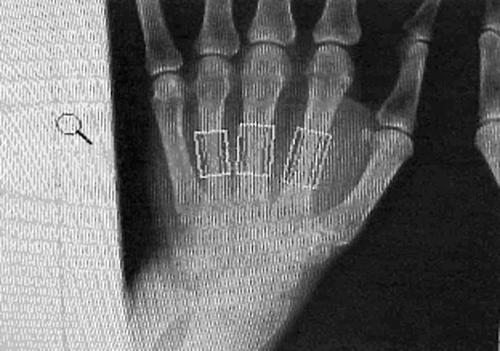
All the hand radiographs were then assessed using the Pronosco X‐Posure system, version 2.0 (Sectra Medical Systems, Sweden).18,21,22,23,24 This system provides the following measurements: BMD; porosity (POR); metacarpal index (MCI); cortical thickness (CT); bone width (BW). The Pronosco X‐Posure system requires plain x ray films. The x rays films of the subjects in this study showed both hands on the same film. To evaluate radiographs using DXR the radiograph of each hand was separately scanned in and then analysed. BMD was calculated using formulae after assessment of the bone volume per area, assuming a cylindrical bone,18 thus negating the need for a phantom. Figure 1 shows regions of interest.
Figure 1 Automatic selection of regions of interest by Pronosco.
All data were entered into a spreadsheet and analysed using SPSS 11.0.1 (SPSS Inc, USA) and Microsoft Excel (Microsoft Corp, USA). Tests used included χ2 tests, independent t tests, multiple linear regression analysis, and partial correlation, as appropriate. The precision was estimated by calculating the smallest detectable difference (SDD) and the standardised coefficient of variation (CV). The reproducibility of the repeated measurements was assessed using Bland and Altman plots and the coefficient of repeatability (CR) was calculated. Correlation coefficients were compared using Fisher's z transformation.
Results
Subject flow and comparison
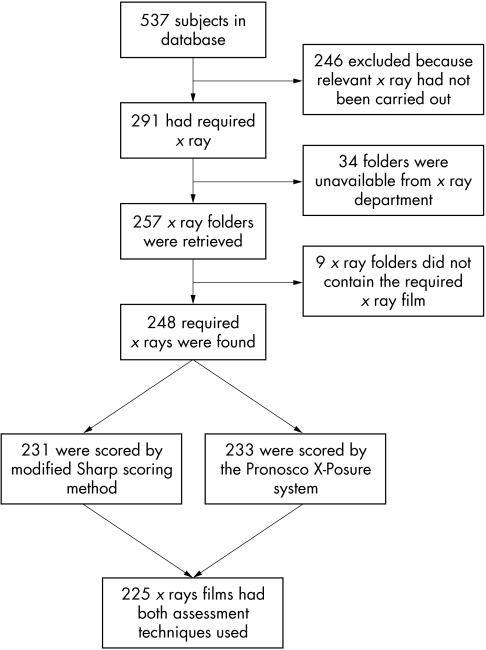
Figure 2 shows the flow of subjects through the study. 537 subjects were on the original database, but only 225 met the entry requirements. Table 1 compares the demographics and disease activity indices for the current study group and the total study group. We found the HAQ score was significantly lower (p = 0.026) in the study group than in the whole group and disease duration was significantly shorter (p = 0.023), but there were no other significant differences between the groups.
Figure 2 Flow of subjects through the study.
Table 1 Comparison between demographic and disease activity indices for all subject's on database and those that were selected for the study, with numbers for each index shown in parentheses.
| Total study group (n = 537)) | Current study group (n = 225) | p Value | |
|---|---|---|---|
| % Female | 76±4 (537) | 71±6 (225) | 0.160 |
| RF seropositive (% ) | 79±4 (482) | 79±6 (217) | 0.842 |
| Age at 1 Jan 2000 (years) | 55.1±0.96 (534) | 54.5±1.54 (225) | 0.521 |
| AGA | 34.5±1.9 (520) | 32.7±2.7 (220) | 0.291 |
| PGA | 40.1±2.1 (520) | 39.9±3.1 (220) | 0.917 |
| VAS pain | 37.4±2.2 (521) | 36.0±3.2 (221) | 0.477 |
| No of swollen joints | 5.2±0.4 (514) | 4.7±0.6 (222) | 0.118 |
| No of tender joints | 3.4±0.4 (514) | 3.3±0.6 (222) | 0.640 |
| EMS (min) | 50.8±5.7 (351) | 50.2±8.6 (165) | 0.909 |
| HAQ | 1.33±0.07 (519) | 1.19±0.10 (221) | 0.026* |
| ESR (min) | 26.7±2.4 (340) | 27.3±3.4 (157) | 0.262 |
| DAS28 | 5.88±0.40 (334) | 5.61±0.57 (156) | 0.447 |
| Disease duration at 2001 (years) | 10.5±0.7 (519) | 8.9±1.1 (223) | 0.023* |
| Weight (kg) | 70.6±1.3 (483) | 71.7±2.1 (210) | 0.362 |
Results shown as mean±95% confidence interval (CI) unless stated otherwise.
Precision
Sharp scores
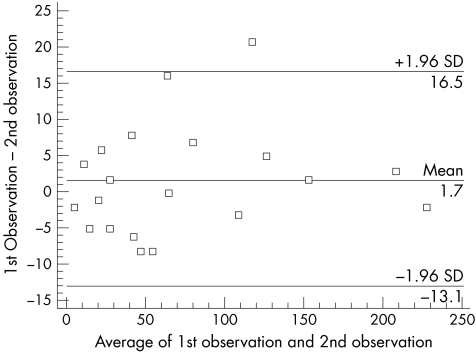
The mean (SD) Sharp score for the study group was 79.38 (53.45). The intraobserver CV for the modified Sharp scoring techniques was 7.2%. The intraobserver Bland and Altman plot (fig 3) shows that variation at the extremes of the modified Sharp scores is smaller than the variation at intermediate Sharp scores. The CR was 14.8.
Figure 3 Bland and Altman plot for the modified Sharp score intraobserver variation as scored by WBJ.
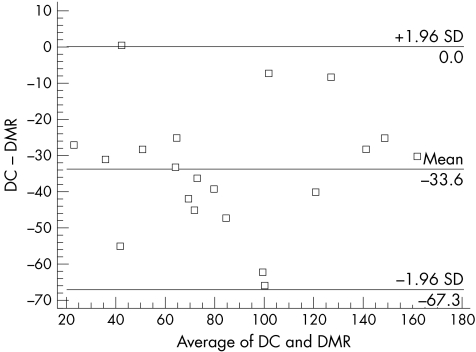
For the interobserver variation the CV was 27.8% and the SDD was calculated at 73.9 units for a mean Sharp score of 94. The CR were as follows; between DC and DMR 33.6 Sharp score units, between WBJ and DMR 33.0 units, and between DC and WBJ 47.6 units. Figure 4 shows an example of an interobserver Bland and Altman plot.
Figure 4 Bland and Altman plot for the modified Sharp score interobserver variation as scored by DC and DMR.
DXR measurements
Table 2 summarises the intraobserver precision values for the different DXR variables scored on the same 20 radiographs assessed on two separate occasions 2 months apart. Of the DXR measurements, POR appears to be the least precise measurement while bone width and cortical thickness are the most precise. The CR ranged from 0.002 to 0.792 for the DXR measurements.
Table 2 Precision of DXR measurements.
| Left hand DXR measurements | |||||
|---|---|---|---|---|---|
| BMD (g/cm2) | POR | MCI | CT (cm) | BW (cm) | |
| CV (%) | 0.304 | 5.869 | 0.322 | 0.412 | 0.087 |
| Mean | 0.480 | 5.466 | 0.345 | 0.147 | 0.853 |
CV, coefficient of variation.
Correlations between Sharp scores and DXR measurements
Table 3 shows the partial correlations between Sharp scores and DXR measurements. In male subjects correlation between DXR‐MCI and the modified Sharp score and its component scores appears to be weaker than that in female subjects, although the differences are not significant (p>0.05 for all coefficients). The derived POR and BW did not relate to any aspect of the Sharp index.
Table 3 Partial correlation analysis between Sharp score and its component scores and DXR measurements, where significant results are shown in bold.
| Sharp score | Erosion score— both hands | Joint space score— both hands | ||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| BMD | ||||||
| r | −0.481 | −0.394 | −0.441 | −0.390 | −0.461 | −0.353 |
| p Value | <0.005 | 0.003 | <0.005 | 0.003 | <0.005 | 0.008 |
| MCI | ||||||
| r | −0.479 | −0.278 | −0.417 | −0.304 | −0.481 | −0.224 |
| p Value | <0.005 | 0.040 | <0.005 | 0.024 | <0.005 | 0.100 |
| CT | ||||||
| r | −0.478 | −0.369 | −0.433 | −0.372 | −0.462 | −0.326 |
| p Value | <0.005 | 0.006 | <0.005 | 0.005 | <0.005 | 0.015 |
| POR | ||||||
| r | +0.133 | −0.155 | +0.135 | −0.124 | +0.116 | −0.166 |
| p Value | 0.102 | 0.259 | 0.099 | 0.367 | 0.158 | 0.225 |
| BW | ||||||
| r | +0.037 | −0.180 | −0.024 | −0.115 | +0.091 | −0.220 |
| p Value | 0.654 | 0.189 | 0.771 | 0.405 | 0.265 | 0.107 |
Age at x ray examination and weight were used as controlling variables.
Correlation of radiological measures of disease severity with clinical disease activity measurements
Table 4 show the correlation between DXR measurements, Sharp scores, and clinical measures of disease activity. In female subjects for DXR measurements the only significant correlations seen were with HAQ scores (r = −0.218, p = 0.008). In contrast, Sharp scores significantly correlated with AGA, swollen and tender joint counts, VAS pain, HAQ, and 28 joint count disease activity score (DAS28) but with low r values of around 0.17–0.30 (p = 0.043–0.01). Sharp scores correlated slightly better with the HAQ than did DXR (r = +0.298, p<0.005). In contrast, for the smaller number of male patients, DXR measurements showed better correlation with certain disease measures, whereas the Sharp scores did not show significant correlation with any of the clinical measures.
Table 4 Partial correlation analysis between structural measures and clinical measures, where bold indicates significant results.
| AGA | PGA | Swollen | Tender | Pain | EMS | HAQ | ESR | DAS28 | |
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| DXR‐BMD | |||||||||
| r | −0.057 | −0.017 | −0.106 | −0.061 | −0.033 | −0.029 | −0.218 | +0.073 | −0.126 |
| p Value | 0.495 | 0.840 | 0.197 | 0.457 | 0.694 | 0.763 | 0.008 | 0.460 | 0.201 |
| DXR‐MCI | |||||||||
| r | −0.031 | +0.002 | −0.051 | −0.002 | −0.028 | −0.047 | −0.170 | +0.048 | −0.064 |
| p Value | 0.712 | 0.982 | 0.536 | 0.981 | 0.732 | 0.628 | 0.039 | 0.626 | 0.515 |
| DXR‐CT | |||||||||
| r | −0.040 | −0.005 | −0.088 | −0.035 | −0.025 | −0.021 | −0.193 | +0.072 | −0.095 |
| p Value | 0.629 | 0.951 | 0.286 | 0.668 | 0.766 | 0.827 | 0.018 | 0.464 | 0.333 |
| Sharp score | |||||||||
| r | +0.212 | +0.143 | +0.170 | +0.190 | +0.166 | −0.050 | +0.298 | +0.100 | +0.246 |
| p Value | 0.010 | 0.083 | 0.038 | 0.020 | 0.043 | 0.607 | <0.005 | 0.311 | 0.011 |
| Men | |||||||||
| DXR‐BMD | −0.292 | −0.296 | +0.000 | −0.214 | −0.277 | −0.215 | −0.348 | −0.024 | −0.167 |
| r | 0.030 | 0.030 | 0.999 | 0.116 | 0.041 | 0.162 | 0.009 | 0.880 | 0.290 |
| p Value | |||||||||
| DXR‐MCI | |||||||||
| r | −0.211 | −0.150 | +0.109 | −0.038 | −0.223 | −0.246 | −0.265 | −0.039 | −0.045 |
| p Value | 0.121 | 0.279 | 0.428 | 0.782 | 0.102 | 0.108 | 0.051 | 0.806 | 0.776 |
| DXR‐CT | |||||||||
| r | −0.272 | −0.266 | +0.045 | −0.163 | −0.254 | −0.238 | −0.332 | −0.038 | −0.145 |
| 0.044 | 0.052 | 0.747 | 0.235 | 0.061 | 0.119 | 0.013 | 0.811 | 0.361 | |
| Sharp score | |||||||||
| r | +0.242 | +0.229 | +0.225 | +0.175 | +0.176 | +0.020 | +0.261 | +0.186 | +0.233 |
| p Value | 0.076 | 0.096 | 0.099 | 0.201 | 0.199 | 0.554 | 0.054 | 0.239 | 0.138 |
Age at x ray and weight were used as controlling variables.
Discussion
The precision results clearly indicate that DXR measurements are much more reproducible than Sharp scores, which vary greatly not only between observers but also when rescored by the same observer even a week later. The precision of DXR measurements in this study is better than that reported by Jorgensen et al.18 This discrepancy is probably due to the fact that we are simply measuring noise in the system, because the same radiograph is used each time. Perhaps a truer estimate would be given if two x ray pictures were taken at the same time and used for calculation of precision. The precision of the modified Sharp score was lower than that reported by Sharp et al,25 who found an interobserver CV of 17%. However, this may be because of the limited experience of two of the scorers or may be owing to the long duration of disease, making reading of the radiograph more difficult. A recent comparison of intra‐ and interobserver variation by Sharp et al has shown figures for the SDD which are very similar to those we achieved.26
Bone loss is an early feature of RA and precedes the permanent and irreversible erosive changes that occur.13,14 The Pronosco device can measure bone loss at the metacarpal joints, which are in the region most commonly affected by RA, and therefore may be able to identify subjects requiring a change in treatment before any irreversible joint damage occurs. It has been well established that BMD is reduced in patients with RA and the degree of loss is related to disease activity. Daragon et al found significantly reduced BMD of the whole hand using DXA at 6 months in patients with RA compared with subjects with other rheumatic diseases.27
This study has shown that both DXR and Sharp score measurements are related more to disease severity than to current disease activity. This has also been shown previously.28 DXR measurements are as good as Sharp scores in predicting HAQ and may even be better in male subjects. Their predictive value may not be so good in women owing to other confounding factors which influence the measurement of bone mass, including menopausal state and pre‐disease bone mass status. Of the various DXR measures available, BMD seems numerically to correlate best with modified Sharp scores, but the differences between the correlation coefficients and the other DXR variables are not statistically significant.
At present, rheumatologists do not routinely use all of the RA measures mentioned in this study in their clinical review of subjects. Less than 10% of rheumatologists use pain scales, fewer of them use functional status questionnaires, and although joint counts are done, few are recorded in medical records.7 Wolfe and Zwillich showed that currently clinicians base their decisions on treatment change mainly on pain and joint count, which are immediately obvious during the subject interview and physical examination.29
In our study DXR measurements are related to long term disease activity in RA and therefore serial measurements in individual subjects may have the ability to assess response to treatment and this may be possible over sufficiently short time periods to improve patient care, owing to the high precision of the technique. Indeed, a recent study by Jensen et al30 has demonstrated that during a 2 year period, measurements of DXR BMD showed more rapid changes in those with active disease than in those with inactive disease and could distinguish those with erosive from those with non‐erosive RA. A recent pilot longitudinal study from our own centre in subjects with early RA has identified how a measurement of the rate of change of DXR BMD in the first year of follow up may be able to identify those who become erosive by 4 years of disease with high specificity and reasonable sensitivity.31 DXR is simple to use, does not require intensive training, and if used in a clinical setting may have the ability quickly and cheaply to identify subjects not responding to standard treatment or may allow selection of subjects who would benefit from more aggressive treatment with newer, more expensive treatments at an earlier stage of the disease. Such a hypothesis will require testing in a formal prospective clinical trial.
In summary, we describe a method of assessing peripheral bone mass in RA that might have use in routine clinical care unlike the time consuming and rather imprecise measurement of joint space narrowing and erosion scores such as the Sharp score. Longitudinal studies will be required to determine the value of the technique in assessing suitability of subjects for expensive biological agents which can effectively limit joint destruction and bone loss.
Acknowledgements
We are grateful to the Arthritis Research Campaign (ARC) for supporting this work by a studentship for WBJ and for infrastructure support to AS and DMR.
Abbreviations
BMD - bone mineral density
CR - coefficient of repeatability
CV - coefficient of variation
AGA - assessor's global assessment
BW - bone width
CT - cortical thickness
DAS28 - 28 joint count disease activity score
DXA - dual energy x ray absorptiometry
DXR - digital x ray radiogrammetry
EMS - early morning stiffness
ESR - erythrocyte sedimentation rate
HAQ - Health Assessment Questionnaire
MCI - metacarpal index
PGA - patient global assessment
POR - porosity
RA - rheumatoid arthritis
SDD - smallest detectable difference
VAS - visual analogue scale
Footnotes
Competing interests: None
References
- 1.Neidel J, Schulze M, Lindschau J. Association between degree of bone‐erosion and synovial fluid‐levels of tumor necrosis factor alpha in the knee‐joints of patients with rheumatoid arthritis. Inflamm Res 199544217–221. [DOI] [PubMed] [Google Scholar]
- 2.Brennan F M, Maini R N, Feldmann M. Role of pro‐inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol 199820133–147. [DOI] [PubMed] [Google Scholar]
- 3.Wong J B, Singh G, Kavanaugh A. Estimating the cost‐effectiveness of 54 weeks of infliximab for rheumatoid arthritis. Am J Med 2002113400–408. [DOI] [PubMed] [Google Scholar]
- 4.Culy C R, Keating G M. Etanercept: an updated review of its use in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis. Drugs 2002622495–2539. [DOI] [PubMed] [Google Scholar]
- 5.Mikuls T R, Moreland L W. Benefit‐risk assessment of infliximab in the treatment of rheumatoid arthritis. Drug Saf 20032623–32. [DOI] [PubMed] [Google Scholar]
- 6.Peno‐Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest 20021221858–1860. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Pincus T, O'Dell J. Evaluation and documentation of rheumatoid arthritis disease status in the clinic: which variables best predict change in therapy. J Rheumatol 2001281712–1717. [PubMed] [Google Scholar]
- 8.Haugeberg G, Orstavik R E, Uhlig T, Falch J A, Halse J I, Kvien T K. Bone loss in patients with rheumatoid arthritis: results from a population‐based cohort of 366 patients followed up for two years. Arthritis Rheum 2002461720–1728. [DOI] [PubMed] [Google Scholar]
- 9.Alenfeld F E, Diessel E, Brezger M, Sieper J, Felsenberg D, Braun J. Detailed analyses of periarticular osteoporosis in rheumatoid arthritis. Osteoporos Int 200011400–407. [DOI] [PubMed] [Google Scholar]
- 10.Roben P, Barkmann R, Ullrich S, Gause A, Heller M, Gluer C C. Assessment of phalangeal bone loss in patients with rheumatoid arthritis by quantitative ultrasound. Ann Rheum Dis 200160670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp J T, Lidsky M D, Collins L C, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum 197114706–720. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D M, van't Hof M, van Riel P L, van de Putte L B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 199320579–581. [PubMed] [Google Scholar]
- 13.Deodhar A A, Woolf A D. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol 199635309–322. [DOI] [PubMed] [Google Scholar]
- 14.Devlin J, Lilley J, Gough A, Huissoon A, Holder R, Reece R.et al Clinical associations of dual‐energy X‐ray absorptiometry measurement of hand bone mass in rheumatoid arthritis. Br J Rheumatol 1996351256–1262. [DOI] [PubMed] [Google Scholar]
- 15.Peel N F, Spittlehouse A J, Bax D E, Eastell R. Bone mineral density of the hand in rheumatoid arthritis. Arthritis Rheum 199437983–991. [DOI] [PubMed] [Google Scholar]
- 16.Martin J C, Munro R, Campbell M K, Reid D M. Effects of disease and corticosteroids on appendicular bone mass in postmenopausal women with rheumatoid arthritis: comparison with axial measurements. Br J Rheumatol 19973643–49. [DOI] [PubMed] [Google Scholar]
- 17.Rosholm A, Hyldstrup L, Backsgaard L, Grunkin M, Thodberg H H. Estimation of bone mineral density by digital X‐ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int 200112961–969. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen J T, Andersen P B, Rosholm A, Bjarnason N H. Digital X‐ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Physiol 200020330–335. [DOI] [PubMed] [Google Scholar]
- 19.Sharp J T, Young D Y, Bluhm G B, Brook A, Brower A C, Corbett M.et al How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 1985281326–1335. [DOI] [PubMed] [Google Scholar]
- 20.Fries J F, Spitz P W, Young D Y. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 19829789–793. [PubMed] [Google Scholar]
- 21.Bouxsein M L, Palermo L, Yeung C, Black D M. Digital x‐ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int 200213358–365. [DOI] [PubMed] [Google Scholar]
- 22.Malich A, Freesmeyer M G, Mentzel H J, Sauner D, Boettcher J, Petrovitch A.et al Normative values of bone parameters of children and adolescents using digital computer‐assisted radiogrammetry (DXR). J Clin Densitom 20036103–111. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J A, Cheng X G, Lu Y, Njeh C, Toschke J, Engelke K.et al Universal standardization of forearm bone densitometry. J Bone Miner Res 200217734–745. [DOI] [PubMed] [Google Scholar]
- 24.Black D M, Palermo L, Sorensen T, Jorgensen J T, Lewis C, Tylavsky F.et al A normative reference database study for Pronosco X‐posure System. J Clin Densitom 200145–12. [DOI] [PubMed] [Google Scholar]
- 25.Sharp J T, Bluhm G B, Brook A, Brower A C, Corbett M, Decker J L.et al Reproducibility of multiple‐observer scoring of radiologic abnormalities in the hands and wrists of patients with rheumatoid arthritis. Arthritis Rheum 19852816–24. [DOI] [PubMed] [Google Scholar]
- 26.Sharp J T, Wolfe F, Lassere M, Boers M, Van Der Heijde D, Larsen A.et al Variability of precision in scoring radiographic abnormalities in rheumatoid arthritis by experienced readers. J Rheumatol 2004311062–1072. [PubMed] [Google Scholar]
- 27.Daragon A, Krzanowska K, Vittecoq O, Menard J F, Hau I, Jouen‐Beades F.et al Prospective X‐ray densitometry and ultrasonography study of the hand bones of patients with rheumatoid arthritis of recent onset. Joint Bone Spine 20016834–42. [DOI] [PubMed] [Google Scholar]
- 28.Haugeberg G, Lodder M C, Lems W F, Uhlig T, Orstavik R E, Kijkmans B A C.et al Hand cortical bone mass and its association with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo‐Truro‐Amsterdam (OSTRA) collaborative study. Ann Rheum Dis 2004631331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe F, Zwillich S H. The long‐term outcomes of rheumatoid arthritis: a 23‐year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum 1998411072–1082. [DOI] [PubMed] [Google Scholar]
- 30.Jensen T, Klarlund M, Hansen M, Jensen K E, Podenphant J, Hansen T M.et al Bone loss in unclassified polyarthritis and early rheumatoid arthritis is better detected by digital x ray radiogrammetry than dual x ray absorptiometry: relationship with disease activity and radiographic outcome. Ann Rheum Dis 20046315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart A, Mackenzie L M, Black A J, Reid D M. Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital x‐ray radiogrammetry: a pilot study, Rheumatology (Oxford) 2004431561–1564. [DOI] [PubMed] [Google Scholar]