Abstract
Some Borrelia species associated with Lyme disease bind the complement-regulatory protein factor H (fH), a process that may aid in immune evasion. In this report we demonstrate that some Borrelia species associated with relapsing fever bind fH, but not those associated with avian borreliosis and epizootic bovine abortion. Cell-bound fH was also found to mediate cleavage of exogenously supplied human C3b, demonstrating the biological relevance of fH binding and its possible importance in the pathogenesis of the relapsing-fever spirochetes.
In North America, tick-borne relapsing fever (TBRF) is caused by Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae (4) and transmitted by Ornithodoros ticks. Lyme disease (worldwide) is caused by Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii and is transmitted by Ixodes ticks. TBRF is characterized by cyclic spirochetemias, a high-grade relapsing fever, and in some cases neurological involvement (3). Antigenic variation is central to the ability of the relapsing-fever spirochetes (RFS) to survive in the host and thrive in the blood (5, 15). In the closely related Lyme spirochetes, immune evasion is thought to be a multifactorial process. One newly delineated mechanism that may contribute to immune evasion and the maintenance of chronic infection is the binding of the complement regulatory protein factor H (fH) to the cell surface (1, 2, 7, 8, 10, 13). Under normal conditions, fH is a complement regulatory protein that serves as a cofactor for the factor I (fI)-mediated cleavage of C3b, a key component of the complement cascade (6). FHL-1, which is derived from the same gene as fH via alternative splicing of the mRNA, plays a role similar to that of fH (6). By increasing the local concentration of fH at the cell surface, bacteria that bind fH can more efficiently promote the degradation of C3b and thereby decrease the efficiency of complement-mediated killing. A recent comprehensive analysis of fH binding to Lyme disease spirochetes revealed that 100% of B. burgdorferi, 83% of B. afzelii, and 25% of B. garinii isolates tested bind fH (12). The binding of fH by B. burgdorferi and B. afzelii may aid in evasion of complement attack and thereby facilitate dissemination (1, 2, 7, 8, 10, 13). The general inability of B. garinii to bind fH may account for the frequency with which this species has been isolated from the CNS, since in this niche the bacteria would be protected from complement (13). Here we demonstrate that some RFS species (B. hermsii and B. parkeri) can bind human fH (hfH) and that bound fH can interact with fI, leading to the cleavage of C3b. Like the neurotropic species B. garinii, the potentially neurotropic RFS species B. turicatae does not appear to bind fH. These analyses indicate that fH binding is of biological relevance in the pathogenesis of RFS and may influence dissemination and tissue localization.
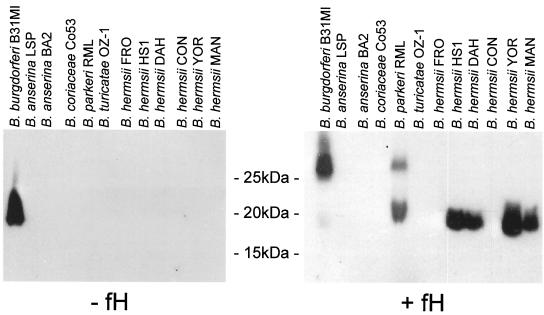
To determine if the RFS can bind fH and to identify potential fH binding proteins (FHBPs), an affinity ligand binding immunoblot assay was employed as previously described (12, 13). Briefly, whole-cell lysates of each isolate were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted. The immunoblots were incubated with purified hfH (Calbiochem), unbound fH was removed by washing with phosphate-buffered saline (PBS), and goat anti-hfH antiserum (Calbiochem; 1:800 dilution) was added to screen for fH bound to Borrelia proteins. Horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin G served as the secondary antibody (Pierce; 1:40,000). Several B. hermsii isolates (DAH, HS1, YOR, and MAN) and B. parkeri bound fH, while B. coriaceae (epizootic bovine abortion), B. anserina (avian borreliosis), B. turicatae, and the B. hermsii isolates CON and FRO did not (Table 1; Fig. 1). All fH binding isolates expressed an FHBP of ∼19 kDa (FHBP19). B. parkeri expressed a second, less dominant FHBP of ∼28 kDa (FHBP28). In contrast, the Lyme disease spirochetes produce multiple, variable FHBPs (2, 12). Here we demonstrate differences in fH binding phenotype among RFS species, a feature that may contribute to the different pathogenesis of individual RFS species. Most B. hermsii isolates and B. parkeri bind fH, while B. turicatae does not. B. hermsii and B. parkeri infections are typically characterized by high bacterial cell densities in the blood and an absence of central nervous system (CNS) invasion. In contrast, B. turicatae tends to infect the CNS. Residence in this niche may serve to protect B. turicatae from complement. It is important to note that the B. hermsii isolates CON and FRO, which were originally recovered from human blood, did not bind hfH. This is contrary to what might be expected for blood isolates. However, it is also important to note that FRO and CON were isolated in 1987 and in the 1960s (specific date unknown), respectively, and have since been passaged extensively in vitro. Exact passage histories for these strains are not known. It is possible that over the course of long-term maintenance in the lab, changes in genome composition (i.e., plasmid loss or genetic rearrangements) that influence the ability of these isolates to bind fH have occurred. Alternatively, this may also reflect natural variation among isolates of a species similar to that reported for the Lyme disease spirochete species, B. garinii and B. afzelii (1, 9, 11, 12, 16). Hence, intraspecies phenotypic variation among B. hermsii isolates is not unexpected.
TABLE 1.
Description of bacteria used in this study
| Isolate | Associated disease | Origin | fH binding |
|---|---|---|---|
| B. hermsii HS1 | TBRF | O. hermsii, Washington | + (FHBP19) |
| B. hermsii YOR | TBRF | Human blood, California | + (FHBP19) |
| B. hermsii CON | TBRF | Human blood, California | − |
| B. hermsii FRO | TBRF | Human blood, Washington | − |
| B. hermsii DAH | TBRF | Human blood, Washington | + (FHBP19) |
| B. hermsii MAN | TBRF | Human blood, California | + (FHBP19) |
| B. parkeri RML | TBRF | Ornithodoros parkeri, origin unknown | + (FHBP19 and FHBP28) |
| B. turicatae OZ-1 | TBRF | Ornithodoros turicata, Texas | − |
| B. coriaceae Co53 | Epizootic bovine abortion | Ornithodoros coriaceus, California | − |
| B. anserina BA2 | Avian borreliosis | Domestic chicken | − |
| B. anserina LSP | Avian borreliosis | Domestic chicken | − |
| B. burgdorferi B31MI | Lyme disease | Ixodes scapularis, New York | + (OspE and FHBP27) |
| B. burgdorferi BL224 | Lyme disease | Human blood isolate, New York | + (OspE and FHBP27) |
FIG. 1.
Immunoblot affinity ligand binding analysis; identification of human FHBPs in the RFS. Whole-cell lysates of each isolate (indicated above each lane) were generated, fractionated by SDS-PAGE, and immunoblotted. The ability of hfH to bind to the immobilized proteins was tested by the affinity ligand binding immunoblot approach as described in the text with saturating amounts of fH. All bacteria analyzed in panel A were cultivated at 33°C. A negative control in which hfH was not included in the assay is presented.
In this study, B. burgdorferi B31MI served as a control in all fH binding assays. B. burgdorferi produces multiple FHBPs that exhibit different fH binding properties. In an earlier study it was demonstrated that the OspE protein of the Lyme disease spirochetes is detected by the goat anti-hfH antisera even when hfH is not included in the affinity ligand fH binding assay (13). This is evident in Fig. 1 of this report. Possible explanations for this observation have been discussed in earlier publications from this lab (12, 13). The importance of this control and its bearing on the interpretation of data pertaining to the specificity of binding of fH to Borrelia proteins had largely been overlooked in earlier studies (14). In brief, fH is present in high concentrations in the sera of all mammals and by extension is a natural component of the goat anti-hfH antiserum used in the binding analyses described here. The detection of OspE-fH complexes even when exogenous hfH is not added in the assays presumably reflects the ability of OspE to bind to the endogenous goat fH. This complex is then detected by the goat anti-hfH antiserum. Although the goat anti-fH antiserum was generated by using hfH, we and others have shown that it recognizes fH from several mammalian species (14). This supports the suggestion that the antiserum recognizes goat fH bound to OspE. The OspE proteins which do not appear to exhibit species specificity with regard to fH binding have been designated as class I FHBPs. In contrast, the class II FHBPs of the Lyme spirochetes exhibit species specificity in their fH binding. These proteins bind hfH but not goat fH (12, 13). The FHBPs of the RFS exhibit binding characteristics that are similar to those of the class II FHBPs of the Lyme disease spirochetes in that they exhibit species specificity in their fH binding properties. The narrower fH binding specificity of the RFS in contrast to that of the Lyme disease spirochetes is consistent with the transmission biology of these bacteria. The RFS remain viable in ticks for several years. This feature enhances the potential for transmission of the spirochetes to mammals. In addition, the RFS can be transovarially transmitted in ticks. In contrast, the Lyme disease spirochetes are not transovarially transmitted and are maintained for relatively shorter periods in ticks. As a consequence, the Lyme spirochetes are highly dependent on the establishment of chronic infection in their mammalian reservoirs, and population maintenance in nature requires that the host range for these bacteria be rather broad. By producing multiple FHBPs that exhibit different binding specificities, the Lyme disease spirochetes can increase their potential host range. In contrast, the biological features of the RFS render them less dependent on mammalian reservoirs in general for population maintenance in nature. This could account for the more limited set of FHBPs produced by these bacteria.
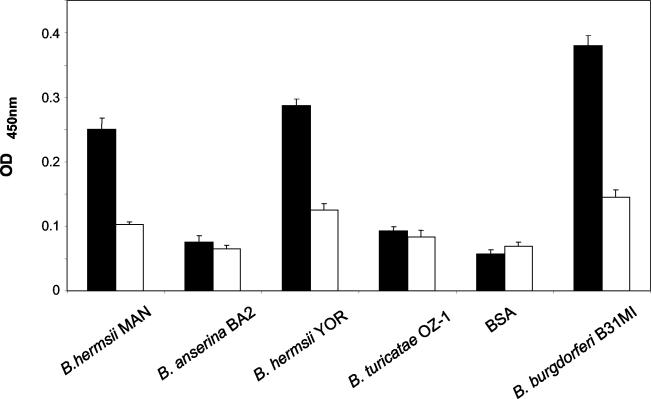
As a second approach to assess fH binding to the RFS, enzyme-linked immunosorbent assays (ELISAs) were conducted as previously described (12). Equivalent numbers of cells of each isolate analyzed were immobilized in the wells of a 96-well microtiter plate. hfH was added to each well (10 ng μl−1; 4°C, 15 h), unbound hfH was removed by washing with PBST (PBS with 0.02% Tween 20), goat anti-hfH antiserum was added (1:800, 4 h 4°C), and then horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin G secondary antibody was added (1:40;000, 1 h, 4°C). The one-step Turbo TMB ELISA kit (Pierce) was used for detection, and the plates were read in a Spectromax 400 plate reader. All samples were analyzed in triplicate, and averages and standard deviations are presented (Fig. 2). B. burgdorferi B31MI, which has previously been shown to bind fH in an ELISA format, served as the positive control. Immobilized bovine serum albumin served as the negative control. Consistent with the affinity ligand binding analyses, B. hermsii MAN and YOR bound fH while B. anserina and B. turicatae OZ1 did not.
FIG. 2.
Analysis of fH binding using an ELISA format. All methods were as described in the text. Bacteria were cultivated at 33°C, immobilized in the wells of ELISA plates, and incubated with fH (black bars) or without fH (white bars), and fH binding was assessed by using anti-fH antiserum.
The similarity in the sizes of FHBP19 of the RFS and the OspE proteins of the Lyme spirochetes raised the possibility that these proteins are genetically or antigenically related. To assess this possibility, an immunoblot of the RFS isolates was screened with polyclonal anti-OspE antisera (13). Except in the B. burgdorferi (positive) controls, immunoreactive proteins were not detected (data not shown). In addition, ospE-related sequences were not detected in the RFS by Southern hybridization with PCR-generated ospE probes (data not shown). Hence, it can be concluded that FHBP19 of the RFS is not an OspE homolog. Based on its lack of homology to OspE and its hfH binding specificity, FHBP19 can be classified as a class II FHBP.
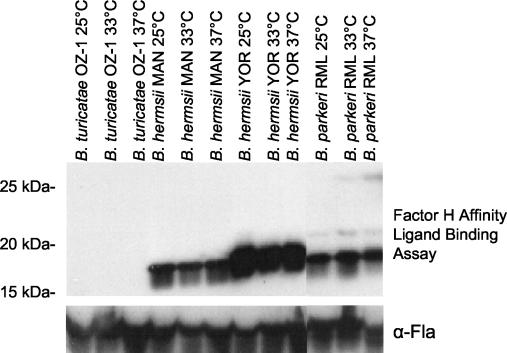
The expression of the OspE proteins (class I FHBPs) of the Lyme spirochetes has been demonstrated to increase with temperature (2). To determine if the expression of the FHBPs of the RFS is also influenced by temperature, affinity ligand binding immunoblot assays were performed with bacteria cultivated at 25, 33, or 36°C in Barbour-Stoenner-Kelly-H medium (12% rabbit sera). In these experiments, saturating amounts of hfH were employed (Fig. 3). As a control, the expression level of the constitutively expressed FlaB protein was compared in these bacteria through immunoblot analysis with rabbit anti-FlaB antisera (kindly provided by Michael Theisen). FlaB expression level was unaffected by growth conditions. Similarly, the relative amount of fH binding by the ∼19-kDa FHBPs of the RFS did not change with growth condition, indicating constitutive expression. However, a minor increase in expression of an FHBP of ∼28 kDa in B. parkeri was observed. The significance of this remains to be determined, as this protein appears to be expressed at a low level. Constitutive expression of most of the FHBPs of the RFS suggests that these proteins are likely to be expressed in ticks. Expression in ticks may serve to protect the spirochetes from the complement ingested by the tick as part of the blood meal.
FIG. 3.
Constitutive expression of the FHBP of the RFS. Bacteria were cultivated at 25, 33, and 37°C, harvested, and tested for fH binding by the affinity ligand binding immunoblot assay (top) as described in the text. Saturating amounts of fH were employed in the assay. An immunoblot of cell lysates screened with anti-Fla antiserum, which recognizes the constitutively expressed Fla protein, confirmed that the loadings in all lanes were equal (bottom).
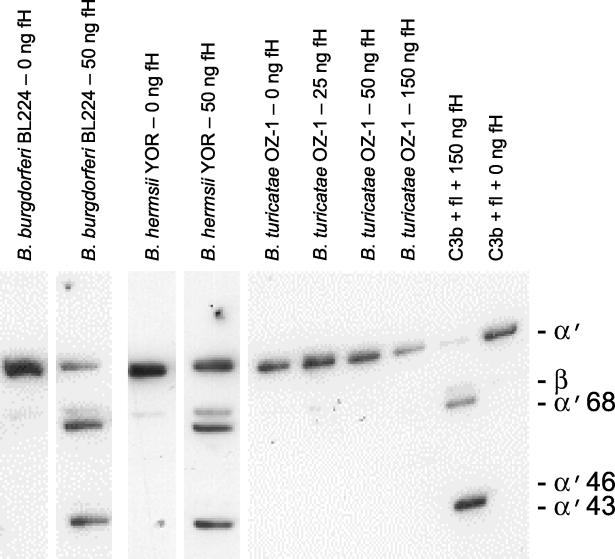
To assess the biological relevance of fH binding in the RFS, C3b cleavage assays were performed using a fH binder (B. hermsii YOR), a nonbinder (B. turicatae OZ1), and a B. burgdorferi positive control (BBL224). Cultures of each were pelleted, washed with ice-cold PBS, resuspended in cold RPMI medium (Gibco-BRL), and incubated with 0 or 50 ng of purified hfH (1 h, 37°C). To remove unbound hfH, the cells were washed with PBS. The cells were then resuspended in 15 μl of PBS, and fI (150 ng; Calbiochem) and C3b (250 ng; Calbiochem) were added. The mixture was incubated for 2 h at 37°C, 15 μl of SDS-PAGE sample buffer was added, and the samples were fractionated by SDS-PAGE, immunoblotted, and screened with anti-human C3b antiserum (Accurate; 1:800 dilution). Immunoblot methods were as previously described (12). As a positive control for the reaction conditions employed, purified fH (150 ng), fI (150 ng), and C3b (250 ng) were incubated together at 37°C in PBS for 2 h. Efficient cleavage of C3b was observed in the control but not when fH or fI was omitted (negative controls). B. hermsii YOR and the positive-control B. burgdorferi isolate cleaved C3b, while B. turicatae OZ-1 did not, even when threefold more fH (150 ng) was used in the assay (Fig. 4). The fH-fI mediated cleavage of C3b generated the characteristic C3b cleavage products, α′43 and α′68. This important observation indicates that surface-bound fH can participate in C3b cleavage and thus may play an important role in immune-system evasion during natural infection in mammals.
FIG. 4.
Demonstration of the ability of cell-bound fH to serve as a cofactor for the fI-mediated cleavage of C3b. The cleavage assay was conducted as described in the text, and the reaction products were fractionated by SDS-PAGE using a 15% acrylamide gel. The proteins were transferred to membranes and screened with anti-C3b antiserum.
In closing, this study presents the first demonstration that some RFS isolates bind fH and cleave C3b. The fH binding specificity of the FHBPs of the RFS is similar to that of the class II FHBPs of the Lyme spirochetes. As discussed above, the B. turicatae OZ-1 isolate tested here did not bind fH. It is tempting to speculate that this phenotype may be of relevance to the CNS localization of this species, as has been hypothesized for the potentially neurotropic Lyme disease spirochete species B. garinii (12, 13). Unfortunately, additional isolates of B. turicatae are not currently available, and therefore it is premature to draw conclusions about the fH binding ability of this species as a whole. Future studies will seek to test the hypothesis that fH binding phenotype influences the pathogenesis and tissue tropism of Borrelia species.
REFERENCES
- 1.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo, A., M. T. H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G. 1987. Immunobiology of relapsing fever. Contrib. Microbiol. Immunol. 8:125-137. [PubMed] [Google Scholar]
- 4.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., S. L. Tessier, and H. G. Stoenner. 1982. Variable major proteins of Borrelia hermsii. J. Exp. Med. 156:1312-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friese, M. A., J. Hellwage, T. S. Jokiranta, S. Meri, H. H. Peter, H. Eibel, and P. F. Zipfel. 1999. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differentially expressed and regulated. Mol. Immunol. 36:809-818. [DOI] [PubMed] [Google Scholar]
- 7.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 8.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 9.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:3393-3401. [DOI] [PubMed] [Google Scholar]
- 10.Kraiczy, P., C. Skerka, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtenbach, K., H.-S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metts, S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in the binding of factor H and OspE targeting antibodies elicited during infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]