Abstract
Objective
To investigate the role of early magnetic resonance imaging (MRI) of the wrist in predicting functional outcome in rheumatoid arthritis.
Methods
MRI scans of the dominant wrist were scored for synovitis, tendon inflammation, bone oedema, and erosion at first presentation (n = 42), at 1 year (n = 42), and at 6 years (n = 31). At 8 years, clinical reassessment (n = 28) was undertaken. Tendon function was graded 0–3 for movement, tendon sheath swelling, and pain on resistance at nine flexor and extensor tendons of the hand. Hand function was also assessed using the Sollerman grip test. The requirement for joint or tendon surgery by 8 years was determined by telephone survey in 39 of the original 42 patients.
Results
At 8 years, tendon function was highly correlated with hand function (Sollerman score, R = −0.51, p = 0.005) and global function (health assessment questionnaire score, R = 0.53, p = 0.004). Using a model incorporating baseline and 1 year MRI scores, the MRI bone oedema score was strongly predictive of tendon function at 8 years (χ22 = 15.3, p = 0.0005), as was the MRI bone erosion score (χ22 = 9.23, p = 0.01). Hand function was also predicted by the baseline MRI erosion score (p = 0.02). MRI variables did not predict the requirement for surgery, but patients who had surgery were more likely to show progression of MRI bone erosion scores between baseline and 1 year (p = 0.008).
Conclusions
Extensive MRI bone oedema and erosions at the wrist in early rheumatoid arthritis predict tendon dysfunction and impaired hand function in the medium term but not the requirement for joint or tendon surgery.
Keywords: magnetic resonance imaging, rheumatoid arthritis, functional outcome
Functional outcome in rheumatoid arthritis is influenced by the extent of structural damage to bones, joints, and tendons plus the severity of joint inflammation.1 It has been estimated that 90% of patients will develop some form of functional disability, and in around 65% this will be moderate to severe.2,3 Hand function is liable to be affected by inflammation and erosion at the small joints of the hand and by tendon rupture. The latter will influence pinch and power grip, resulting in limitation of hand movement and power. Predictors of global function have been identified in rheumatoid arthritis and include indicators of disease activity such as high erythrocyte sedimentation rate (ESR) and C reactive protein levels4,5 but there have been no studies investigating specific predictors of hand or tendon function.
Magnetic resonance imaging (MRI) is emerging as an important imaging tool in rheumatoid arthritis. It combines the ability to image structural damage such as bony erosion and tendon rupture with excellent soft tissue resolution, revealing the presence of inflammation in the synovium, tenosynovium, and probably the periarticular bone.6 We have reported that early MRI evidence of bone marrow oedema at the carpus is a predictor of global function in the medium term, as measured by the physical function component of the medical outcomes short form 36 (PF SF‐36).7 We have also recently shown that MRI evidence of tendinopathy is a predictor of tendon rupture after six years.8 In this study, we recalled the same patients at eight years to assess outcome in terms of tendon function in the dominant hand. We compared this with hand function, as measured by the Sollerman grip test,9 and with measures of global function. We then used data obtained prospectively in these patients to determine whether tendon function at eight years was predicted by abnormalities on early MRI. We also examined data for predictors of the requirement for orthopaedic surgery in this cohort at eight years.
Methods
Patient population and clinical assessments
Recruitment, demographics, and assessment of this patient population have been described previously.10 Briefly, patients with a diagnosis of rheumatoid arthritis11 were recruited between 1994 and 1996 from an early arthritis clinic. Approval for this study was granted by the Auckland ethics committee. The inception cohort numbered 42 patients, but because of patient drop out, full clinical data were available for only 28 patients at eight years (in 2004). At eight years, the median age of the study cohort was 55 years (range 39 to 78), 65% being female. Their demographic variables and drug treatment profiles are shown in table 1. For information regarding surgical outcome, attempts were made to contact all patients from the inception cohort by telephone. Three had emigrated and could not be contacted, but a surgical history (of orthopaedic procedures related to rheumatoid arthritis) was obtained in 39 of the original 42 patients. This included one patient who had died before this eight year follow up study took place but for whom a review of the clinical records provided the necessary information.
Table 1 Demographic, clinical, and drug treatment profiles of patients with rheumatoid arthritis at eight years (n = 28).
| Female : male (n) | 19 : 9 | |
| Age at 8 year follow up (y) (median (range)) | 55 (39 to 78) | |
| European : Maori : Pacific Islander (n) | 22 : 5 : 1 | |
| Functional assessments (median (range)) | ||
| Sollerman score | 72.5 (64 to 80) | |
| HAQ score | 0.27 (0 to 1.75) | |
| PF SF‐36 score | 67.5 (0 to 100) | |
| DAS score | 2.21 (0.58 to 5.47) | |
| Tendon function score | 1 (0 to 17) | |
| Disease activity (median (range)) | ||
| ESR (mm/h) | 26 (2 to 104) | |
| C reactive protein (mg/l) | 5.7 (0.1 to 78.7) | |
| Swollen joint count | 3 (0 to 25) | |
| Tender joint count | 3 (0 to 32) | |
| Drug treatment (n (%)) | ||
| NSAIDs | 15 (54%) | |
| DMARDs | 17 (61%) | |
| hydroxychloroquine | 4 | |
| sulfasalazine | 5 | |
| methotrexate | 10 | |
| leflunomide | 2 | |
| Ever DMARD use | 25 (89%) | |
| Cytotoxic agents | 3 (11%) | |
| Prednisone | 9 (32%) | |
| Steroids | 5 (18%) | |
| Analgesics | 3 (11%) |
DAS, disease activity score; DMARD, disease modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; NSAID, non‐steroidal anti‐inflammatory drug; PF SF‐36, physical function component of the medical outcomes short form 36 item health survey; RF, rheumatoid factor; y, years.
MRI and radiography
MRI scans of the dominant wrist were obtained at first presentation (baseline), then again at one and six years using a 1.5 Tesla MR scanner (GE Signa Horizon) as described previously.10 The small field of view (8 cm) was chosen to optimise resolution, and included the distal radioulnar, radiocarpal, and mid‐carpal joints as well as the metacarpal bases, but did not include the metacarpo‐phalangeal joints. T1 weighted pre‐ and post‐contrast (Gd‐DTPA) sequences were done in coronal and axial planes, interrupted by a T2 weighted axial fat suppressed fast spin echo sequence for better definition of bone marrow oedema. All MR images were assigned a score for synovitis, tendonitis, bone oedema, and bone erosion by two musculoskeletal radiologists in a blinded fashion. The two sets of values were averaged to obtain the final scores. Conventional radiography of the hands and feet was also done and scored for erosion and joint space narrowing using the Sharp‐van der Heidje system, as previously described.12
Assessments of disease activity and global function
Disease activity measures—including 66 and 68 swollen and tender joint counts, analogue pain scores, physician and patient global assessments, and C reactive protein and ESR—were obtained. The three‐variable disease activity score (DAS) was derived according to the method described by van der Heidje et al.13 Patients were asked to complete self‐administered questionnaires including the Stanford health assessment questionnaire (HAQ)14 and the SF‐36 survey.15
Tendon integrity and function score
Nine extensor and flexor tendons of the dominant hand, including extensor pollicis longus and brevis, extensor carpi radialis and ulnaris, extensor digitorum and indicis, extensor digiti minimi, flexor carpi radialis and ulnaris, and flexor digitorum superficialis and profundus were scored 0–3 for ability to perform a dictated manoeuvre (function), tendon sheath swelling, and pain upon resistance, to give a maximum (worst) possible score of 27 for all tendons. Where tendon rupture was confirmed clinically or according to surgical notes, the full disability score for that tendon was assigned.
Hand function assessments
Hand function was measured using the Sollerman grip function test9 which examines seven of the most commonly used hand grips, unilaterally and bilaterally, by asking patients to complete a series of 20 tasks within a given time. Figure 1 shows the Sollerman test kit and a sample activity. Pinch and volar grip capabilities were tested with simple activities such as turning a door handle, picking up coins, using a screwdriver, and doing up a zip. A score of 0–4 was given depending on the time and ease with which each task was completed, with a total score out of 80 (maximum score indicating full hand function).
Figure 1 Photograph of a patient undertaking one of the tasks in the Sollerman grip function test, showing his ability to use a screwdriver to turn a screw into the board.
Statistical analysis
A Spearman's correlation was carried out on the eight year tendon function, Sollerman, and HAQ scores to assess correlations between these functional measures. The distribution of the eight year tendon function score was such that parametric methods were not applicable; thus it was categorised into 0, 0–5, and >5. Logistic ordinal regression models (for the categorised tendon score) and multiple linear regression models (for Sollerman scores) were used to investigate whether MRI scores for bone oedema, bone erosion, tendonitis, and synovitis at baseline and one year predicted functional scores at eight years. Statistical models incorporated baseline variables alone initially, and then year 1 variables were added to investigate whether predictive ability was improved. To determine whether there was an association between six year MRI tendinopathy and eight year tendon function at each site, scores were reduced to present or absent and a generalised linear model with a logit link was used. For surgical outcomes, patients were separated into two sets of two groups: + or − for joint replacement surgery, and + or − for general rheumatoid arthritis related surgery (including tendon repair). Logistic regression was then used to investigate the association between early MRI scores and the probability of having surgery.
Results
Tendon function correlates with hand function and HAQ scores
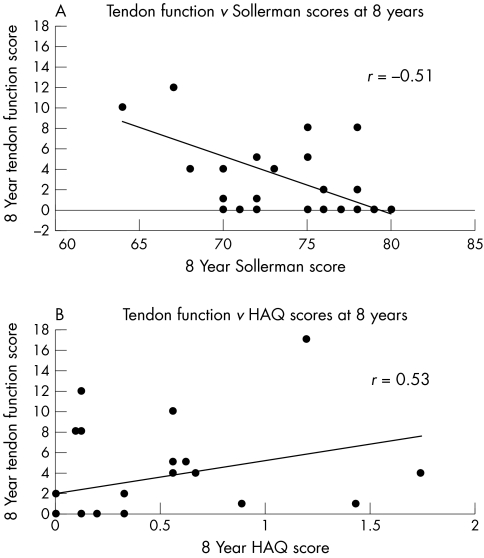
Each of the nine flexor and extensor tendons at the wrist was examined and scored for pain on movement, swelling (of the tendon sheath), and ability to perform a dictated manoeuvre. Scores were then summed to give a total tendon function score at eight years. Tendon function scores ranged from 0 (normal) to 17 in the 28 patients examined, and were highly correlated with scores for hand function (Sollerman: R = −0.51, p = 0.005) and global function (HAQ: R = 0.53, p = 0.004) (fig 2).
Figure 2 (A) Correlation between the tendon function score and hand function (Sollerman score) (R = −0.51, p = 0.005). (B) Correlation between the tendon function score and global function (HAQ score) (R = 0.53, p = 0.004). HAQ, health assessment questionnaire.
MRI bone oedema strongly predicts tendon function
Using a χ2 model, we investigated whether early MRI scores (quantifying synovitis, tendinopathy, bone oedema, and bone erosion at the wrist) could predict eight year tendon function. When the model included only baseline MRI variables, none of the individual measures was predictive (p>0.2 for all). However, when baseline and one year values were included, the MRI bone oedema score was strongly predictive of function (χ22 = 15.3, p = 0.0005) (table 2). The MRI bone erosion score was also predictive (χ22 = 9.23, p = 0.01), and there was a trend towards prediction by MRI tendonitis and synovitis scores (χ22 = 5.38, p = 0.07 and χ22 = 5.03, p = 0.08, respectively). When all baseline and one year MRI variables were used in the model, 61.6% of the variability of the eight year tendon function data were predicted.
Table 2 Predictors of tendon function and hand function at eight years.
| Variables* | Tendon | Sollerman hand | ||
|---|---|---|---|---|
| function score | function score | |||
| Statistic | p Value | Statistic | p Value | |
| MR bone oedema | χ22 = 15.3 | 0.0005 | F2,19 = 0.33 | 0.7 |
| MR bone erosion | χ22 = 9.23 | 0.01 | F2,19 = 0.84 | 0.4 |
| MR tendonitis | χ22 = 5.38 | 0.07 | F2,19 = 0.02 | 0.97 |
| MR synovitis | χ22 = 5.03 | 0.08 | F2,19 = 0.26 | 0.7 |
| All MR variables | R2 = 0.616 | R2 = 0.261 | ||
*Model includes MR variables at baseline and one year.
MR, magnetic resonance.
A similar analysis was undertaken using the eight year Sollerman hand function score as an outcome measure. When all baseline and one year MRI scores were included in the model, 26% of the variability of the eight year Sollerman score was predicted but no single MRI variable reached significance (table 2). When baseline MRI variables were tested individually, the MRI bone erosion score was predictive of the eight year Sollerman score (F1,26 = 6.20, p = 0.02) but when all baseline MRI variables were included in the model, significance was lost, as all variables were highly correlated with each other. Interestingly, the Sharp score from baseline was not predictive of eight year tendon function or hand function (p>0.2).
MRI tendinopathy at six years was not associated with tendon function at eight years
Data were available from a previous study of this cohort documenting MRI evidence for tendinopathy at six years.8 No association was found between the six year MRI tendinopathy score and the eight year tendon function score when this was examined at individual tendon sites. This result may have been influenced by the effects of surgery as five patients had had tenosynovectomy or tendon transfer operations by the eight year time point (table 3).
Table 3 Demographic, clinical, and surgical details of 12 patients requiring orthopaedic surgery by eight years.
| Patient No/ | Baseline clinical scores | Baseline MRI scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| age (y)/sex | HAQ | DAS | ESR | CRP | Sw/Td jnt | Tendonitis | Synovitis | Bone erosion | Bone oedema |
| 21/46/F | 1.3 | 4.77 | 41 | 27 | 16/32 | 3 | 4.5 | 0 | 0.5 |
| 27/45/M | 0.1 | 2.88 | 57 | 10 | 6/4 | 2.5 | 15.5 | 0 | 0 |
| 34/49/F | 0.7 | 4.74 | 46 | 19 | 18/26 | 2.5 | 8 | 1.5 | 2 |
| 36/46/M | 0 | 4.24 | 35 | 18 | 15/17 | 3 | 0 | 0.5 | 0 |
| 39/68/F | 0.6 | 4.01 | 30 | 9 | 10/26 | 0.5 | 6 | 2 | 6 |
| 41/81/F | 1.8 | 5.16 | 103 | 16 | 11/29 | 1 | 4 | 0 | 0 |
| 47/58/F | 0.7 | 4.02 | 22 | 39 | 14/16 | 1 | 6 | 0.5 | 0 |
| 58/51/F | 1.6 | 7.57 | 131 | 23 | 38/44 | 2.5 | 7 | 3 | 8 |
| 61/62/M | 1.3 | 4.31 | 27 | 60 | 12/25 | 1 | 14.5 | 9 | 9 |
| 62/60/F | 1.1 | 5.97 | 73 | 53 | 22/42 | 2 | 8 | 5.5 | 0.5 |
| 68/71/F | 1 | 4.15 | 79 | 99 | 5/13 | 1 | 12.5 | 1.5 | 3.5 |
| 73/72/F | 0 | 4.87 | 100 | 66 | 22/18 | 3 | 12.5 | 8.5 | 1.5 |
Type of surgical procedure:
Patient 21: Left flexor synovectomy.
Patient 27: Right wrist fusion + Sauve Kapanji procedure.
Patient 34: Right knee yttrium synovectomy + later TKJR.
Patient 36: Lateral arthrotomy right elbow + synovectomy; capsular release; radial head excision; posterior arthrotomy + olecranon osteotomy + synovectomy.
Patient 39: Left THJR.
Patient 41: TKJR, failed with sepsis proceeded to arthrodesis.
Patient 47: Right wrist Steinman pin fusion.
Patient 58: Dorsal tenosynovectomy; synovectomy distal radioulnar joint; EI to EPL tendon transfer; Sauve Kapandji procedure left wrist; right THJR.
Patient 61: Right wrist fusion; dorsal tenosynovectomy + reconstruction of extensor tendon right wrist; Darrach's procedure left wrist; dorsal extensor tenosynovectomy left wrist
Patient 62: Carpal tunnel release/exploration; fusion of IPJ of right thumb; dorsal extensor synovectomy of right wrist.
Patient 68: Multiple joint replacements including fingers, hip, elbow, and shoulder.
Patient 73: Left dorsal tenosynovectomy + flexor tenosynovectomy for little, ring, and middle fingers
CRP, C reactive protein; DAS, disease activity score; EI, extensor indicis; EPL, extensor pollicis longus; ESR, erythrocyte sedimentation rate; F, female; HAQ, health assessment questionnaire; IPJ, interphalangeal joint; M, male; MRI, magnetic resonance imaging; Sw/Td jnt, swollen and tender joint scores; THJR, total hip joint replacement; TKJR, total knee joint replacement; y, years.
Prediction of orthopaedic outcome
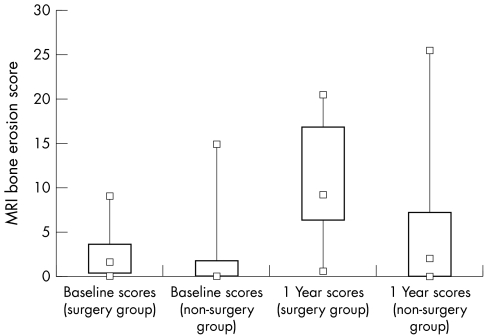
Twelve of the 39 patients (31%) for whom surgical data were available had undergone orthopaedic rheumatoid arthritis related surgery by eight years, 10 having had joint replacement operations. A description of their demographics, baseline clinical and MRI scores, and orthopaedic procedures is given in table 3. There was a greater increase in the median bone erosion score from baseline to one year in the surgery group than in the non‐surgery group (2.0 v 0.0 at baseline and 9.0 v 2.5 at year 1, p = 0.008) (fig 3). However, no single MRI measure was capable of predicting either joint replacement or general rheumatoid arthritis related surgery at eight years (p>0.2 for all).
Figure 3 MRI bone erosion score at baseline and one year for surgery and non‐surgery groups. Top and bottom squares joined by lines indicate highest and lowest values; large rectangular box marks out the 25th and 75th centiles; small box within represents the median value. The median MRI bone erosion score increased more from baseline to one year in the surgery group than in the non‐surgery group (2.0 v 0 at baseline and 9.0 v 2.5 at year 1 follow up, p = 0.008). MRI, magnetic resonance imaging.
Discussion
Hand function is an important aspect of global function, influencing more than half the activities mentioned in the HAQ score. In an orthopaedic population, loss of hand function has been directly linked to loss of earning capacity, emphasising its significance as an indicator of disability.16 Tendon function at the wrist and hand has not been explored as an outcome measure in rheumatoid arthritis, but tendon rupture clearly has a significant impact on function, and a previous study on this cohort showed that rupture had occurred in four of 34 patients (12%) by six years.8 The current analysis deals with outcome at eight years, by which time the number of patients in our cohort available for examination had fallen to 28 from the original 42. We quantified tendon function at the dominant hand by devising a score that measured tendon inflammation, integrity, and function and used the same tendon groups as had been examined on MRI scans taken previously.8,10 The Sollerman grip test was selected as a measure of hand function as it was designed to reflect the commonest grips used in daily activities, has established validity and reliability in rheumatoid arthritis, and focuses on the hand.17
We found that the eight year tendon function score was strongly predicted by the MRI bone oedema score derived from baseline and one year scans, and to a lesser extent by the MRI bone erosion score. The bone oedema score has stood out as a predictor of adverse outcome in several studies from this cohort of patients, at different stages of disease and using different outcome measures. A site specific analysis showed that bone oedema was a pre‐erosive change at one year18 and six years.12 The baseline bone oedema score was also a predictor of radiographic progression (Δ Sharp score)12 and of global functional outcome (PF SF‐36) in these patients at six years.7 Despite a loss of power for the current analysis with attrition of patient numbers by eight years, the bone oedema score still figures as the most important predictor of outcome, this time measured by the tendon function score. Our studies and those of others have revealed that MRI bone oedema is common in early rheumatoid arthritis and potentially affects many joints.10,19,20 Its histopathological equivalent has yet to be determined but several lines of evidence suggest it may represent an inflammatory infiltrate in subchondral bone.21 If so, it may be a marker for that subset of patients who develop ongoing active joint inflammation, affecting bone and soft tissues such as tendons. Persistent tenosynovitis has been linked to a late risk of tendon rupture.22 Additionally, the role of bone oedema as a harbinger of erosions could be linked to tendon dysfunction, as the other mechanism postulated for tendon rupture in rheumatoid arthritis is friction of the tendon against sharp eroded bony surfaces.22
Results were not as positive for prediction of hand function, as measured by the Sollerman grip test. There was a significant association between the baseline MRI bone erosion score and the eight year Sollerman score when individual MRI measures were examined, but this disappeared when all MRI scores were included in the model owing to the interrelatedness of the variables. However, the combination of all MRI scores at baseline and one year did predict 26% of the variability of the eight year hand function score. One possible reason for the disparity between tendon function and hand function as outcome measures in this analysis would be that hand function had been influenced by surgical procedures in many patients by eight years. For example, patient No 58 underwent extensive surgery to the wrist in 1997, including tenosynovectomy and a tendon transfer procedure rerouting the extensor indicis to extensor pollicis longus (which had been ruptured). Her hand function at eight years was relatively good (Sollerman 75/80) but as tendon rupture had been confirmed at surgery her tendon function score (for EPL) was high. A similar explanation may account for our inability to find an association between the six year MRI tendinopathy and eight year tendon function scores at individual sites.
Joint replacement surgery is eventually required (within 22 years of onset) in 25% of rheumatoid patients.23 Wolfe et al found a series of variables reflecting disease activity were predictive of total joint arthroplasty and these included the ESR, global severity, and HAQ score. We examined surgical outcome in our cohort at eight years and found that 12 of 39 patients (31%) had had some form of rheumatoid arthritis related orthopaedic surgery, including 10 (26%) who had had joint replacement or arthrodesis. We consider our cohort to represent the spectrum of patients with more severe disease as they were enrolled on the basis of fulfilling ACR criteria for rheumatoid arthritis at presentation, when symptoms had been present for a median of four months. In very early rheumatoid arthritis, these criteria are known to be relatively insensitive and to select for a more severe disease population.24 We found no evidence that MRI variables reflecting the extent of joint erosion and inflammation in early disease could predict surgery at the eight year point. Unfortunately, it is difficult to interpret this result as the small size of our cohort would preclude detection of anything less than a very major effect. Furthermore, the eight year point is relatively early in terms of joint replacement surgery, and studying the same cohort at 15 and 20 years might be necessary to obtain a true picture of this outcome measure. However, there was clear evidence that those in the surgical group had faster progression of MRI erosive change from baseline to one year, indicating that they were destined for more severe articular damage in the long term.
Conclusions
We have presented data suggesting that MRI bone oedema, bone erosion, and to a lesser extent synovitis and tendonitis detected at the wrist in early rheumatoid arthritis have prognostic significance in terms of hand and specifically tendon function in the medium term. Studies conducted over longer periods including larger numbers of patients are required to determine whether early MRI can help to predict surgical outcome.
Acknowledgements
We wish to acknowledge the assistance of the following clinicians who have referred patients for this study: Dr Mike Butler, Dr David Caughey, Dr Nora Lynch, Dr Alan Doube, Dr Hamish Hart, Dr Peter Gow, Dr Raoul Stuart, Dr Terry Macedo, Dr Max Robertson, Dr Roger Reynolds, and Dr Bob Grigor. The Megan Wynn Trust for Rheumatology Research provided funds for a University of Auckland summer studentship for SZ.
References
- 1.Scott D L, Pugner K, Kaarela K, Doyle D V, Woolf A, Holmes J.et al The links between joint damage and disability in rheumatoid arthritis. Rheumatology 200039122–132. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan E, Tugwell P, Fries J F. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI). Arthritis Res Ther 20046R505–R513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce B, Fries J F. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakala M, Nieminen P, Koivisto O. More evidence from a community based series of better outcome in rheumatoid arthritis. Data on the effect of multidisciplinary care on the retention of functional ability. J Rheumatol 1994211432–1437. [PubMed] [Google Scholar]
- 5.Smolen J S, Aletaha D. Patients with rheumatoid arthritis in clinical care. Ann Rheum Dis 200463221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterfy C G. MRI of the wrist in early rheumatoid arthritis. Ann Rheum Dis 200463473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benton N, Stewart N, Crabbe J, Robinson E, Yeoman S, McQueen F M. MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann Rheum Dis 200463555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQueen F M, Beckley V, Crabbe J, Robinson E, Yeoman S, Stewart N. Magnetic resonance imaging evidence of tendinopathy in early RA predicts tendon rupture at 6 years. Arthritis Rheum 200552744–751. [DOI] [PubMed] [Google Scholar]
- 9.Sollerman C, Ejeskär A. Sollerman Hand Function Test. A standardised method and its use in tetraplegic patients. Scand J Plast Reconstr Surg Hand Surg 199529167–176. [DOI] [PubMed] [Google Scholar]
- 10.McQueen F M, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan P L.et al Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom‐onset. Ann Rheum Dis 199857350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The ARA 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 12.McQueen F M, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S.et al Bone oedema scored on magnetic resonance scans of the dominant carpus at presentation predicts radiographic joint damage at the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum 2003481814–1827. [DOI] [PubMed] [Google Scholar]
- 13.van der Heijde D M, van't Hof M, van Riel P L, van de Putte L B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 199320579–581. [PubMed] [Google Scholar]
- 14.Fries J F, Spitz P, Kraines R G, Holman H R. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 15.Ware J E, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 16.Hung L K, Ho K K, Leung P C. Impairment of hand function and loss of earning capacity after occupational hand injury: prospective cohort study. Hong Kong Med J 19995245–250. [PubMed] [Google Scholar]
- 17.O'Connor D, Kortman B, Smith A, Ahern M, Smith M, Krishnan J. Correlation between objective and subjective measures of hand function in patients with rheumatoid arthritis. J Hand Ther 199912323–329. [DOI] [PubMed] [Google Scholar]
- 18.McQueen F M, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan P L.et al Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis 199958156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostendorf B, Scherer A, Modder U, Schneider M. Diagnostic value of magnetic resonance imaging of the forefeet in early rheumatoid arthritis when findings on imaging of the metacarpophalageal joints of the hands remain normal. Arthritis Rheum 2004502094–2102. [DOI] [PubMed] [Google Scholar]
- 20.Savnik A, Malmskov H, Thomsen H S, Graff L B, Nielsen H, Danneskiold‐Samsoe B.et al MRI of the wrist and finger joints in inflammatory joint diseases at 1‐yr interval: MRI features to predict bone erosions. Eur Radiol 2002121203–1210. [DOI] [PubMed] [Google Scholar]
- 21.McQueen F M, Robinson E. The synovium in rheumatoid arthritis: evidence for (at least) two pathologies: comment on the article by Kirwan JR. Arthritis Rheum 2004503734–3735.15529382 [Google Scholar]
- 22.Moore J R, Weiland A, Valdata L. Tendon ruptures in the rheumatoid hand: analysis of treatment and functional results in 60 patients. J Hand Surg 1987129–14. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Zwillich S H. The long‐term outcomes of rheumatoid arthritis: a 23‐year prospective, longitudinal study of total joint replacement and its predictors in 1600 patients with rheumatoid arthritis. Arthritis Rheum 1998411072–1082. [DOI] [PubMed] [Google Scholar]
- 24.Green M, Marzo‐Ortega H, McGonagle D, Wakefield R, Proudman S, Conaghan P.et al Persistence of mild, early inflammatory arthritis: the importance of disease duration, rheumatoid factor, and the shared epitope. Arthritis Rheum 1999422184–2188. [DOI] [PubMed] [Google Scholar]