Abstract
Whether arthropod vectors retain competence for transmission of infectious agents in the long-term absence of vector-pathogen interaction is unknown. We addressed this question by quantifying the vector competence of two tick vectors, with mutually exclusive tropical- versus temperate-region distributions, for genetically distinct tropical- and temperate-region strains of the cattle pathogen Anaplasma marginale. The tropical cattle tick Boophilus microplus, which has been eradicated from the continental United States for over 60 years, was able to acquire and transmit the temperate St. Maries (Idaho) strain of A. marginale. Similarly, the temperate-region tick Dermacentor andersoni efficiently acquired and transmitted the Puerto Rico strain of A. marginale. There were no significant quantitative differences in infection rate or number of organisms per tick following feeding on cattle with persistent infections of either A. marginale strain. In contrast, the significantly enhanced replication of the Puerto Rico strain in the salivary gland of B. microplus at the time of transmission feeding is consistent with adaptation of a pathogen strain to its available vector. However, the transmission of both strains by B. microplus demonstrates that adaptation or continual interaction between the pathogen and vector is not required for retention of vector competence. Importantly, the results clearly show that reestablishment of acaricide-resistant B. microplus in the United States would be associated with A. marginale transmission.
Anaplasma marginale is the most globally prevalent tick-borne pathogen of cattle, with regions of endemicity on the six populated continents (22). Although A. marginale has a global distribution, the prevalence and incidence are highest in regions where the tropical cattle fever tick Boophilus microplus is endemic (19, 21, 25, 28). Larval, nymphal, and adult stages of this tick all preferentially feed on cattle, and each can efficiently acquire and transmit A. marginale (1, 24). This high vectorial capacity of B. microplus results in most calves in subtropical and tropical regions being infected within the first year of life, and this high incidence represents a severe constraint on animal health and production (11). Remarkably, B. microplus was eradicated from the continental United States by a mandatory acaricide-based program initiated in 1906, and eradication status is maintained by required acaricide treatment of cattle entering or resident within a quarantine zone along the U.S.-Mexico border (10). Since Boophilus eradication, tick transmission of A. marginale in the United States is mediated by Dermacentor andersoni and Dermacentor variabilis, species with less vectorial capacity because larval and nymphal stages prefer small mammals and only the adults acquire and transmit by feeding on cattle (27). Corresponding with this change in the vector species, A. marginale prevalence in the United States since B. microplus eradication is markedly lower than in regions where B. microplus is the primary vector (17, 30, 32).
The likelihood of B. microplus reestablishing its previous range in the U.S. has been markedly increased by the emergence in Mexico of ticks resistant to multiple acaricides, including those used to maintain the 80-mile quarantine region (3, 4). Reestablishment of B. microplus, with its high vectorial capacity for A. marginale, threatens U.S. cattle herds with no or minimal levels of immunity due to the low transmission rate associated with Dermacentor spp. (17, 23, 30). Assessment of this risk requires determining whether B. microplus has retained vector competence: that is the ability to acquire and transmit to the United States temperate-region strains of A. marginale in the long-term absence of pathogen-vector interaction. A. marginale strains have well-characterized genotypic and phenotypic differences (23): notably, strains naturally transmitted by B. microplus form a distinct genetically defined clade as compared to temperate-region strains transmitted by Dermacentor spp. (5). In the 60 years since eradication of the cattle fever tick in the 1940s, any A. marginale strains dependent upon tick transmission by B. microplus would have been lost from the cattle population, as is suggested by the distinct clade structures.
The ability of the tick vector to acquire A. marginale from a persistently infected animal is influenced by the level of rickettsemia during feeding, and not all fed ticks become infected (7). The failure of the ticks to become infected appears to occur at the level of the midgut (26), consistent with the importance of a midgut barrier to infection. Successful acquisition of A. marginale, subsequent to receptor-mediated invasion of the midgut epithelium (5), is thus a first determinant of vector competence. Whether the efficiency of initial acquisition differs among competent vectors of A. marginale is unknown; however, a markedly reduced ability of Boophilus to acquire temperate-region strains of A. marginale during feeding on persistently infected cattle would result in the reduction or loss of vector competence. Following invasion of the midgut epithelium, A. marginale undergoes initial intracellular replication in the midgut epithelial cells and progresses to invasion of the salivary glands (14). Within the salivary glands, a second round of replication occurs and culminates in development of infectious organisms during transmission feeding (15, 18, 26). In adult male D. andersoni ticks infected with the temperate-region South Idaho strain of A. marginale, replication continues during the first 72 h of transmission feeding, reaching approximately 105 organisms per salivary gland (18). This replication is required for transmission, and the levels influence transmission efficiency (9). As a result, differences among vectors in pathogen replication in the salivary gland would be expected to have a significant impact on transmission. Thus, two critical determinants of vector competence are the ability of the ticks to acquire the infection and pathogen replication within the tick salivary gland. In the present study, we examine these two determinants of vector competence for B. microplus and D. andersoni fed on cattle persistently infected with either a tropical-region strain or a temperate-region strain and determine whether pathogen transmission by each vector is restricted to the genetically and geographically defined A. marginale clades.
MATERIALS AND METHODS
Pathogen and vector strains.
B. microplus and D. andersoni naturally transmit the Puerto Rico and St. Maries (Idaho) strains of A. marginale, respectively. The sequence of the St. Maries msp4 gene assigns it to a clade of temperate-region strains (6), while the sequence of the Puerto Rico msp4 gene (obtained from infected ticks [GenBank accession no. AY191827]) places it within a clade of seven strains from regions of B. microplus endemicity as reported by de la Fuente et al. (6). The La Minita strain of B. microplus and the Reynolds Creek strain of D. andersoni, free of A. marginale and other pathogens, were maintained at the USDA Agricultural Research Service tick unit in Moscow, Idaho. Larvae were fed for 14 to 16 days on calves to produce fully engorged nymphs. Once the nymphs engorged, they were removed and induced to molt to an adult stage within 48 h at 26°C and 92% relative humidity. Adult males were used for acquisition feeding, because terminally developed male D. andersoni and B. microplus ticks feed more efficiently than either nymphs or adult females and are at an epidemiologically relevant stage for transmission by both vectors (16).
Acquisition feeding on persistently infected cattle.
Persistent infection is defined by control of the acute rickettsemia and maintenance of rickettsemia levels of ≤106 infected erythrocytes per ml of blood (7, 8, 12). To develop carriers for tick feeding, Holstein calves (8 to 12 months of age) confirmed to be A. marginale negative by the msp5 competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA) (13, 28) were each inoculated intravenously with 109 organisms of either the Puerto Rico (calves 908 and 929) or St. Maries (calves 928 and 931) strain of A. marginale. During acute infection, Giemsa-stained blood smears were examined daily, following resolution of the acute stage, and weekly during persistence. Ticks were fed only after no infected erythrocytes had been observed by light microscopy (minimum of 15,000 erythrocytes examined) for 3 consecutive weeks, consistent with a rickettsemia level of ≤106 organisms per ml of infected blood. The actual rickettsemia level during acquisition feeding was determined by quantitative real-time PCR (see the following section). The rickettsemia level on each day, expressed as mean log10 (± standard deviation) of A. marginale per milliliter, was determined from triplicate assays and used to calculate the mean rickettsemia level during the complete 7-day period of acquisition feeding. The latter was expressed as the mean log10 (± standard error) of A. marginale per milliliter. The D. andersoni and B. microplus ticks were simultaneously fed on each calf by using separate cloth patches to ensure exposure of both vectors to the same level of inoculum during persistent infection and to control for individual host differences in age, genetics, and innate immunity. Ticks were allowed to acquisition feed for 7 days, engorged ticks were removed, and the fed ticks were incubated for 48 h at 26°C at 93% relative humidity with a 12-h photoperiod. Incubation for 48 h under these conditions allows the blood meal to be cleared from the midgut, thus preventing false-positive detection of ticks as infected, and allows replication within the tick (7, 18, 26). Ticks of each species were dissected, and DNA was extracted from isolated individual salivary glands and midguts as previously described (18).
Transmission feeding on naïve cattle.
Eight MSP5 CI-ELISA-negative Holstein calves (calves 919, 920, 945, 948, 951, 952, 954, and 955) were used for the transmission feeding of B. microplus and D. andersoni. Adult male B. microplus or D. andersoni ticks, previously acquisition fed and incubated, were allowed to transmission feed on individual calves for 6 days (B. microplus on calves 919, 945, 952, and 954 and D. andersoni on calves 920, 948, 951, and 955). Fed ticks were removed after the transmission feed, and DNA was extracted from isolated individual salivary glands and midguts. Giemsa-stained blood smears of recipient calves were examined daily for microscopically detectable A. marginale. The genotype of the transmitted strain was identified by cloning and sequencing of msp1α (see the following section). Serum samples of calves were monitored daily by using the MSP5 CI-ELISA to confirm seroconversion.
Determination of tick infection rates.
Tick infection was determined by PCR of individual ticks followed by Southern hybridization and was confirmed by immunohistochemistry. Amplification of the A. marginale msp5 gene and hybridization with a digoxigenin-labeled msp5 probe were done as previously described (28). A plasmid containing msp5 was used as a positive control (28), and DNA samples isolated from the salivary glands of uninfected D. andersoni or B. microplus ticks were used as negative controls. The PCR-amplified fragments were size separated by gel electrophoresis, visualized in a 1.5% agarose gel following electrophoresis and staining with ethidium bromide, and then, to confirm the identity of the amplicons, hybridized with a digoxigenin-labeled 343-bp msp5 probe by Southern blotting as previously described (28). For immunohistochemical detection, individual ticks were fixed in 10% glutaraldehyde and embedded in paraffin. Replicate 4-μm sections were cut from individual ticks, stained with 0.1 μg of monoclonal antibody ANA8A per ml, and blocked in hydrogen peroxide before counterstaining with Mayer's hematoxylin.
Determination of the A. marginale infection level of tick salivary glands.
Real-time PCR for the conserved, single-copy gene msp5 (18, 29, 31) was used to determine the number of organisms in the salivary glands of individual B. microplus and D. andersoni ticks. For real-time amplification, forward 5′-GCCAAGTGATGGTGAATCGAC-3′ and reverse 5′-AGAATTAAGCATGTCACCGCTG-3′ primers were used, and for detection of target-specific product, a 22-bp msp5 Taqman probe, AACGTTCATGTACCTCATCAA, was generated. The Taqman assay consisted of 55 cycles of 95°C for 10 min, melting at 95°C for 15 s, and annealing at 50°C for 45 s, with extension at 72°C for 7 min. Standard curves were constructed by amplification of 107, 106, 105, 104, 103, and 102 copies of full-length msp5 cloned into pMAL-2 vector (New England Biolabs). Amplification of the unknown salivary gland DNA samples was conducted simultaneously with a set of standards and, as an internal standard for amplification efficiency, an infected D. andersoni salivary gland containing 105 A. marginale organisms (18). Each sample was analyzed in triplicate, and the number of A. marginale organisms was determined by using the standard curve and presented as mean log10 (± standard deviation) number of A. marginale organisms per salivary gland pair. The mean value for 10 individual ticks was calculated and expressed as the mean log10 (± standard deviation) number of A. marginale organisms per salivary gland pair. The difference in the mean numbers of A. marginale organisms per salivary gland between the two tick vectors was analyzed by Student's t test.
Verification of strain identity.
The stability of the A. marginale genotype during persistent infection, replication, and development within the tick and subsequent transmission was confirmed by PCR amplification and sequencing of the msp1α strain marker. DNA was extracted from the persistently infected calves used for acquisition feeding, salivary glands of the infected ticks, and blood of acutely infected cattle following tick transmission. Primers in the conserved regions flanking the strain-specific repeat region of msp1α (forward, 5′-CATTTCCATATACTGTGCAG-3′; and reverse, 5′-CTTGGAGCGCATCTCTCTTGCC-3′) were used in PCR amplification as previously described (23). Amplified fragments were cloned into the PCR-4 TOPO vector using the TOPO-TA cloning kit (Invitrogen), and TOP10 Escherichia coli competent cells were transformed. Plasmid DNA was isolated from individual transformed colonies, the presence of inserts was confirmed by EcoRI digestion, and inserts were sequenced in both directions by using the Big Dye kit and ABI Prism automated sequencer. Sequences were compiled and analyzed with VECTOR NTI (InforMax).
RESULTS
Rickettsial levels in persistently infected calves.
A. marginale levels were determined in individual persistently infected calves by real-time PCR analysis during the entire period of acquisition feeding. The log10 mean numbers of infected erythrocytes per milliliter of blood over the 7-day feeding period were 6.23, 5.78, 6.06, and 5.92 for calves 908, 929, 928, and 931, respectively (Table 1), consistent with these levels being below microscopic detection (log10 7 to 8 infected erythrocytes per ml) and representative of long-term persistent infection (8).
TABLE 1.
A. marginale infection rate in B. microplus and D. andersoni ticks
| A. marginale straina | Rickettsemia (mean log10 ± SE)b | % of fed ticks with A. marginale (no. positive/no. fed)
|
|
|---|---|---|---|
| B. microplus | D. andersoni | ||
| Puerto Rico | |||
| Calf 908 | 6.23 ± 0.22 | 89 (140/155) | 84 (131/155) |
| Calf 929 | 5.78 ± 0.10 | 92 (138/150) | 89 (134/150) |
| St. Maries | |||
| Calf 928 | 6.06 ± 0.18 | 92 (138/150) | 90 (135/150) |
| Calf 931 | 5.92 ± 0.13 | 89 (134/150) | 89 (134/150) |
Shown are the A. marginale strain and the number of the persistently infected calf used for acquisition feeding.
Mean rickettsemia (log10 infected erythrocytes per milliliter of blood) in the persistently infected calf during the 7-day acquisition feeding period.
Tick infection rates following acquisition feeding.
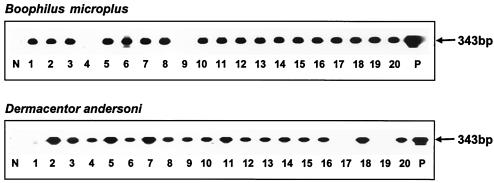
A. marginale infection rates of ticks that had acquisition fed for 7 days on persistently infected calves were determined by PCR amplification of A. marginale msp5 in genomic DNA extracted from isolated individual midguts and salivary gland pairs, followed by Southern hybridization with an msp5-specific probe (Fig. 1). A minimum of 150 individual B. microplus and D. andersoni ticks were examined from each acquisition feeding on calves with persistent infections with the St. Maries strain or the Puerto Rico strain. Tick infection rates were calculated by dividing the number of infected ticks by the total number of fed ticks probed. Using the PCR and Southern hybridization method, which has a detection limit of 10 A. marginale organisms per sample (28), A. marginale infection rates of B. microplus adult males during replicate acquisition feeding experiments were essentially identical for the Puerto Rico strain and the St. Maries strain (Table 1). Similarly, infection rates within D. andersoni adult males did not differ between the Puerto Rico and St. Maries strains (Table 1). There were no statistically significant differences in the infection rates between B. microplus and D. andersoni for either strain (P > 0.05). Notably, the infection rates were high in all vector-pathogen combinations (Table 1), supporting the importance of persistently infected cattle as reservoirs.
FIG. 1.
Detection of A. marginale in salivary glands isolated from individual adult male B. microplus or D. andersoni ticks. DNA of individual tick salivary glands was PCR amplified with msp5 primers and amplicons hybridized with a digoxigenin-labeled msp5 probe. P is the positive control and represents an amplicon of an msp5 plasmid. N is the negative control and represents salivary glands of individual B. microplus or D. andersoni ticks fed on an uninfected calf. Lanes 1 to 20 designate amplicons of individual ticks fed on calf 908, persistently infected with the Puerto Rico strain. A minimum of 150 individual B. microplus and D. andersoni ticks were examined from each feeding on calves with persistent infections with the St. Maries strain or the Puerto Rico strain.
A. marginale levels in infected tick salivary glands following acquisition feeding.
The number of A. marginale organisms per salivary gland pair of individual ticks was determined by real-time PCR in triplicate assays. The efficiency of standard curves obtained from amplification of dilution series of the full-length msp5 gene in each experiment was 0.96 to 0.99. The numbers of A. marginale per salivary gland, expressed as mean log10 ± standard deviation, were similar in B. microplus and D. andersoni following acquisition feeding and infection with either the St. Maries or Puerto Rico strain. The levels in the post-acquisition-fed ticks reflected the rickettsemia level of the individual persistently infected calf on which they fed (Table 2). For example, Puerto Rico strain-infected calves 908 and 929 had, respectively, log10 6.23 ± 0.22 and log10 5.78 ± 0.10 organisms per ml during tick feeding, and the levels in salivary glands of acquisition-fed B. microplus were significantly higher in those fed on calf 908 (log10 4.68 ± 0.31 organisms per ml) than those fed on calf 929 (log10 3.58 ± 0.54 organisms per ml). A similar relationship occurred with acquisition-fed D. andersoni (Table 2). There were no statistically significant differences in the number of salivary gland organisms between acquisition-fed B. microplus and D. andersoni ticks (P > 0.05).
TABLE 2.
A. marginale levels in infected salivary glands of acquisition-fed and transmission-fed B. microplus and D. andersoni ticks
| A. marginale straina and persistently infected calfa | Rickettsemia (mean log10 ± SE)b | No. of organisms/salivary gland (log10 ± SD)c
|
|||
|---|---|---|---|---|---|
| Acquisition fed
|
Transmission fed
|
||||
| B. microplus | D. andersoni | B. microplus | D. andersoni | ||
| Puerto Rico | |||||
| Calf 908 | 6.23 ± 0.22 | 4.68 ± 0.31 | 4.43 ± 0.46 | 6.39 ± 0.57 | 5.04 ± 0.63 |
| Calf 929 | 5.88 ± 0.10 | 3.58 ± 0.54 | 3.68 ± 0.50 | 6.64 ± 0.52 | 4.27 ± 0.47 |
| St. Maries | |||||
| Calf 928 | 6.06 ± 0.18 | 3.54 ± 0.27 | 2.98 ± 0.48 | 3.67 ± 0.36 | 4.23 ± 0.51 |
| Calf 931 | 5.92 ± 0.13 | 3.18 ± 0.29 | 3.19 ± 0.58 | 3.55 ± 0.31 | 4.12 ± 0.68 |
Shown are the A. marginale strain and the number of the persistently infected calf used for acquisition feeding.
Data are expressed as the mean number (± standard error) of infected erythrocytes per milliliter of blood in each calf on 7 consecutive days.
Results are represented as the mean (± standard deviation) of triplicate values obtained from salivary glands of 10 individual ticks.
Transmission-fed B. microplus ticks had significantly higher levels of the A. marginale Puerto Rico strain organisms in the salivary glands than those in identically fed D. andersoni ticks (P < 0.05).
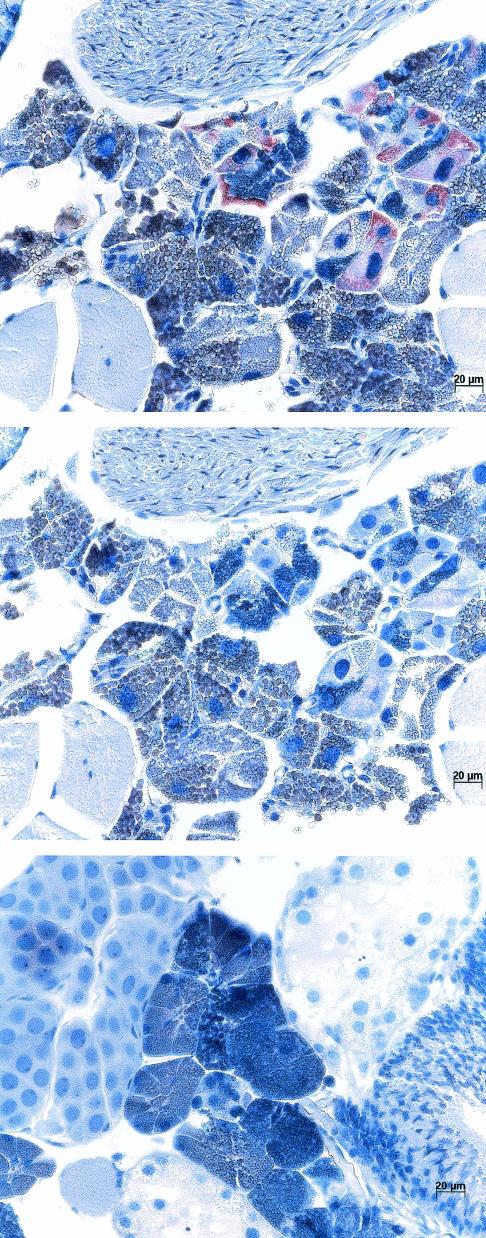
A. marginale colonization of the salivary gland acinar cells, the site of replication upon transmission feeding, was confirmed for each pathogen strain in both vector species by immunohistochemistry and examination by light microscopy. Colonization by the temperate-region St. Maries strain in the tropical tick B. microplus is shown in Fig. 2. Colonies were detected in salivary gland acinar cells of infected ticks following probing with A. marginale-specific monoclonal antibody ANA8A (Fig. 2, top panel), but not in sequential sections probed with a negative control monoclonal antibody to Trypanosoma brucei, Tryp1E1 (Fig. 2, middle panel). Stained colonies were not seen in control uninfected B. microplus ticks probed with ANA8A under identical conditions (Fig. 2, bottom panel).
FIG. 2.
Colonization of the temperate-region A. marginale strain in salivary glands of the tropical tick B. microplus. Colonies were detected in B. microplus ticks fed on calves infected with the St. Maries strain by immunohistochemistry with A. marginale-specific monoclonal antibody ANA8A (top panel), but not when an isotype control antibody to an unrelated pathogen, Trypanosoma brucei (middle panel), was used.B. microplus ticks identically fed on uninfected calves were unreactive with the A. marginale-specific antibody ANA8A (bottom panel). The sections were counterstained with hematoxylin; arrows designate the colonies.
A. marginale levels in infected tick salivary glands following transmission feeding.
In contrast to the results in the acquisition-fed ticks, there were larger numbers of Puerto Rico strain A. marginale organisms in the salivary glands of transmission-fed B. microplus ticks than in identically-transmission-fed D. andersoni ticks. This enhanced replication of the Puerto Rico strain in the salivary glands of transmission-fed B. microplus was observed and was statistically significant (P < 0.05) in replicate feedings done with additional calves and ticks of each species. Similarly, there were significantly higher numbers of the St. Maries strain in transmission-fed D. andersoni than in identically-transmission-fed B. microplus (P < 0.05).
Transmission.
B. microplus successfully transmitted both the Puerto Rico and St. Maries strains of A. marginale in replicate experiments. A. marginale-infected erythrocytes were detected microscopically after feeding with B. microplus adult males infected with the Puerto Rico strain (calves 919 and 954) or the St. Maries strain (calves 945 and 952). Similarly, D. andersoni transmitted both strains in replicate experiments: the Puerto Rico strain to calves 920 and 955 and the St. Maries strain to calves 948 and 951. Following microscopic detection of A. marginale infection, all calves seroconverted from negative to positive, as determined with the MSP5 CI-ELISA (15). The identity of the transmitted strain was confirmed based on the msp1α genotype. Sequence analysis confirmed three 87-bp repeats (Fig. 3) of type J/B/B in the St. Maries strain, consistent with the previously described genotype (23). The Puerto strain contained six 84-bp repeats, including one E form, and five novel repeats designated type Z (Fig. 3 [GenBank accession no. AY191826]). Notably, the msp1α genotypes for each strain were identical in samples obtained during persistent infection, in tick salivary glands of both tick vectors, and following transmission in the infected recipient calves.
FIG. 3.
MSP1a N-terminal repeat region sequences from the St. Maries and Puerto Rico A. marginale strains. The msp1α genotype was determined for each strain by PCR amplification and sequencing of the 5′ repeat region and reported as the amino acid sequence according to the convention of Allred et al. (2). The 28- or 29-amino-acid repeat regions are underlined, and the start of each of repeat is in boldface. The number and sequence of each repeat are used to designate a unique genotype for each strain (2). The nucleotide and derived amino acid sequence of each strain were identical in A. marginale organisms obtained from persistent infection, the salivary glands of fed ticks, and the blood of the acutely infected calves following transmission.
DISCUSSION
In the western hemisphere, the predominant vector species of A. marginale, members of the genera Dermacentor and Boophilus, occupy diverse ecological niches, with the latter dependent on warmer temperatures and higher humidity. Because the natural distribution of B. microplus prior to eradication in the 1940s was limited to the southern United States, and D. andersoni does not occur in Puerto Rico, the transmission of the St. Maries (Idaho) and Puerto Rico strains of A. marginale by both tick genera demonstrated that vector competence is retained without requiring continual exposure or coadaptation. Quantitative analysis of vector competence in replicate trials demonstrated that there were no significant differences in the percentage of fed B. microplus ticks that acquired the St. Maries strain versus those that acquired the Puerto Rico strain. Approximately 90% of B. microplus ticks had A. marginale within the salivary gland, regardless of the pathogen strain, at the time of transmission feeding; this is a critical determinant, because colonization of the salivary gland is required for transmission. Similarly, D. andersoni ticks, obtained from Idaho, were efficiently infected with both the tropical- and temperate-region pathogen strains, resulting in colonization of the salivary gland. These data suggest that expansion of B. microplus into its former range within the United States would reestablish a competent vector for existing temperate-region strains of A. marginale, despite its absence for over 60 years. Importantly, B. microplus as a one-host tick with preferential feeding for cattle has a much greater vectorial capacity for A. marginale than the Dermacentor spp. presently responsible for transmission in the United States.
Temperate-region strains of A. marginale, typified by the St. Maries strain, form a genetically distinct clade as compared to a cluster of tropical strains isolated from Mexico, the Caribbean region, and Central and South America (6). This genetic distance between the St. Maries and Puerto Rico strains, combined with their isolation from within well-defined regions in which only one of the tick vector species is present, supports the belief that pathogen-vector coadaptation would be strictly limited to B. microplus for the tropical pathogen strain and to D. andersoni for the temperate strain. The only evidence for coadaptation of the pathogen and its vector is seen with the increase in the numbers of A. marginale organisms in the salivary glands at the time of transmission feeding as compared to the numbers during acquisition feeding. This increase is presumed to reflect replication within the salivary gland, although differences in the release of organisms into the saliva during transmission feeding may also influence the number of A. marginale organisms detected in the salivary gland at this time (20). There were statistically significant increases in the numbers of Puerto Rico strain organisms in the salivary gland of B. microplus and in St. Maries strain organisms in D. andersoni following transmission feeding. In contrast, there was no statistically significant increase in organisms between the acquisition feed levels and the transmission feed levels for the St. Maries strain within B. microplus and for the Puerto Rico strain within D. andersoni (Table 2), consistent with only minimal replication within the salivary gland. Because replication and development of infectivity within the salivary gland triggered by tick feeding are key determinants of transmission, adaptation of an A. marginale strain to its sole available vector, resulting in increased replication, may be expected to provide the pathogen strain with a competitive advantage in regions of endemicity where numerous A. marginale strains exist (23). However, this does not appear to limit the ability of a vector to acquire and transmit a strain from outside the region of endemicity, at least in the absence of competition among strains.
B. microplus ticks resistant to multiple acaricides have emerged throughout the world, including Mexico and the Caribbean. As a one-host tick—that is, a tick that preferentially completes its life cycle on a single host species—B. microplus develops acaricide resistance at a much higher rate than do ticks of the three-host Dermacentor spp. (20). The emergence of resistance to the acaricides used to maintain the quarantine zone represents a significant risk that this tick will reestablish itself within the United States (3, 4). Critically, if allowed to reestablish in its former range, the high intrinsic vectorial capacity of B. microplus combined with its having retained vectorial competence for temperate-region strains of A. marginale would be expected to dramatically increase transmission of A. marginale in the U.S. population of cattle.
Acknowledgments
This research was supported by USDA-ARS-CRIS 5348-32000-016-00D and 5348-32000-016-03S (58-5348-8-044), NIH R01 AI44005, and a Fulbright Scholarship to J. E. Futse.
The excellent technical assistance of Peter Hetrick, Ralph Horn, Bev Hunter, and Carla Robertson is gratefully acknowledged.
REFERENCES
- 1.Aguirre, D. H., A. B. Gaido, A. E. Vinabal, S. T. De Echaide, and A. A. Guglielmone. 1994. Transmission of Anaplasma marginale with adult Boophilus microplus ticks fed as nymphs on calves with different levels of rickettsaemia. Parasite 1:405-407. [DOI] [PubMed] [Google Scholar]
- 2.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockelman, C. R. 1990. Prevalence and impact of anaplasmosis and babesiosis in Asia. Anaplasmosis Babesiosis Newsl. 20:3. [Google Scholar]
- 4.Davey, R. B., and J. E. George. 1998. In vitro and in vivo evaluations of a strain of Boophilus microplus (Acari: Ixodidae) selected for resistance to permethrin. J. Med. Entomol. 35:1013-1019. [DOI] [PubMed] [Google Scholar]
- 5.Davey, R. B., and J. E. George. 2002. Efficacy of macrocyclic lactone endectocides against Boophilus microplus (Acari: Ixodidae) infested cattle using different pour-on application treatment regimes. J. Med. Entomol. 39:763-769. [DOI] [PubMed] [Google Scholar]
- 6.De la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan. 2001. Differential adhesion of major surface proteins 1a and 1b of the ehrlichial cattle pathogen Anaplasma marginale to bovine erythrocytes and tick cells. Int. J. Parasitol. 31:145-153. [DOI] [PubMed] [Google Scholar]
- 7.De la Fuente, J., R. A. Van Den Bussche, J. C. Garcia-Garcia, S. D. Rodriguez, M. A. Garcia, A. A. Guglielmone, A. J. Mangold, L. M. Friche Passos, M. F. Barbosa Ribeiro, E. F. Blouin, and K. M. Kocan. 2002. Phylogeography of New World isolates of Anaplasma marginale based on major surface protein sequences. Vet. Microbiol. 88:275-285. [DOI] [PubMed] [Google Scholar]
- 8.Eriks, I. S., D. Stiller, and G. H. Palmer. 1993. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J. Clin. Microbiol. 31:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge, N. L., K. M. Kocan, E. F. Blouin, and G. L. Murphy. 1996. Developmental studies of Anaplasma marginale (Rickettsiales:Anaplasmataceae) in male Dermacentor andersoni (Acari:Ixodidae) infected as adults by using nonradioactive in situ hybridization and microscopy. J. Med. Entomol. 33:911-920. [DOI] [PubMed] [Google Scholar]
- 11.Graham, O. H., and J. L. Hourrigan. 1977. Eradication programs for the arthropod parasites of livestock. J. Med. Entomol. 13:629-658. [DOI] [PubMed] [Google Scholar]
- 12.Guglielmone, A. A. 1995. Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 57:109-119. [DOI] [PubMed] [Google Scholar]
- 13.Hodgson, J. L. 1991. Ph.D. thesis. Washington State University, Pullman.
- 14.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58:1117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles, D., S. Torioni de Echaide, G. Palmer, T. McGuire, D. Stiller, and T. McElwain. 1996. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 34:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocan, K. M. 1986. Development of Anaplasma marginale Theiler in Ixodid ticks: coordinated development of rickettsial organism and its tick host, p. 472-505. In J. Sauer and J. A. Hair (ed.), Morphology, physiology, and behavioral biology of ticks. John Wiley & Sons, Inc., New York, N.Y.
- 17.Kocan, K. M., D. Stiller, W. L. Goff, P. L. Claypool, W. Edwards, S. A. Ewing, T. C. McGuire, J. A. Hair, and S. J. Barron. 1992. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am. J. Vet. Res. 53:499-507. [PubMed] [Google Scholar]
- 18.Kocan, K. M., W. L. Goff, D. Stiller, W. Edwards, S. A. Ewing, P. L. Claypool, T. C. McGuire, J. A. Hair, and S. J. Barron. 1993. Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am. J. Vet. Res. 54:107-112. [PubMed] [Google Scholar]
- 19.Lincoln, S. D., J. L. Zaugg, and J. Maas. 1987. Bovine anaplasmosis: susceptibility of seronegative cows from an infected herd to experimental infection with Anaplasma marginale. J. Am. Vet. Med. Assoc. 190:171-173. [PubMed] [Google Scholar]
- 20.Löhr, C. V., F. R. Rurangirwa, T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Specific expression of Anaplasma marginale major surface protein 2 salivary gland variants occurs in the midgut and is an early event during tick transmission. Infect. Immun. 70:114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love, J. N. 1972. Cryogenic preservation of A. marginale with dimethyl sulfoxide. Am. J. Vet. Res. 3 3:2557-2560. [PubMed] [Google Scholar]
- 22.Mekonnen, S., N. R. Bryson, L. J. Fourie, R. J. Peter, A. M. Spickett, R. J. Taylor, T. Strydom, and I. G. Horak. 2002. Acaricide resistance profiles of single- and multi-host ticks from communal and commercial farming areas in the Eastern Cape and North-West Provinces of South Africa. Onderstepoort J. Vet. Res. 69:99-105. [PubMed] [Google Scholar]
- 23.Nicholls, M. J., G. Ibata, and F. V. Rodas. 1980. Prevalence of antibodies to Babesia bovis and Anaplasma marginale in dairy cattle in Bolivia. Trop. Anim. Health Prod. 12:48-49. [DOI] [PubMed] [Google Scholar]
- 24.Palmer, G. H., A. F. Barbet, A. J. Musoke, J. M. Katende, F. R. Rurangirwa, V. Shkap, E. Pipano, W. C. Davis, and T. C. McGuire. 1988. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int. J. Parasitol. 18:33-38. [DOI] [PubMed] [Google Scholar]
- 25.Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain. 2001. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro, M. F., and J. D. Lima. 1996. Morphology and development of Anaplasma marginale in midgut of engorged female ticks of Boophilus microplus. Vet. Parasitol. 61:31-39. [DOI] [PubMed] [Google Scholar]
- 27.Smith, R. D. 1977. Current world research on ticks and tick-borne disease of food-producing animals. Interciencia 2:335-344. [Google Scholar]
- 28.Stiller, D., K. M. Kocan, W. Edwards, S. A. Ewing, and J. A. Barron. 1989. Detection of colonies of Anaplasma marginale in salivary glands of three Dermacentor spp infected as nymphs or adults. Am. J. Vet. Res. 50:1381-1385. [PubMed] [Google Scholar]
- 29.Stiller, D., and M. E. Coan. 1995. Recent developments in elucidating tick vector relationships for anaplasmosis. Vet. Parasitol. 57:97-108. [DOI] [PubMed] [Google Scholar]
- 30.Torioni de Echaide, S., D. P. Knowles, T. C. McGuire, G. H. Palmer, C. E. Suarez, and T. F. McElwain. 1998. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay with recombinant major surface protein 5. J. Clin. Microbiol. 36:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visser, E. S., T. C. McGuire, G. H. Palmer, W. C. Davis, V. Shkap, E. Pipano, and D. P. Knowles, Jr. 1992. The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect. Immun. 60:5139-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaugg, J. L., D. Stiller, M. E. Coan, and S. D. Lincoln. 1986. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am. J. Vet. Res. 47:2269-2271. [PubMed] [Google Scholar]