Abstract
Background
Rheumatoid arthritis is associated with an unexplained increased risk of cardiovascular disease (CVD). Antibodies against human 60 kDa heat shock protein (anti‐HSP60) are associated with the presence and severity of CVD.
Objectives
To investigate whether anti‐HSP60 antibodies are associated with prevalent CVD in patients with rheumatoid arthritis.
Methods
In a nested case–control design, anti‐HSP60 antibody levels were measured in the serum samples of 192 rheumatoid patients. In a regression analysis the association between prevalent CVD and anti‐HSP60 antibodies was examined, along with the possible influence on this association of several demographic, rheumatoid arthritis, and CVD related variables.
Results
In a random sample of 326 patients with rheumatoid arthritis, 48 cases were identified who also suffered from CVD. Three controls per case with rheumatoid arthritis but without CVD (n = 144) were matched for sex, age, disease duration, and smoking habits. A regression analysis showed no significant association between prevalent CVD and anti‐HSP60 antibodies (odds ratio = 1.00 (95% confidence interval, 0.997 to 1.004)). After correcting for possible confounding variables, still no association was found.
Conclusions
In contrast to the general population, anti‐HSP60 antibody titres are not associated with prevalent CVD in patients with rheumatoid arthritis. These findings could be the result of an altered immune response to HSP60 in rheumatoid arthritis.
Keywords: rheumatoid arthritis, cardiovascular disease, heat shock protein 60 antibodies
Heat shock protein 60 (HSP60) is involved in intracellular transport and in the folding of intracellular proteins. It is produced by endothelial cells, smooth muscle cells, and macrophages. HSP60 protects the cell against denaturation by associating with other intracellular proteins (chaperonins) when the cell is under stress because of inflammation, oxidising agents, hypercholesterolaemia, hypertension, and certain agents in cigarette smoke.1 Human HSP60 has a similarity of approximately 55% in amino acid sequence to mycobacterial HSP65, to which both the humoral and the cellular immune system reacts by activating T cells and producing antibodies. It is postulated that a cross reaction may occur, causing the immune system to attack human HSP60.2 Immune reactivity against HSP60 is thought to be proatherogenic. Antibodies may bind to HSP60 expressed on endothelial cells and may have a cytotoxic effect which subsequently leads to endothelial lesions and ultimately to the formation of atherosclerotic plaques.3,4 HSP60 is detected on endothelial cells in atherosclerotic lesions of blood vessels, whereas no expression of HSP60 is observed in disease‐free control vessels.5 In line with this hypothesis, it has been found in some epidemiological studies that antibodies against HSP60 (anti‐HSP60) are associated with cardiovascular disease (CVD), although some studies have failed to show this.6,7
Rheumatoid arthritis is an inflammatory joint disease associated with an increased cardiovascular risk for which a clear explanation is lacking.8,9 It has been shown that rheumatoid patients have increased HSP60 levels.10 It is thought that chronic inflammation in rheumatoid arthritis is responsible for this increased expression of HSP60. In addition, increased levels of anti‐HSP60 have been reported11 but the relation with an increased cardiovascular risk has not yet been studied. In the present study, we hypothesised that increased anti‐HSP60 levels are associated with CVD in rheumatoid arthritis. In addition, we assessed whether anti‐HSP60 antibody levels in rheumatoid patients were associated with demographic, clinical, or laboratory variables.
Methods
Patients
Demographic, clinical, and laboratory data on the patients were collected cross sectionally as part of an ongoing prospective cohort study assessing CVD in rheumatoid arthritis. The patients' age ranged from 50 to 75 years at the time of inclusion, and rheumatoid arthritis was diagnosed in accordance with the 1987 American College of Rheumatology criteria.12 The various data and sera were obtained by a systematic review of the medical notes and during a visit to our outpatient clinic.
A nested case‐control study was undertaken comparing rheumatoid patients with prevalent CVD with those without CVD. Each case was compared with three controls, who were patients with rheumatoid arthritis without CVD, matched for sex, age (±5 years), disease duration (longer or shorter than the median of four years), and current smoking habits. To establish a frame of reference the anti‐HSP60 antibody levels were also determined in healthy donors. Furthermore, the longitudinal stability of the antibody levels was tested in serial samples of 10 rheumatoid patients at time points 0, 12, and 24 months.
CVD and risk factors to CVD
CVD was defined as a medical history of coronary, cerebral, or peripheral arterial disease.13 Coronary artery disease included a myocardial infarct, a coronary artery bypass graft procedure, percutaneous transluminal coronary angioplasty, or ischaemic abnormalities on the ECG (Minnesota codes 1‐1 and 1‐2). Cerebral arterial disease was defined as a cerebral vascular accident, a transient ischaemic attack, or carotid endarterectomy. Peripheral arterial disease included an aneurysm of the abdominal aorta, a peripheral arterial bypass operation, an ankle/brachial blood pressure index of less than 90% (independent of symptoms), and an amputation of the (lower) leg.
Risk factors for CVD that were assessed were age, male sex, hypertension, hypercholesterolaemia, diabetes, C reactive protein levels, and smoking habits. Diabetes and hypercholesterolaemia were considered to be present if patients received treatment for these conditions, or needed treatment according to the recommendations of the Dutch National Consensus Committee, at the time of inclusion of this study.14 Hypertension was defined as receiving blood pressure lowering treatment or as a measured systolic blood pressure of more than 155 mm Hg or a diastolic blood pressure of more than 95 mm Hg. Smoking was assessed by questionnaire at the time of inclusion in the study. Current and past smoking habits were recorded and as a cumulative measure we calculated the number of pack‐years—that is, the number of years over which the patient had smoked a pack of 20 cigarettes a day on average.
Enzyme linked immunosorbent assay for detecting antibodies against human HSP60
Recombinant human HSP60 (Stressgen, Victoria, Canada) was diluted to 2 μg/ml in coating buffer containing 0.1 M NaHCO3− (pH 9.6), and 50 μl/well (that is, 0.1 μg/well) were incubated in wells of microplates (Nunc MaxiSorp™) overnight at 4°C. Control wells were incubated with coating buffer alone. Wells were then incubated overnight at 4°C with 100 μl/well coating buffer containing 1% grade V bovine serum albumin (BSA; Sigma, St Louis, Missouri, USA) to block non‐specific binding. Plates were then washed three times with phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBS‐T). Plates were preincubated at room temperature with 200 μl/well of preincubation buffer, containing 0.05% Tween 20 and 1% BSA in PBS, for one hour. After washing twice with PBS‐T, wells were incubated in triplicate with patient serum in a previously established optimum dilution of 1:50, 50 μl/well, in incubation buffer and kept overnight at 4°C. The next day, plates were washed five times with PBS‐T and incubated with goat F(ab′)2 anti‐human IgG (Fc)‐horseradish peroxidase conjugate (Cappel‐ICN Immunobiologicals, Costa Mesa, California, USA), diluted 1:2000 in incubation buffer, for two hours at room temperature. After washing five times with PBS‐T, 100 μl of freshly made substrate (containing 0.5 mg/ml o‐phenylenediamine (Sigma) and 0.03% H2O2 in citrate/phosphate buffer, pH 4.9) was added to each well. After 10 minutes, the reaction was stopped with 50 μl of 4N H2SO4 and plates were read at 490 nm. A standard positive serum sample with anti‐HSP60 activity was run in every assay at seven serial dilutions from 1:10 to 1:640. Optical density (OD) values were derived from triplicate determinations corrected for non‐specific binding by subtraction of OD in uncoated wells. A standard curve was created with the seven OD values, and the undiluted serum sample was arbitrarily assigned 100 units/ml of activity. The OD values of each test serum were then read off the standard curve to determine the number of units of anti‐HSP60 antibody activity. Results are expressed as anti‐HSP60 antibody levels in arbitrary units (AU)/ml.
Other laboratory variables
Fasting serum total cholesterol (normal <5.0 mmol/l) and triglycerides (normal <1.90 mmol/l) were analysed by an enzymatic method (Roche Diagnostics, Basel, Switzerland) in accordance with the instructions of the manufacturer. PEG‐modified enzymes were used for assessing the high density lipoprotein cholesterol levels (HDLc, normal >0.9 mmol/l). The low density lipoprotein cholesterol (LDLc) levels were calculated using the Friedewald formula. In addition, we calculated the atherogenic index—that is, the total cholesterol to HDLc ratio, an important prognostic indicator for future cardiovascular disease. C reactive protein (normal <10 mg/l) was measured using a latex enhanced highly sensitive assay (Roche Diagnostics).
IgM rheumatoid factor (IgM‐RF) and anti cyclic citrullinated peptides (anti‐CCP) antibodies were measured using in‐house enzyme linked immunosorbent assays on an ES 300 analyser (Roche Diagnostics) as described previously.15
Statistics
We compared the group of patients with rheumatoid arthritis and prevalent CVD with the group of rheumatoid patients without CVD. Comparison of continuous variables between the two groups was conducted with the use of a Student's t test for variables with a normal distribution and the Mann–Whitney U test for variables with a non‐normal distribution. Dichotomous variables were compared with the use of a Pearson χ2 test.
To estimate the cut off value of anti‐HSP60 the receiver operator characteristics (ROC) curve was determined.
In a logistic regression analysis we examined the association between having CVD or not having CVD and anti‐HSP60 antibody levels, and we adjusted for possible influence of demographic, clinical, and laboratory variables.
A linear regression model was used to examine whether the anti‐HSP60 antibody levels were associated with any of the demographic, clinical, or laboratory variables.
A probability (p) value of 0.05 or less was considered statically significant, and all tests were undertaken using the SPSS 11.5 software package for windows.
Results
Patient characteristics
We identified 48 rheumatoid patients with prevalent CVD (RA‐CVD group) and matched these with three rheumatoid patients per case without CVD—a total of 192 subjects. The characteristics and comparison of demographic, rheumatoid arthritis related, and CVD related variables between the two groups are shown in table 1. There were no significant differences between the two groups in demographic and rheumatoid arthritis related variables. As expected, statins were used more often in the RA‐CVD group (p<0.000) and consequently the total cholesterol and the (calculated) LDLc levels were significantly lower in this group (p = 0.03, p = 0.002, respectively) compared with the control rheumatoid patients without CVD. Furthermore, a significantly larger number of rheumatoid patients with CVD received treatment for hypertension and their median cigarette pack‐years was higher than in the controls without CVD (p = 0.000 and p = 0.004, respectively).
Table 1 Characteristics of rheumatoid arthritis patients with and without cardiovascular disease.
| RA with CVD (n = 48) | RA without CVD (n = 144) | p Value | |
|---|---|---|---|
| Demographic variables | |||
| Age (years) (mean (SD)) | 66.2 (7.0) | 64.6 (7.0) | 0.17 |
| Female | 54% | 56% | 0.80 |
| Male | 46% | 44% | |
| RA related variables | |||
| Disease duration (years) (mean (SD)) | 7.2 (3.5) | 7.7 (4.1) | 0.41 |
| Erosive disease | 80% | 85% | 0.43 |
| IgM‐RF | 72% | 71% | 0.96 |
| IgM‐RF titre (IU/l) (median (IQR)) | 71 (14 to 200) | 32 (10 to 150) | 0.33 |
| Anti‐CCP titre (AU/l) (median (IQR)) | 50 (10 to 262) | 62 (10 to 450) | 0.57 |
| ESR (mm/h) (median (IQR)) | 16 (9 to 29) | 19 (8 to 34) | 0.49 |
| C reactive protein (mg/dl) (median (IQR)) | 6 (2 to 16) | 8 (4–25) | 0.12 |
| DAS28 (mean (SD)) | 3.9 (1.2) | 4.0 (1.5) | 0.74 |
| Number of DMARDs used (median (IQR)) | 2 (2 to 4) | 3 (2 to 3) | 0.40 |
| DMARD‐naive patients | 13% | 9% | 0.49 |
| Current methotrexate users | 69% | 57% | 0.15 |
| Current sulfasalazine users | 15% | 20% | 0.39 |
| Current prednisone users | 19% | 21% | 0.83 |
| CVD related variables | |||
| Current smokers | 36% | 31% | 0.53 |
| History of smoking | 89% | 82% | 0.23 |
| Pack‐years (median (IQR)) | 38 (18 to 50) | 22 (6 to 37) | 0.004 |
| Hypertension, measured | 19% | 25% | 0.41 |
| Hypertension, on drug treatment | 49% | 22% | 0.000 |
| Diabetes mellitus | 6% | 4% | 0.39 |
| Current statin users | 46% | 6% | 0.000 |
| Body mass index (kg/m2) (mean (SD)) | 25.9 (5.5) | 26.8 (4.7) | 0.33 |
| Waist/hip ratio (mean (SD)) | 0.9 (0.08) | 0.9 (0.09) | 0.15 |
| Total cholesterol (mmol/l) (mean (SD)) | 5.5 (1.3) | 5.9 (1.0) | 0.03 |
| HDLc (mmol/l) (mean (SD)) | 1.4 (0.6) | 1.4 (0.4) | 0.77 |
| Atherogenic index (mean (SD)) | 4.4 (1.6) | 4.7 (1.5) | 0.37 |
| LDLc (mmol/l) (mean (SD)) | 3.4 (1.2) | 3.9 (0.9) | 0.002 |
| Triglycerides (mmol/l) (median (IQR)) | 1.3 (0.9 to 2.0) | 1.8 (1.0 to 1.8) | 0.83 |
| HSP | |||
| Anti‐HSP60 (AU/ml) (median (IQR)) | 31.1 (13 to 98) | 34.8 (15 to 69) | 0.87 |
| Anti‐HSP60 titre ⩾80 AU/l | 27% | 22% | 0.43 |
Comparisons were made using Student's t test or the Mann–Whitney U test for comparison of continuous variables and Pearson's χ2 test for dichotomous variables. Significant differences in bold.
AU, arbitrary unit; CCP, cyclic citrullinated peptide; DAS28, 28 joint disease activity score; DMARD, disease modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HDLc, high density lipoprotein cholesterol; HSP60, heat shock protein 60; IQR, interquartile range; LDLc, low density lipoprotein cholesterol RF, rheumatoid factor; TC, total cholesterol.
The mean (SD) age of the healthy donors was 39 (9.0) years and 56% were male. The healthy donors did not suffer from known cardiovascular or other disease, and were not receiving treatment for cardiovascular risk factors.
Anti‐HSP60 antibody levels
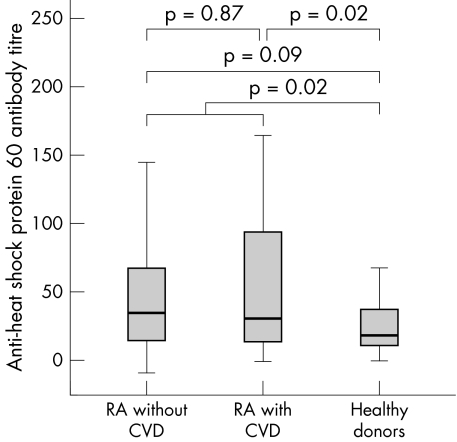
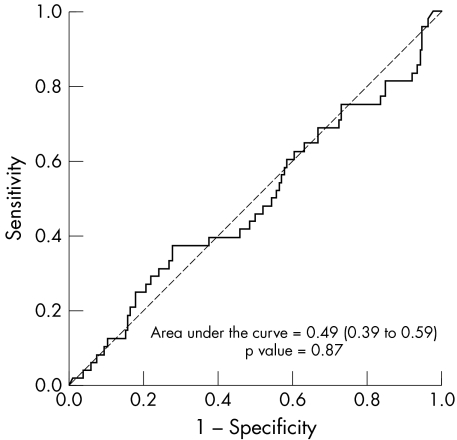
The median anti‐HSP60 antibody levels of rheumatoid patients with and without CVD were not significantly different, at 31.1 v 34.8 AU/l, respectively (Mann–Whitney U test: p = 0.87, fig 1). Using an ROC curve, no cut off value could be determined (fig 2). Subsequently, the mean titre +2SD ( = 80 AU/l) of healthy donors was taken as the cut off point. This resulted in 27% of the rheumatoid group with CVD and 22% of the rheumatoid group without CVD being positive for anti‐HSP60 (NS; Pearson's χ2 test: p = 0.43).
Figure 1 Box plot of the anti‐heat shock protein 60 antibody titres for RA patients with and without CVD and for healthy donors. The p values were calculated using a Mann–Whitney U test for non‐normally distributed variables. CVD, cardiovascular disease; RA, rheumatoid arthritis.
Figure 2 Receiver operating characteristic curve to estimate the cut off value of anti‐HSP60.
The median anti‐HSP60 antibody level of the healthy donors was significantly lower than in the rheumatoid patients (19.0 AU/l; Mann–Whitney U test: p = 0.02). A comparison between all three the groups separately is shown in fig 1. The percentage of healthy donors exceeding the cut off point of 80 AU/l was 13, which was not significantly lower than in all rheumatoid patients, rheumatoid patients with CVD, or rheumatoid patients without CVD (p = 0.16, p = 0.10, and p = 0.23, respectively).
The logistic regression analysis showed no association between the presence of CVD and anti‐HSP60 antibody levels (odds ratio = 1.00 (95% confidence interval (CI), 0.997 to 1.004)). When adjusting for potential confounding variables such as age, sex, IgM‐RF titre, presence of bony erosions on radiographs, rheumatoid arthritis disease duration, ESR, C reactive protein, 28 joint disease activity score,16 total cholesterol, HDLc, LDLc, triglycerides, atherogenic index, smoking habits, hypertension, diabetes, and prednisone or statin use, separately or together, the odds ratio remained 1.00 (95% CI, 0.994 to 1.003).
With univariate linear regression analyses no significant associations between anti‐HSP60 and any of the other variables were found, except for the IgM‐RF titre (b = 0.044; p = 0.05). However, this association disappeared in a multivariate analysis.
When testing the stability of the anti‐HSP60 antibody levels in serial samples of 10 rheumatoid patients there was a fall in the antibody level in one patient at one of the time points. This patient suffered from multiple co‐morbidity, including diabetes mellitus and severe nephropathy, providing possible explanation for the fall in antibody titre. The titres in the nine other rheumatoid patients at the various time points remained stable (data not shown).
Discussion
To our knowledge, this is the first study in which the relation between the occurrence of antibodies against HSP60 and CVD was investigated in patients with rheumatoid arthritis. Anti‐HSP60 antibody levels in rheumatoid patients were increased compared with healthy donors, but the mean level was not significantly different between rheumatoid patients with and without CVD. An association was found between the anti‐HSP60 antibody levels and IgM‐RF titres. Contrary to earlier findings in healthy controls and in patients with CVD, no association was found between age, diabetes, and acute phase proteins.6
The mean anti‐HSP60 antibody levels found in our rheumatoid arthritis appeared to be higher than that in a group of healthy controls. This is consistent with findings of others,11 although it was also reported that there was no difference between rheumatoid patients and healthy donors.17 Rheumatoid arthritis is known to be associated with the development of several autoantibodies such as antibodies against rheumatoid factor and cyclic citrullinated peptides.18 It can be hypothesised that the development of autoantibodies against HSP60 is part of the rheumatic disease per se and should consequently not be seen as a marker for CVD. Hence, we suggest that the formation of anti‐HSP60 antibodies in rheumatoid patients is part of the autoimmune response or the result of inflammation, both of which are fundamental features of rheumatoid arthritis. The fact that we found a significant association between anti‐HSP60 antibody levels and IgM‐RF supports this hypothesis.
There are various alternative hypotheses for the explanation of the absence of an association between anti‐HSP60 antibody levels and CVD in rheumatoid arthritis. First, there may be a difference in functionality between anti‐HSP60 antibodies formed in rheumatoid patients compared with those formed in patients who do not have rheumatoid arthritis (different epitopes of anti‐HSP60 antibodies). The “rheumatoid arthritis‐anti‐HSP60 antibodies” may be less cytotoxic to the endothelial cells to which these antibodies are directed and therefore they may be less atherogenic. Whether this is the case remains to be determined by further investigation into the functionality of anti‐HSP60 in rheumatoid patients compared with non‐rheumatoid patients. These investigations could show that there are several epitopes of anti‐HSP60 antibodies, some being more and other less atherogenic.
Second, HSP60 expression on the cell membranes occurs not only on endothelial cells but also on macrophages. As rheumatoid arthritis is characterised by proliferation of macrophages in synovial tissue, it can be hypothesised that this may result in an increased load of the potential antigen, HSP60, which could lead to increased antibody formation. This could explain the higher levels of anti‐HSP60 antibodies that we found in our rheumatoid population compared with the healthy donors, as well as the lack association with CVD.
Associations between anti‐HSP antibodies and CVD in non‐rheumatoid populations reported previously were obtained from incident cases of CVD. Whether the design of the present study, in which prevalent cases of CVD in rheumatoid patients were investigated, could explain the absence of such an association is unclear. Furthermore, it has been shown that statins have pleiotropic effects on endothelial function, inflammation, and immunomodulation19,20; therefore, one may suggest that the more prevalent use of particular lipid lowering agents in the group of patients with rheumatoid arthritis and CVD may have an effect on anti‐HSP60 levels. However, we did not find any effect of statins on anti‐HSP levels in our rheumatoid patients (data not shown). Finally, some have debated the association of coronary heart disease with human HSP60 antibody levels and showed an association with antibodies to chlamydial HSP60, suggesting that bacterial HSPs may be more important than human HSP in the pathogenesis of coronary heart disease.21
In summary, anti‐HSP60 antibodies are not associated with prevalent CVD in patients with rheumatoid arthritis and cannot therefore be used as a marker for cardiovascular risk in these patients.
Acknowledgements
We gratefully acknowledge Margret de Koning and Ruud Theunissen for their technical assistance.
Abbreviations
CVD - cardiovascular disease
HDLc - high density lipoprotein cholesterol
HSP - heat shock protein
LDLc - low density lipoprotein cholesterol
ROC - receiver operating characteristic
References
- 1.Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J 19961010–19. [PubMed] [Google Scholar]
- 2.Ranford J C, Henderson B. Chaperonins in disease: mechanisms, models, and treatments. Mol Pathol 200255209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q.et al Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 1999991560–1566. [DOI] [PubMed] [Google Scholar]
- 4.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol 200422361–403. [DOI] [PubMed] [Google Scholar]
- 5.Kleindienst R, Xu Q, Willeit J, Waldenberger F R, Weimann S, Wick G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol 19931421927–1937. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Quyyumi A A, Rott D, Csako G, Wu H, Halcox J.et al Antibodies to human heat‐shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation 20011031071–1075. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S.et al Association of serum antibodies to heat‐shock protein 65 with carotid atherosclerosis. Lancet 1993341255–259. [DOI] [PubMed] [Google Scholar]
- 8.Solomon D H, Karlson E W, Rimm E B, Cannuscio C C, Mandl L A, Manson J E.et al Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 20031071303–1307. [DOI] [PubMed] [Google Scholar]
- 9.Goodson N J, Wiles N J, Lunt M, Barrett E M, Silman A J, Symmons D P. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum 2002462010–2019. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Miyata M, Kasukawa R. Expression of heat shock protein on lymphocytes in peripheral blood and synovial fluid from patients with rheumatoid arthritis. J Rheumatol 1996232027–2032. [PubMed] [Google Scholar]
- 11.Yokota S I, Hirata D, Minota S, Higashiyama T, Kurimoto M, Yanagi H.et al Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones 20005337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 13.Hoogeveen E K, Kostense P J, Beks P J, Mackaay A J, Jakobs C, Bouter L M.et al Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non‐insulin‐dependent diabetes mellitus: a population‐based study. Arterioscler Thromb Vasc Biol 199818133–138. [DOI] [PubMed] [Google Scholar]
- 14.Dutch Institute for Healthcare Improvement Consensus Cholesterol, tweede herziening. Utrecht: CBO, 1998
- 15.Nielen M M, van Schaardenburg D, Reesink H W, van de Stadt R J, van der Horst‐Bruinsma I E, de Koning M H.et al Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 200450380–386. [DOI] [PubMed] [Google Scholar]
- 16.Prevoo M L, 't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 17.Horvath L, Czirjak L, Fekete B, Jakab L, Prohaszka Z, Cervenak L.et al Levels of antibodies against C1q and 60 kDa family of heat shock proteins in the sera of patients with various autoimmune diseases. Immunol Lett 200175103–109. [DOI] [PubMed] [Google Scholar]
- 18.Schellekens G A, Visser H, de Jong B A, van den Hoogen F H, Hazes J M, Breedveld F C.et al The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 200043155–163. [DOI] [PubMed] [Google Scholar]
- 19.Kwak B R, Mulhaupt F, Mach F. Atherosclerosis: anti‐inflammatory and immunomodulatory activities of statins. Autoimmun Rev 20032332–338. [DOI] [PubMed] [Google Scholar]
- 20.Werner N, Nickenig G, Laufs U. Pleiotropic effects of HMG‐CoA reductase inhibitors. Basic Res Cardiol 200297105–116. [DOI] [PubMed] [Google Scholar]
- 21.Mahdi O S, Horne B D, Mullen K, Muhlestein J B, Byrne G I. Serum immunoglobulin G antibodies to chlamydial heat shock protein 60 but not to human and bacterial homologs are associated with coronary artery disease. Circulation 20021061659–1663. [DOI] [PubMed] [Google Scholar]