Abstract
Objective
To examine whether human endogenous retrovirus K10 is associated with autoimmune rheumatic disease.
Design
A novel multiplex reverse transcription polymerase chain reaction (RT‐PCR) system was developed to investigate HERV‐K10 mRNA expression in patients with rheumatoid arthritis.
Methods
40 patients with rheumatoid arthritis, 17 with osteoarthritis, and 27 healthy individuals were recruited and total RNA was extracted from peripheral blood mononuclear cells (PBMCs) and analysed using multiplex RT‐PCR for the level of HERV‐K10 gag mRNA expression. Southern blot and DNA sequencing confirmed the authenticity of the PCR products.
Results
Using the histidyl tRNA synthetase (HtRNAS) gene as a housekeeping gene in the optimised multiplex RT‐PCR, a significantly higher level of HERV‐K10 gag mRNA expression was found in rheumatoid arthritis than in osteoarthritis (p = 0.01) or in the healthy controls (p = 0.02).
Conclusion
There is enhanced mRNA expression of the HERV‐K10 gag region in rheumatoid arthritis compared with osteoarthritis or healthy controls. This could contribute to the pathogenesis of rheumatoid arthritis.
Keywords: human endogenous retroviruses, rheumatoid arthritis, peripheral blood mononuclear cells, histidyl tRNA synthetase
The aetiology of most autoimmune diseases such as rheumatoid arthritis has yet to be established, but indirect evidence indicates that a trigger operating on the background of hormonal and genetic predisposing factors may be involved. Human endogenous retroviruses (HERVs) have been suggested as potential aetiological agents for certain autoimmune disorders, in particular the rheumatic diseases.1,2,3 The human genome contains many types of retrovirus‐like elements.4 HERVs represent footprints of previous retroviral infection and have been coined “fossil viruses.” They are transmitted vertically through the germline and thus inherited by successive generations in a Mendelian manner. Full length HERVs share the LTR‐gag‐pol‐env‐LTR structure of infectious retroviruses. HERVs incorporate reverse transcriptase into their replicative cycle and may code for retrovirus‐like particles, but are generally not infectious; in fact, most sequences are defective because of accumulation of mutations, frame shifts, and deletions. About 8% of the human genome is derived from HERV sequences.5 Over time, HERVs have been subjected to repeated amplification and transposition events, giving rise to single and multi copy proviruses that are distributed within the DNA of all cells. Over 26 families of HERVs have been identified during the past two decades.5 While many are defective through the accumulation of mutations, deletions, and termination signals within coding sequences, a limited number retain the potential to produce viral products and indeed form viral particles (HERV‐K). Furthermore, some HERVs have been implicated in certain autoimmune diseases and cancer; hence endogenous viruses may play a potential role in the aetiology and pathology of disease. Mechanisms whereby HERVs could influence autoimmunity include molecular mimicry (that is, HERVs sharing amino acids common to host proteins), superantigen motifs which bypass the normal major histocompatibility complex (MHC) restrictive process of T cell stimulation, aberrant expression of antigens, and the presence of neoantigens perhaps as a result of HERV or exogenous viral combinations.6,7,8
Previous investigations of rheumatoid patients have demonstrated antibodies to HIV‐1 and HTLV‐I/‐II retroviral products in the serum.9,10 One such study by Nelson et al11 showed a significant association of anti‐retroviral antibodies in patients with rheumatoid arthritis, systemic lupus erythematosus (SLE), and polymyositis, yet polymerase chain reaction (PCR) failed to detect exogenous retroviruses when using specific oligonucleotide primers. To explain this paradox, HERVs—which possess regions of sequence homology to exogenous retroviruses—have been suggested as potential aetiological triggers of disease.6,12,13
Retroviral antigens have also been detected at the site of disease: 45% of rheumatoid synovial sections were shown to be positive using antibodies to HTLV‐I p24 and p19, and retrovirus‐like particles have been observed in a human T cell line co‐cultured with salivary gland tissue from a patient with primary Sjögren's syndrome.14 Despite the fact that patients in these studies partially seroconverted to HIV/HTLV, again no evidence of past or active infection by either virus was detected. This may suggest the presence of an unknown exogenous or endogenous retrovirus with homology to HIV/ HTLV.
This ambiguity in the serological evidence indicated a need to clarify the role of putative retroviral agents in the pathogenesis of rheumatic diseases. To date there have been few reports on the detection of retroviral agents in these disorders using direct molecular approaches such as PCR. Additionally, to our knowledge no simple quantitative comparison has been undertaken on the level of HERV mRNA expression in health and disease states. Consequently, we sought to develop a novel multiplex reverse transcription polymerase chain reaction (RT‐PCR) system to identify possible candidate agents in patients with rheumatoid arthritis.
Methods
Patients and controls
The rheumatoid arthritis patients and the disease control group were selected from the rheumatology clinic at Heartlands Hospital in Birmingham and New Cross Hospital in Wolverhampton. The normal healthy donors were a volunteer group made up of hospital workers.
Blood samples were taken from 40 rheumatoid patients of average age 61 years (range 34 to 85) and of average disease duration 75 months. Samples collected from 17 osteoarthritis patients were used as a disease control. These patients had an average age of 67 years (range 40 to 92), and average disease duration of 39 months. Blood was collected from 27 normal healthy donor volunteers of average age 47 years (range 21 to 80).
All rheumatoid patients satisfied the American College of Rheumatology (ACR) criteria for the diagnosis of rheumatoid arthritis and had clinically active disease as defined by serological and clinical indices. All were IgM rheumatoid factor positive. The patients were taking non‐steroidal anti‐inflammatory drugs (NSAIDs) plus weekly methotrexate. The osteoarthritis patients had clinically active disease as defined by diagnostic indices and the absence of serological abnormalities. The patients were either on no drug treatment or were taking analgesics or NSAIDs. All samples were collected with full ethical approval from each site and with the patients' consent. Disease groups were not age or sex matched.
Separation of PBMCs and total RNA extraction
Histopaque‐1077 (Sigma, Poole, UK) was used to separate PBMCs from heparinised whole blood. Total RNA was extracted using TRI Reagent (Sigma). The crude extracts were subjected to RNase‐free RQ‐DNase I digestion at 37°C for 30 minutes. The total RNA concentrations were measured photometrically using a GeneQuant spectrophotometer. The extracts were analysed for possible structural damage and genomic DNA contamination by horizontal submarine gel electrophoresis, using 1% agarose loening gel, which contained ethidium bromide (0.5 mg/l). Electrophoresis was carried out in 1×loening buffer (35.9 mM Tris base, 34 mM sodium dihydrogen orthophosphate, 1 mM EDTA in HPLC water, pH 7.7). The gels were then visualised under a BioVisionTM gel imager (Biogene, Huntingdon, UK).
Multiplex RT‐PCR
One microgram of total RNA was converted into cDNA using the reverse transcriptase (RT) system kit with random hexamer primers and the avian myeloblastosis virus (AMV) RT enzyme (Promega, Southampton, UK). RT cycles consisted of 10 minutes at room temperature, one hour at 42°C, five minutes at 99°C, and were then stored at 4°C until used in the PCR reaction. PCR was carried out on a TouchDown thermal cycler (Hybaid, UK) using primers for HERV‐K10 reference sequence M14123 (sense: 5′‐GCAAGTAGCCTATCAATACTGC‐3′ (nt 1729 to 1751); antisense: 5′‐GCAGCCCTATTTCTTCGGACC‐3′ (nt 2241 to 2261)). Intron spanning primers for histidyl tRNA synthetase (HtRNAS) were, sense: 5′‐CTTCAGGGAGAGCGCGTGCG‐3′; antisense: 5′‐CCTTCAGGTCATAGATAAGC‐3′). PCR cycling conditions were as follows: 94°C for three minutes; 30 cycles of one minute at 94°C, one minute at 59°C, and two minutes at 72°C, with a final extension step of 10 minutes at 72°C. The PCR mix contained the two sets of primers at final concentrations of 0.5 µM (for HERV‐K10) and 1 µM (for HtRNAS), 0.2 mM dNTP mix, 1.25 U Taq DNA polymerase (Promega), and 3 mM MgCl2 in total volume of 50 μl. Controls (omitting RNA templates and RT) were also included in each experiments to detect any traces of genomic DNA contamination. The PCR products were analysed by loading aliquots of products onto a 2% agarose‐Tris Borate‐EDTA (TBE) gel containing ethidium bromide, which then underwent electrophoresis. The bands on the gel were visualised under an ultraviolet (UV) transilluminator (Biogene). By comparing the size of amplified products with a molecular weight standard (pGEM DNA marker, Promega), it was possible to confirm the size and identity of PCR products. PCR bands were analysed semiquantitatively by measuring their pixel densities using Scion imageTM internet software. A testicular carcinoma cell line known to harbour HERV‐K10 (Tera‐1) was used as a positive control for HERV‐K10 PCR development, optimisation, and testing.
Southern blotting
A specific 5′‐biotin labelled HERV‐K10 gag probe (5′‐AAGGAGATACTGAGGCATGG‐3′; position 1931–1950; 20 nt), selected from HERV‐K10 sequence published by Ono et al15 (manufactured by Oswel Research Products, Southampton, UK) was designed to confirm the specificity of the HERV‐K10 PCR product. RT‐PCR products were run on agarose‐TBE gel electrophoresis. The DNA bands were denatured by soaking the gel in 1 M NaCl/0.5 M NaOH, neutralised by 0.5 M Tris/pH 7.5, 1.5 M NaCl, and transferred onto a porablot nylon membrane (Amersham Pharmacia, Amersham, UK) overnight. The labelled probe was added to the hybridisation solution (5% Denhardt's solution, 0.5% SDS, 5×SSC) and a UV crosslinked membrane for overnight hybridisation at 65°C. Probe concentration during the hybridisation was 20 ng/ml (300 ng of probe in 15 ml of hybridisation solution). The bands on the nylon membrane were detected using enhanced chemiluminescent (ECL) detection system (Amersham Pharmacia).
DNA sequencing
Authenticity of the HERV‐K10 PCR products were further characterised by DNA sequencing and any resulting sequence data compared with the published HERV‐K10 reference sequence.15
Statistical evaluation
Statistical analysis was carried out on the ratio of pixel intensities between bands for HERV‐K10 and HtRNAS mRNA expression. Wilcoxon (Mann–Whitney) rank‐sum test and two tailed unpaired Student t test were applied to the results. Probability (p) values <0.05 were considered significant.
Results
Multiplex RT‐PCR: detection and semiquantification of HERV‐K10 expression
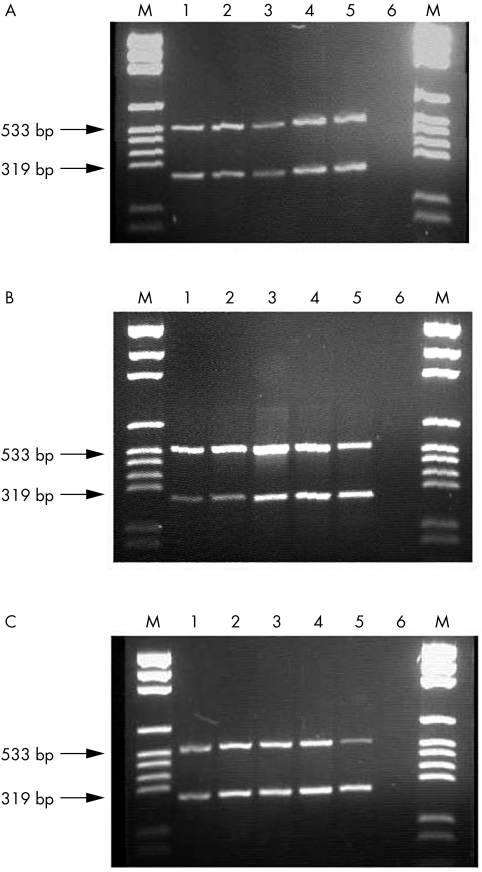
In order to detect and relatively quantify HERV‐K10 mRNA expression in rheumatoid patients, total RNA was initially extracted from PBMCs. The extracts were validated for possible structural damage and genomic DNA contamination by horizontal submarine agarose gel electrophoresis (fig 1). Using two sets of primers specific for HERV‐K10 gag2 and HtRNAS genes, amplicons of 533 base pairs (bp) and 319 bp, respectively, were amplified from healthy and diseased samples (fig 2).
Figure 1 A good quality total RNA band pattern in healthy samples. No top band (DNA contamination) was seen. Two central bands (28S + 18S rRNA) were visible, with the upper 28S band twice as bright as the 18S fraction. Between 28S and 18S bands, mRNA (weak smear) was visible. Degraded RNA bands were weak. Total RNA extractions were of high quality and suitable for reverse transcription polymerase chain reaction.
Figure 2 Multiplex reverse transcription polymerase chain reaction (RT‐PCR) system using HERV‐K10 gag and HtRNAS primers in (A) healthy individuals, (B) osteoarthritis patients, and (C) rheumatoid arthritis patients. Lane M, pGEM DNA markers; lanes 1–5, individual samples tested in each group; lane 6, RT‐ PCR control (no RNA template).
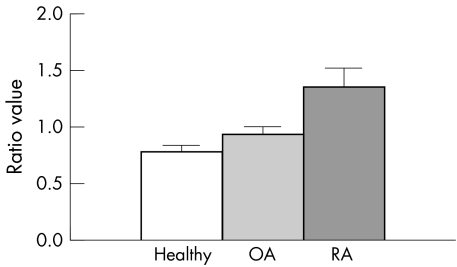
PCR results of the PBMCs showed a significant increase in HERV‐K10 expression when compared with expression of the housekeeping gene—histidyl tRNA synthetase in rheumatoid patients. Of PBMC samples taken from rheumatoid patients, 68% (27/40) showed significantly increased HERV‐K10 expression levels (p = 0.02) when compared with disease controls (fig 3). Just 2% (2/40) of the rheumatoid patients showed levels comparable with the housekeeper gene—that is, the level of baseline cellular expression; 17% (3/17) of the disease controls (osteoarthritis) had increased HERV‐K10 expression, and the other controls (normal healthy donors) had comparable levels (18.5%; 5/27). There was no significant difference in levels of HERV‐K10 expression between osteoarthritis and normal healthy donor controls (p = 0.34). Levels of the HtRNAS were consistent in all samples tested.
Figure 3 The average ratio of pixel intensity of the polymerase chain reaction (PCR) bands for HERV‐K10 to HtRNAS in rheumatoid patients was significantly higher than patients with osteoarthritis (p = 0.02) or healthy subjects (p = 0.01). There was no significant difference in mean ratio of pixel intensity of HERV‐K10/HtRNAS mRNA expression between osteoarthritis and healthy subjects (p = 0.34). OA, osteoarthritis; RA, rheumatoid arthritis.
Southern blot and DNA sequencing
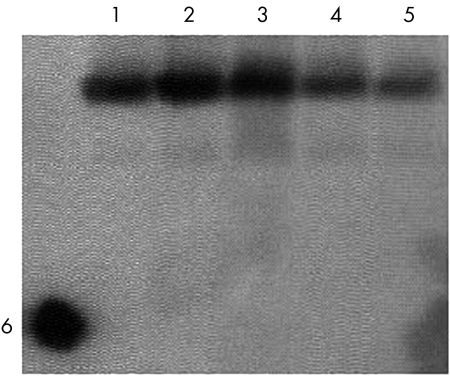
A 5′‐biotin labelled probe specific for HERV‐K10 gag confirmed the specificity of the PCR and identity of the PCR products (fig 4). The authenticity of the HERV‐K10 PCR product was further characterised by DNA sequencing. Sequence analysis showed that PCR product had 96% homology with HERV‐K1015 using the blastn2 program from the National Center for Biotechnology Information (http://www.ncbi.nlm.gov/blast/bl2seq).
Figure 4 Southern blot using specific HERV‐K10 gag biotin labelled probe to verify the HERV‐K10 PCR products. Lanes 1–3 are polymerase chain reaction products from rheumatoid patients; lanes 4 and 5 are from osteoarthritis patients; lane 6 is the dot blot of probe only (control).
Discussion
Rheumatoid arthritis is a systemic disease producing inflammation in diarthroidal joints as well as affecting lungs, heart, nervous system, and skin. It affects about 0.5–1.0% of the adult population and is found worldwide.16 Of the myriad of agents, environmental and genetic, implicated in rheumatoid disease mechanisms and pathology, considerable evidence indicates that viruses may be important environmental triggers. Viruses associated with arthritis‐like symptoms include rubella, hepatitis, alphaviruses, and parvovirus B19.17
Several viruses, including EBV,18 parvovirus B19,19 and cytomegalovirus,20 have already been identified within the joint, although no clear link has been established definitively linking these pathogens with rheumatoid arthritis. One such study, by Stahl and colleagues,21 detected the presence of multiple viral DNA in synovial tissue and fluid taken from patients in the early stages of idiopathic arthritis. Up to two thirds of patients tested were positive for at least one virus, with 20% of those patients positive for two or more.
One group of viruses in particular has attracted a large amount of interest over the past two decades with respect to rheumatoid arthritis. These are the retroviruses.10,22 Among the mounting evidence implicating retroviruses in rheumatoid arthritis are the parallels that can be drawn between human and animal retroviral infections. Animal retroviral pathogens such as caprine arthritis encephalitis virus (CAEV) and maedi visna virus (MVV) cause a chronic arthritis in sheep and goats.23 Both diseases show similarities to human rheumatoid arthritis in their pathology. Furthermore, the spontaneous development of rheumatoid arthritis‐like disease in mice transgenic for the HTLV‐1 tax gene hints at retroviral involvement.24 Also evident is the increased prevalence of rheumatic diseases in areas endemic for retroviruses—for example, HTLV in Japan and southern Central America.25
Seemayer et al26 conducted an elaborate series of experiments involving the co‐culture of rheumatoid patient synovial tissue samples with cell lines known to be permissive for retroviruses. This approach, however, failed to implicate any exogenous retroviral agents in rheumatoid arthritis, although the investigators did not exclude any potential endogenous retroviral activity. Neidhart and colleagues also isolated and identified a retroviral transcript from rheumatoid synovial fluid cells corresponding to ORF2 of the L1‐retrotransposon.27 Additionally human retrovirus‐5 (HRV‐5) has also been implicated as playing a role in rheumatoid arthritis disease pathogenesis,28 although these conclusions were not supported by follow up studies.29
In the present study we showed that levels of HERV‐K10 expression were significantly increased in rheumatoid PBMC samples compared with both disease controls and normal healthy donors (p = 0.02). There was no significant difference between osteoarthritis and healthy individuals (p = 0.34). These data were supported by previous evidence from Nakagawa et al, who also showed that HERV‐K10 levels were increased in rheumatoid patients compared with normal healthy donors.30 Furthermore, our data confirm earlier work which showed that normal healthy donors have a baseline level of HERV expression in the peripheral blood.31,32 Consequently it is possible that differential expression of HERVs may be critical in disease states.
It was also shown that HERV‐K10 expression in rheumatoid patients was high at the site of disease (n = 17), with levels of expression almost twice as high as those seen in the peripheral blood (data not shown). These findings should be noted as preliminary, and require further verification with additional synovial samples.
In the context of the disease process, the precise role of HERV‐K10 in rheumatoid arthritis remains unclear. It is plausible that the virus could be triggered by the ongoing immune response, or is indeed itself a trigger for rheumatoid arthritis.33 Evidently cells within the microenvironment of the joint (for example, B cells) may harbour HERVs themselves34 and therefore also have the potential to play a role in the disease pathology. It is also possible that external factors may contribute in its activation, leading to changes in HERV expression through a process of bystander activation. Many exogenous viruses such as EBV,35 HIV,36 and HSV37 are known to interact, either directly or indirectly, with endogenous retroviruses. Infection with many of these viruses also leads to an activation of specific host cells (for example, T cells with reference to HTLV‐I) during the course of infection, thus furthering the potential for immune dysregulation. Numerous cytokines are also known to modulate viral expression.38 Thus it is possible that host cells infected and activated by exogenous or endogenous viruses could migrate into the synovium, releasing pro‐inflammatory cytokines such as interleukin 639 and tumour necrosis factor α.40 Such events are likely to activate HERVs within the joint and may explain why levels of HERV activity were increased in the synovial fluid.
Conclusions
We have optimised a novel multiplex RT‐PCR system for the simple detection and semiquantification of HERV‐K10 gag gene against HtRNAS in rheumatoid patients versus controls. Overall there appear to be higher levels of K10 expression in peripheral blood and synovial fluid cells in rheumatoid patients. The possible contribution of HERV‐K10 to rheumatoid arthritis is intriguing and merits further investigation in a larger cohort of patients. Furthermore, the advent of new technologies such as quantitative RT‐PCR and microarrays, applied to this field of research, will undoubtedly help our understanding of the role of these HERVs in rheumatoid arthritis.
Acknowledgements
The authors are extremely grateful to Professor Alan Nevill, at the University of Wolverhampton, for his assistance with experimental design and statistical analysis. We would also like to thank the rheumatology departments at New Cross Hospital, Wolverhampton and Heartland Hospital, Birmingham for providing detailed patient information. This work was funded through a University of Wolverhampton PhD studentship.
Abbreviations
HERV - human endogenous retrovirus
HTLV - human T cell lymphotropic virus
HtRNAS - histidyl tRNA synthetase
NSAID - non‐steroidal anti‐inflammatory drug
PBMC - peripheral blood mononuclear cell
RT‐PCR - reverse transcription polymerase chain reaction
References
- 1.Nelson P N. Retroviruses in rheumatoid diseases. Ann Rheum Dis 199554441–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urnovitz H B, Murphy W H. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin Microbiol Rev 1996972–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lower R. The pathogenic potential of endogenous retroviruses: facts and fantasies. Trends Microbiol 19997350–356. [DOI] [PubMed] [Google Scholar]
- 4.Nelson P N, Carnegie P R, Martin J, Davari Ejtehadi H, Hooley P, Roden D.et al Demystified. Human endogenous retroviruses. Mol Pathol 20035611–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gifford R, Tristem M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes 200326291–315. [DOI] [PubMed] [Google Scholar]
- 6.Nelson P N, Lever A M L, Smith S, Pitman R, Murray P, Perera S A.et al Molecular investigations implicate human endogenous retroviruses as mediators of anti‐retroviral antibodies in autoimmune rheumatic disease. Immunol Invest 199928277–289. [DOI] [PubMed] [Google Scholar]
- 7.Larsson E, Andersson G. Beneficial role of human endogenous retroviruses: facts and hypotheses. Scand J Immunol 199848329–338. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa K, Harrison L C. The potential roles of endogenous retroviruses in autoimmunity. Immunol Rev 1996152194–236. [DOI] [PubMed] [Google Scholar]
- 9.Talal N, Dauphinee M J, Dany M.et al Detection of serum antibodies to retroviral proteins in patients with primary Sjögren's syndrome (autoimmune exocrinopathy). Arthritis Rheum 199033774–781. [DOI] [PubMed] [Google Scholar]
- 10.Krieg A M, Gourley M F, Perl A. Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J 199262537–2544. [DOI] [PubMed] [Google Scholar]
- 11.Nelson P N, Lever A M L, Bruckner F E, Isenberg D A, Kessaris N, Hay F C. Polymerase chain reaction fails to incriminate exogenous retroviruses HTLV‐I and HIV‐1 in rheumatological diseases although a minority of sera cross‐react with retroviral antigens. Ann Rheum Dis 199453749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giovine F, Bailly S, Bootman J, Almond N, Duff G W. Absence of lentiviral and human T cell leukaemia viral sequences in patients with rheumatoid arthritis. Arthritis Rheum 199437349–358. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann M, Kalden J R. PCR and reverse dot hybridisation for the detection of endogenous retroviral transcripts. J Virol Methods 199446333–348. [DOI] [PubMed] [Google Scholar]
- 14.Garry R F, Fermin C D, Hart D J, Alexander S S, Donehower L A, Luo‐Zhang H. Detection of a human intracisternal A‐type retroviral particle antigenically related to HIV. Science 19902501127–1129. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Yasunaga T, Miyata T, Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol 198660589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firestein G S. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol 200336372–378. [DOI] [PubMed] [Google Scholar]
- 17.Perl A. Mechanisms of viral pathogenesis in rheumatic disease. Ann Rheum Dis 199958454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takei M, Mitamura K, Fujiwara S, Horie T, Ryu J, Osaka S.et al Detection of Epstein‐Barr virus‐encoded small RNA 1 and latent membrane protein 1 in synovial lining cells from rheumatoid arthritis patients. Int Immunol 19979739–743. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Murai C, Shibata S, Munakata Y, Ishii T, Ishii K.et al Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci USA 1998958227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehraein Y, Lennerz C, Ehlhardt S, Remberger K, Ojak A, Zang K D. Latent Epstein‐Barr virus (EBV) infection and cytomegalovirus (CMV) infection in synovial tissue of autoimmune chronic arthritis determined by RNA‐ and DNA‐in situ hybridization. Mod Pathol 200417781–789. [DOI] [PubMed] [Google Scholar]
- 21.Stahl H D, Hubner B, Seidl B, Liebert U G, van der Heijden I M, Wilbrink B.et al Detection of multiple viral DNA species in synovial tissue and fluid of patients with early arthritis. Ann Rheum Dis 200059342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naides S J. Viral arthritis including HIV. Curr Opin Rheumatol 19957337–342. [DOI] [PubMed] [Google Scholar]
- 23.Michaels F H, Banks K L, Reitz M S. Lessons from caprine and ovine retrovirus infections. Rheum Dis Clin North Am 1991175–23. [PubMed] [Google Scholar]
- 24.Iwakura Y, Saijo S, Kioka Y, Nakayama‐Yamada J, Itagaki K, Tosu M.et al Autoimmunity induction by human T cell leukemia virus type 1 in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. J Immunol 19951551588–1598.7636219 [Google Scholar]
- 25.Poiesz B J, Poiesz M J, Choi D. The human T‐cell lymphoma/leukemia viruses. Cancer Invest 200321253–277. [DOI] [PubMed] [Google Scholar]
- 26.Seemayer C A, Kolb S A, Neidhart M, Ohshima S, Gay R E, Michel B A.et al Absence of inducible retroviruses from synovial fibroblasts and synovial fluid cells of patients with rheumatoid arthritis. Arthritis Rheum 2002462811–2813. [DOI] [PubMed] [Google Scholar]
- 27.Neidhart M, Rethage J, Kuchen S, Kunzler P, Crowl R M, Billingham M E.et al Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum 2000432634–2647. [DOI] [PubMed] [Google Scholar]
- 28.Brand A, Griffiths D J, Herve C, Mallon E, Venables P J. Human retrovirus‐5 in rheumatic disease. J Autoimmun 199913149–154. [DOI] [PubMed] [Google Scholar]
- 29.Gaudin P, Ijaz S, Tuke P W, Marcel F, Paraz A, Seigneurin J M.et al Infrequency of detection of particle‐associated MSRV/HERV‐W RNA in the synovial fluid of patients with rheumatoid arthritis. Rheumatology (Oxford) 200039950–954. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa K, Brusic V, McColl G, Harrison L C. Direct evidence for the expression of multiple endogenous retroviruses in the synovial compartment in rheumatoid arthritis. Arthritis Rheum 199740627–638. [DOI] [PubMed] [Google Scholar]
- 31.Krieg A M, Gourley M F, Klinman D M, Perl A, Steinberg A D. Heterogeneous expression and coordinate regulation of endogenous retroviral sequences in human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses 199281991–1998. [DOI] [PubMed] [Google Scholar]
- 32.Brodsky I, Foley B, Haines D, Johnston J, Cuddy K, Gillespie D. Expression of HERV‐K proviruses in human leukocytes. Blood 1993812369–2374. [PubMed] [Google Scholar]
- 33.Nelson P N, Hooley P, Roden D, Davari Ejtehadi H, Rylance P, Warren P.et al Human endogenous retroviruses: transposable elements with potential? Clin Exp Immunol 20041381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejthadi H D, Martin J H, Junying J, Roden D A, Lahiri M, Warren P.et al A novel multiplex RT‐PCR system detects human endogenous retrovirus‐K in breast cancer. Arch Virol 2005150177–184. [DOI] [PubMed] [Google Scholar]
- 35.Sutkowski N, Chen G, Calderon G, Huber B T. Epstein‐Barr virus latent membrane protein LMP‐2A is sufficient for transactivation of the human endogenous retrovirus HERV‐K18 superantigen. J Virol 2004787852–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIntosh E M, Haynes R H. HIV and human endogenous retroviruses: an hypothesis with therapeutic implications. Acta Biochim Pol 199643583–592. [PubMed] [Google Scholar]
- 37.Lee W J, Kwun H J, Kim H S, Jang K L. Activation of the human endogenous retrovirus W long terminal repeat by herpes simplex virus type 1 immediate early protein 1. Mol Cells 20031575–80. [PubMed] [Google Scholar]
- 38.Walev I, Dienes H P, Bohl J, Podlech J, Falke D. Correlation of virus replication, cytokine (TNF‐alpha and IL‐1) producing cells, neuronal necrosis and inflammation after intranasal infection of mice with herpes simplex virus strains of different virulence. Arch Virol 19951401957–1967. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara Y, Tsukazaki T, Osaki M, Nakashima M, Hasui K, Shindo H. Altered expression of inflammatory cytokines in primary osteoarthritis by human T lymphotropic virus type I retrovirus infection: a cross‐sectional study. Arthritis Res Ther 20046R347–R354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Ishii K K, Munakata Y, Saitoh T, Kaku M, Sasaki T. Regulation of tumor necrosis factor alpha promoter by human parvovirus B19 NS1 through activation of AP‐1 and AP‐2. J Virol 2002765395–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]