Abstract
Objective
To explore the in vivo effects of PD‐0200347, an α2δ ligand of voltage gated Ca2+ channels, on cell signalling in osteoarthritic (OA) chondrocytes from an experimental dog model, and examine the effect of PD‐0200347 on the major signalling pathways involved in OA cartilage degradation.
Methods
OA was surgically induced in dogs by sectioning the anterior cruciate ligament. OA dogs were divided into three groups and treated orally with (a) placebo; (b) 15 mg/kg/day PD‐0200347, or (c) 90 mg/kg/day PD‐0200347. The animals were killed 12 weeks after surgery. Cartilage specimens from femoral condyles and tibial plateaus were processed for immunohistochemistry. Specific antibodies against the phosphorylated form of PKCα, Ras, c‐Raf, the MAP kinases Erk1/2, p38, JNK, and the transcription factors, CREB and Elk‐1, were used.
Results
Levels of all the tested signalling mediators were increased in the placebo treated (OA) group compared with the normal group. PD‐0200347 treatment significantly reduced the levels of the active forms of PKCα, c‐Raf, Erk1/2, and Elk‐1; however, the levels of the active forms of Ras, p38, JNK, and CREB were not affected by the PD‐0200347 treatment.
Conclusion
The action of PD‐0200347 on OA chondrocytes is probably mediated through the inhibition of Erk1/2 activation via a Ras independent mechanism. This effect is associated with reduction of the activation of transcription factors such as Elk‐1, which leads to the inhibition of the induction of the major catabolic factors involved in the degradation process of OA cartilage.
Keywords: chondrocytes, osteoarthritis, calcium channels, PD‐0200347, PKC
Gabapentin, an anticonvulsant, and pregabalin have been successfully used for relieving spontaneous and paroxysmal pain in patients with neuropathy and other diseases.1,2,3,4,5 Gabapentin is also effective in animal models of pain, including the hyperalgesia associated with knee joint inflammation.6 The mechanisms responsible for the analgesic activity of gabapentin and pregabalin have been the subject of numerous studies. Although gabapentin is a structural analogue of γ‐aminobutyric acid (GABA), it does not interact with either GABAA or GABAB receptors. On the other hand, it was found to alter the non‐synaptic release of GABA in brain tissues in vitro.7 Both drugs bind with high affinity to the α2δ subunit of voltage gated Ca2+ channels,8 and can inhibit both the neuronal influx of calcium9 and the release of monoamines10 and glutamate.11 Voltage‐gated channels are multisubunit complexes found not only in the central nervous system but also in peripheral tissues such as cartilage.12 These channels consist of the voltage sensing α1‐pore‐forming subunit and the modulating accessory subunit—that is, α2δ, β, and δ.13 Voltage gated calcium influx could lead to the activation of critical intracellular signal pathways, including the activation of protein kinase C (PKC). This factor has been demonstrated to act on the regulation of matrix metalloproteinase (MMP) expression by PKC. In neurones, the effectiveness of the action of gabapentin correlates with the increase in the level of PKC activity.14,15
Many studies have tried to elucidate the role of ions, especially Ca2+, in chondrocyte biology and in osteoarthritis (OA) because their modulation could raise new treatment concepts for this disease. It has been demonstrated that Ca2+ and Ca2+ channels have a role in chondrocyte metabolism16,17 and, more particularly, in major cell functions such as replication and matrix formation.18 An increase of intracellular Ca2+ was demonstrated when different stress conditions were applied to chondrocytes.19 This means that variations of intracellular Ca2+ concentration could lead to the modification of cartilage formation.20 Of note, it was shown that Ca2+ is involved in the regulation of the synthesis of catabolic factors such as inducible nitric oxide synthase (iNOS)21,22 and MMPs.23,24
PD‐0200347 is a compound related to the gabapentin and pregabalin families. We demonstrated in a previous study,25 the potency of this compound in reducing the progression of experimental OA in dogs. We showed that PD‐0200347 treatment could inhibit the expression and synthesis of major OA catabolic factors—that is, iNOS and MMP‐1, ‐3, and ‐13. This inhibitory effect would explain the ability of PD‐0200347 to reduce the progression of experimental OA cartilage lesions.
The factors responsible for the appearance and progression of structural changes in OA have been the subject of intensive research in the past few decades. Significant progress has been made in understanding the pathophysiological pathways responsible for some of these changes26,27; however, much remains to be done to establish a therapeutic intervention that can effectively decelerate or arrest progression of the disease.
Among the different intracellular signalling pathways occurring in articular chondrocytes, the MAP kinase pathways are of importance. They include the extracellular signal regulated protein kinase (Erk1/2), the c‐jun N‐terminal kinases or stress activated protein kinases (JNK/SAPK), and the p38 kinases. They have been extensively described as well as shown to be implicated in the cellular responses of chondrocytes,28,29,30 especially during OA. More particularly, they were shown to be involved in the induction of the transcription of OA major catabolic factors such as MMPs.31 These cell signalling pathways act down stream of the activation of PKC.32,33,34,35
The goal of this study was to document the mechanism of action of PD‐0200347 on chondrocytes in experimental dog OA. We studied the effect of PD‐0200347 on the major signalling pathway intermediaries, PKCα, Ras, c‐Raf, the MAP kinases, Erk1/2, p38, JNK, and the transcription factors, CREB and Elk‐1.
Materials and methods
Experimental groups
Specimens were obtained from the experimental groups that had been included in a previous study.25 Twenty six adult crossbred dogs (2–3 years old), each weighing 20–25 kg, were used in this study. They included five normal dogs that were used as controls and killed at the same time as the OA dogs. The surgical sectioning of the anterior cruciate ligament of the right knee through a stab wound was performed on 21 dogs, as previously described.36,37 Before surgery, the animals were intravenously anaesthetised with pentobarbital sodium (25 mg/kg) and then intubated. After surgery, the dogs were kept at a housing farm where they could exercise freely in a large pen.
The OA dogs were randomly divided into three treatment groups. Group 1 (n = 7 dogs) consisted of dogs that received a placebo (encapsulated methylcellulose); Groups 2 and 3 (n = 7 dogs in each group) received encapsulated PD‐0200347 at a total dose of 15 or 90 mg/kg/day, respectively, beginning the day after surgery. Dogs receiving PD‐0200347 were dosed three times a day (at 6 hour intervals) with either 5 or 30 mg/kg throughout the duration of the study. Animal care personnel were unaware of the treatment groups. All dogs were killed 12 weeks after surgery. The study protocol was approved by the Clinical Research Ethics Committee at the Notre‐Dame Hospital of the University of Montreal Hospital Centre.
Immunohistochemistry
Cartilage specimens (n = 4–5) were processed for immunohistochemical analysis, as previously described.38,39 Specimens were fixed in TissuFix No 2 (Chaptec, Montreal, Quebec, Canada) for 24 hours and then embedded in paraffin. Sections (5 μm) of paraffin embedded specimens were placed on Superfrost Plus slides (Fisher Scientific, Nepean, Ontario, Canada), deparaffinised in toluene, rehydrated in a reverse graded series of ethanol, and either preincubated with chondroitinase ABC 0.25 units/ml (Sigma‐Aldrich Canada, Oakville, Ontario, Canada) in phosphate buffered saline (PBS) pH 8.0 for 60 minutes at 37°C (for all tested antibodies except anti‐Erk1/2) or heated in citrate buffer 10 mM pH 6.0 at 68°C for 20 minutes (anti‐Erk1/2). The specimens were subsequently washed in PBS, incubated in 0.3% Triton X‐100 for 20 minutes, and then placed in 3% hydrogen peroxide/PBS for 15 minutes. Slides were further incubated with a blocking serum (Vectastain ABC kit; Vector Laboratories, Inc, Burlingame, CA) for 60 minutes, after which they were blotted, and then overlaid with the primary antibody against the phosphorylated (activated) form of PKCα (dilution 1/75, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), Ras (dilution 1/10, Cell Signaling, Beverly, MA, USA), c‐Raf (dilution 1/20, Biosource, Nivelles, Belgium), Erk1/2 (dilution 1/25, Cell Signaling), p38 (dilution 1/25, Biosource), JNK (dilution 1/25, Santa Cruz), CREB (dilution 1/25, Santa Cruz), and Elk‐1 (dilution 1/10, Cell Signaling) for 18 hours at 4°C in a humidified chamber.
Each slide was washed three times in PBS (pH 7.4) and stained using the avidin‐biotin complex method (Vectastain ABC kit), which entails incubation in the presence of the biotin conjugated secondary antibody for 45 minutes at room temperature, followed by the addition of the avidin‐biotin‐peroxidase complex for 45 minutes. All incubations were carried out in a humidified chamber at room temperature and the colour was developed with 3,3′‐diaminobenzidine (DAKO Diagnostics Canada Inc, Mississauga, Ontario, Canada) containing hydrogen peroxide. Slides were counterstained with eosin.
To determine the specificity of staining, two control procedures were employed according to the same experimental protocol: (a) omission of the primary antibody; and (b) substitution of the primary antibody with an autologous preimmune serum. Controls showed only background staining.
Several sections were made from each block of cartilage, and slides from each specimen were processed for immunohistochemical analysis. Each section was examined under a light microscope (Leitz Orthoplan; Leica Inc, St. Laurent, Quebec, Canada) and photographed with a CoolSNAP cf Photometrics camera (Roper Scientific, Rochester, NY, USA).
Morphometric analysis
The different antigens present in the cartilage were quantified using our previously published method25,38,40 and estimated by determining the number of chondrocytes that stained positive in the entire thickness of cartilage. Three sections from each femoral condyle and tibial plateau were examined and each one was separately scored. The resulting data were integrated as a mean for each specimen. The cartilage was divided into six microscopic fields (three in the superficial zone and three in the deep zone) (×40; Leica Inc), and the results were averaged. Before evaluation, it was ensured that an intact cartilage for each arthritic specimen surface could be detected and used as a marker for the validation of the morphometric analysis. The superficial zone of cartilage corresponds to the superficial and the upper intermediate layers, and the deep zone to the lower intermediate and the deep layers. The total number of chondrocytes and those staining positive for the specific antigen were determined. The final results were expressed as the percentage of chondrocytes staining positive for the antigen (cell score), with the maximum score being 100%. Each slide was examined by two independent readers who were unaware of the treatment groups. The final score was reached by consensus between the two readers. For the statistical analysis, the data obtained from the medial and lateral femoral condyles and tibial plateaus were considered together. The staining in the deep zone of cartilage was negligible for each of the studied antibodies; therefore, the data presented are from the superficial zone of cartilage.
Statistical analysis
Values are expressed as the median and the range. Statistical analysis was performed using the Mann‐Whitney U test. Values of p<0.05 were considered significant.
Results
Levels of PKCα, Ras, and c‐Raf
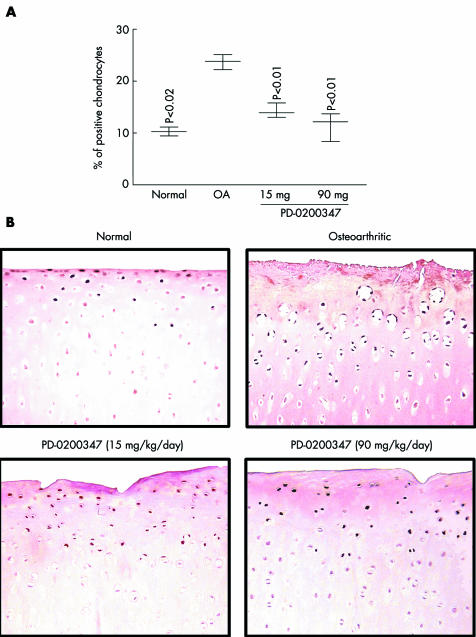
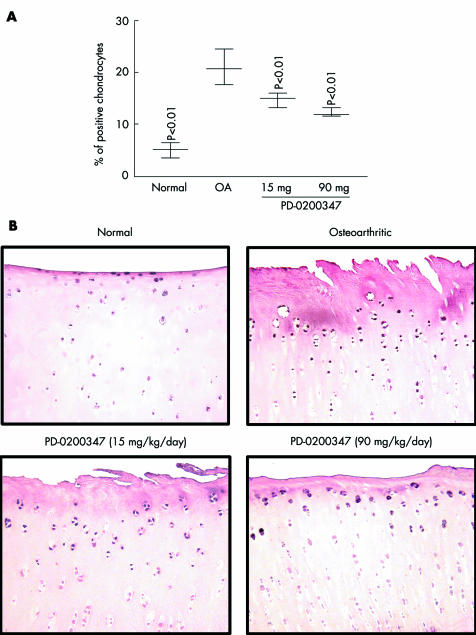
The phosphorylated (activated) form of PKCα, Ras, and c‐Raf were significantly increased in the OA group compared with the normal group (p<0.02, p<0.03, and p<0.01, respectively; figs 1 and 2, table 1). Treatment with PD‐0200347 significantly decreased activation of PKCα (p<0.01 for both tested doses) and c‐Raf (p<0.01 for both tested doses) in a dose dependent fashion (figs 1 and 2) but did not decrease activation of Ras (table 1).
Figure 1 Phospho‐PKCα detection by immunohistochemistry. (A) Morphometric analysis of phospho‐PKCα immunostaining in the superficial zone of cartilage. Data are expressed as median and range and are presented as a box and whisker plot, where the median line corresponds to the 1st and 3rd quartiles and to the median, and the lines outside represent the spread of the values. p Values were compared with the placebo treated (OA) group by the Mann‐Whitney U test. (B) Representative phospho‐PKCα immunohistochemical sections of tibial plateaus (original magnification ×250). No specific staining was detected in the OA cartilage in the control slides (data not shown).
Figure 2 Phospho‐c‐Raf detection by immunohistochemistry. (A) and (B) for phospho‐c‐Raf are as described in the legend of fig 1 for phospho‐PKCα.
Table 1 Morphometric analysis of immunostaining for phospho‐Ras, ‐JNK, ‐p38, and ‐CREB.
| Ras | p38 | JNK | CREB | |
|---|---|---|---|---|
| Normal | 7.0 (5.3–7.8) p<0.03 | 6.5 (5.4–8.2) p<0.03 | 7.3 (5.7–9.0) p<0.03 | 6.2 (4.7–9.6) p<0.03 |
| OA placebo treated | 20.4 (17.5–24.7) | 19.8 (16.5–20.5) | 17.8 (17.1–20.3) | 18.5 (13.7–19.6) |
| PD‐0200347 (90 mg/kg/day) | 21.4 (16.5–24–8) | 18.5 (16.6–20.9) | 19.7 (14.5–21.7) | 19.4 (11.3–26.0) |
Data were analysed by the Mann‐Whitney U test and p<0.05 versus the placebo treated (OA) group was considered significant.
Data are the median (range) of the percentage of positive chondrocytes for the specific antibody (n = 4).
Levels of MAP kinases: Erk1/2, p38, and JNK
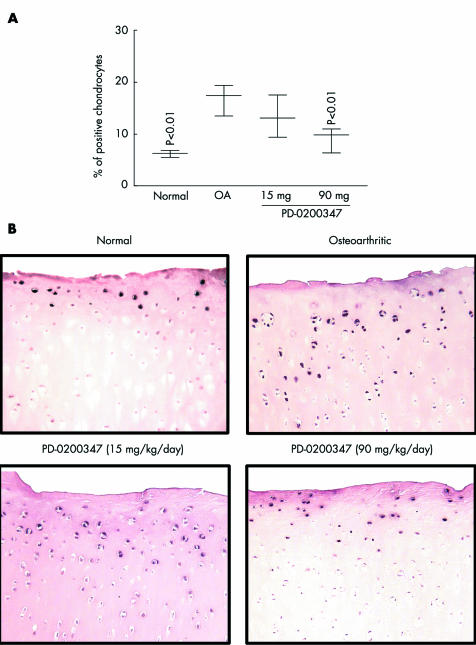
The effect of PD‐0200347 on the activation levels of three MAP kinase signalling pathways—namely, p38, JNK, and Erk1/2, was analysed. The phosphorylated (activated) forms of p38, JNK, and Erk1/2 were found to be increased in the OA group compared with the normal group (p<0.03, p<0.03, and p<0.01, respectively; table 1, fig 3). The highest tested dose of PD‐0200347 (90 mg/kg/day) significantly reduced Erk1/2 activation (p<0.01). The treatment was ineffective for p38 and JNK activation (table 1).
Figure 3 Phospho‐Erk1/2 detection by immunohistochemistry. (A) and (B) for phospho‐Erk1/2 are as described in the legend of fig 1 for phospho‐PKCα.
Levels of transcription factors: CREB and Elk‐1
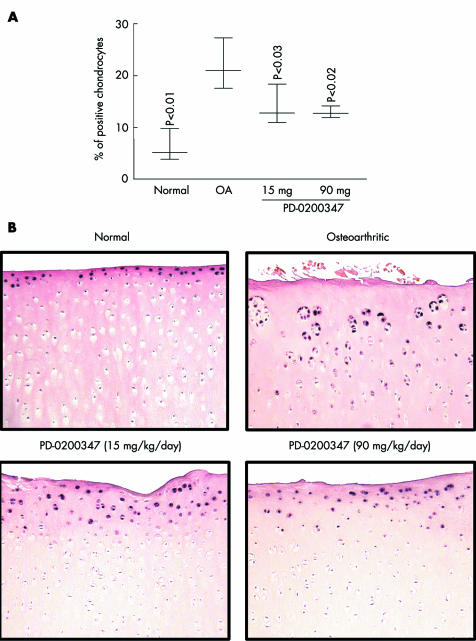
The levels of the active forms of the transcription factors, CREB and Elk‐1 were found to be increased in the OA group compared with the normal group (p<0.03 and p<0.01, respectively; fig 4, table 1). PD‐0200347 treatment significantly reduced activation of the Elk‐1 transcription factor at both doses (p<0.03, 15 mg/kg/day and p<0.02, 90 mg/kg/day) (fig 4). However, PD‐0200347 treatment had no effect on CREB activation (table 1).
Figure 4 Phospho‐Elk‐1 detection by immunohistochemistry. (A) and (B) for phospho‐Elk‐1 are as described in the legend of fig 1 for phospho‐PKCα.
Discussion
This study explored the mechanism of action of the in vivo effect of PD‐0200347 on chondrocytes in experimental dog OA. Emerging data demonstrate that PD‐0200347 inhibited the activation of PKCα and subsequently the MAP kinase Erk1/2 through a Ras independent mechanism. The downstream inhibition of major transcription factors, such as Elk‐1, could provide an explanation for the disease modifying OA drug effect of PD‐0200347 treatment in experimental dog OA. We demonstrated here that PD‐0200347 inhibits the PKCα/c‐Raf/Erk1/2/Elk‐1 signalling pathway.
We previously showed that PD‐0200347 treatment with dosages within the therapeutic range reduced the progression of experimental OA in dogs by inhibiting the synthesis of several major catabolic factors, iNOS and MMP‐1, ‐3, and ‐13, in cartilage from the lesional areas in the weightbearing zones of the OA knee.25 Because these catabolic factors have been previously shown to be preferentially increased in cartilage from lesional areas,25 it was therefore also relevant to explore the effects of the treatment on the cell signalling pathways in these areas of interest. However, because no significant staining was detected in any specimens with the studied proteins in the deep zone (data not shown), only the results obtained from the superficial zone are presented.
PKCα, an important signalling pathway in OA pathophysiology, has already been shown to be implicated in the gabapentin effect.14,41 The link between each of the intermediaries, PKC, Raf, and Erk1/2, has been well documented,42 and PKC and Erk1/2 pathways in chondrocytes have been specifically described.34 PKC has been shown as acting either up stream or on the transcription factors AP‐1 and NF‐κB.43 Both these pathways play a part in the regulation of MMP expression.44,45,46,47 Moreover, it has recently been demonstrated that PKCα and PKCζ play a part in the regulation of MMP‐1 and MMP‐3 syntheses.48 Together, these studies strongly support the role played by PKC in the up regulation of MMP expression and synthesis. c‐Raf, also known as Raf‐1, is a member of the serine/threonine‐specific kinases and found to be involved in the activation of major cellular signalling pathways, such as the activation of Erk1/2. The present study suggests that PD‐0200347 inhibition of c‐Raf activation is independent of the Ras pathway, which is in contrast to the generalisation that c‐Raf activation is dependent on Ras activation. However, it concurs with recent data that demonstrated in erythropoietin responsive cell lines that c‐Raf is activated independently of Ras.49 Interestingly, in the same study,49 the transcription factor Elk‐1 was shown to act downstream of Erk1/2, again in agreement with our results.
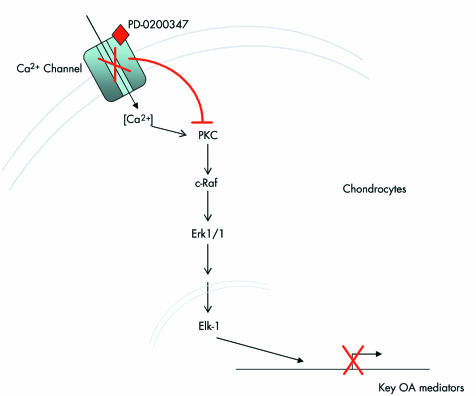
Although not completely elucidated, the pharmacological properties of PD‐0200347 are mainly associated with its specific binding to the voltage gated Ca2+ channel. Consequently, it was proposed (fig 5) that the effects of PD‐0200347 on the activation of PKCα and Erk1/2 pathways in OA chondrocytes occur through the binding of the drug to the α2δ subunit of voltage gated Ca2+ channels. This is possible as gabapentin has been shown to bind to the Ca2+ channels8 with a significant reduction of the current amplitude of Ca2+ in vitro in neurones and muscles in culture,41,50,51 and in vivo in animal models of neuropathic pain.52 A voltage dependent calcium influx could lead to the activation of critical intracellular signal pathways, including PKC. One must, however, exert caution in coming to any firm conclusions at this time about the mode of action of PD‐0200347 on OA pathophysiological pathways. The effect of the drug should be analysed in the context of a chronic treatment, which may influence several of the pathways that, on their own, have an impact on the intracellular signalling pathways. For instance, the structural protective effect of the drug in the dog anterior cruciate ligament model which was used in this study may very well have down regulated the synthesis of a number of catabolic factors, which also may have contributed indirectly to the effect of the drug found on the signalling pathways. Additional experiments are currently underway in our laboratory to explore and confirm these hypotheses.
Figure 5 Hypothetical mechanism of action of PD‐0200347 on articular chondrocytes during the OA process. One mechanism might be a reduced calcium influx in chondrocytes subsequent to PD‐0200347 binding to the α2δ protein of the calcium channel. Red lines indicate the inhibitory effect of gabapentin or PD‐0200347, on cell signalling. PKC, protein kinase C; Erk1/2, extracellular regulated kinase 1/2; Elk‐1, transcription factor; OA, osteoarthritis.
Alternative hypotheses to the action of gabapentin on OA chondrocytes might also be suggested, such as the inhibition of signalling pathways through alternative or unidentified mechanisms of action. Such hypotheses might include the ability of the drug to inhibit the activation of cell signalling pathways through other mechanism(s), which may not be exclusive of the first mechanism proposed. For instance, studies have demonstrated that stimulation of cells with Ca2+ ionophores initiates the generation of NO and reactive oxygen species (ROS),21 which are known to be capable of inducing PKC and Erk1/2 activation.53,54,55 Because chondrocytes can overproduce NO and ROS,56,57,58,59 this situation might possibly apply to OA and it is therefore possible to believe that treatment with PD‐0200347 has inhibited NO and ROS production, which might also have contributed to the inhibition of the cell signalling. Moreover, the recent demonstration that diltiazem, a Ca2+ channel antagonist, can block the synthesis of MMP‐1 in smooth muscle cells by a mechanism independent of c‐jun/AP‐1 expression24 suggests one additional hypothesis for the potential mode of action of PD‐0200347.
Conclusions
This study provides new insights into some of the possible mechanisms of the action of PD‐0200347 on reduction of the development of experimental OA lesions. This drug inhibits one of the major signalling pathways, the PKCα, and the Ras independent Erk1/2 activation, which might be implicated in the induction of major catabolic factors. Other effects that occur through an effect on Ca2+ channels, which remain unidentified at this time, may also explain the findings.
Acknowledgements
We thank François Mineau, BSc, for his exceptional technical support, and Santa Fiori and Martha Evans for their invaluable assistance in the preparation of this manuscript.
This study was supported by grants from Pfizer Global Research and Development, Ann Arbor, MI, USA, and Groupe de Recherche des Maladies Rhumatismales du Québec, Montreal, Quebec, Canada.
Abbreviations
GABA - γ‐aminobutyric acid
iNOS - inducible nitric oxide synthase
MMP - matrix metalloproteinase
OA - osteoarthritis
PBS - phosphate buffered saline
PKC - protein kinase C
ROS - reactive oxygen species
Footnotes
Competing interests: Denis Schrier and Craig Flory are employees of Pfizer Global Research and Development.
Jean‐Pierre Pelletier and Johanne Martel‐Pelletier are consultants for Pfizer Global Research and Development.
References
- 1.Rosner H, Rubin L, Kestenbaum A. Gabapentin adjunctive therapy in neuropathic pain states. Clin J Pain 19961256–58. [DOI] [PubMed] [Google Scholar]
- 2.Magnus L. Nonepileptic uses of gabapentin. Epilepsia. 1999;40: 66–72; discussion S73–4, [DOI] [PubMed]
- 3.Pappagallo M. Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. Clin Ther 2003252506–2538. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin R H, Corbin A E, Young J P, Jr, Sharma U, LaMoreaux L, Bockbrader H.et al Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo‐controlled trial. Neurology 2003601274–1283. [DOI] [PubMed] [Google Scholar]
- 5.Attal N, Brasseur L, Parker F, Chauvin M, Bouhassira D. Effects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot study. Eur Neurol 199840191–200. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Westlund K N. Gabapentin attenuates nociceptive behaviors in an acute arthritis model in rats. J Pharmacol Exp Ther 1999290214–219. [PubMed] [Google Scholar]
- 7.Kocsis J D, Honmou O. Gabapentin increases GABA‐induced depolarization in rat neonatal optic nerve. Neurosci Lett 1994169181–184. [DOI] [PubMed] [Google Scholar]
- 8.Gee N S, Brown J P, Dissanayake V U, Offord J, Thurlow R, Woodruff G N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem 19962715768–5776. [DOI] [PubMed] [Google Scholar]
- 9.Fink K, Dooley D J, Meder W P, Suman‐Chauhan N, Duffy S, Clusmann H.et al Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 200242229–236. [DOI] [PubMed] [Google Scholar]
- 10.Dooley D J, Donovan C M, Meder W P, Whetzel S Z. Preferential action of gabapentin and pregabalin at P/Q‐type voltage‐sensitive calcium channels: inhibition of K+‐evoked. Synapse 200235171–190. [DOI] [PubMed] [Google Scholar]
- 11.Maneuf Y P, Hughes J, McKnight A T. Gabapentin inhibits the substance P‐facilitated K(+)‐evoked release of [(3)H]glutamate from rat caudial trigeminal nucleus slices. Pain 200193191–196. [DOI] [PubMed] [Google Scholar]
- 12.Poiraudeau S, Lieberherr M, Kergosie N, Corvol M T. Different mechanisms are involved in intracellular calcium increase by insulin‐like growth factors 1 and 2 in articular chondrocytes: voltage‐gated calcium channels, and/or phospholipase C coupled to a pertussis‐sensitive G‐protein. J Cell Biochem 199764414–422. [PubMed] [Google Scholar]
- 13.Maneuf Y P, Gonzalez M I, Sutton K S, Chung F Z, Pinnock R D, Lee K. Cellular and molecular action of the putative GABA‐mimetic, gabapentin. Cell Mol Life Sci 200360742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Huang L Y. Gabapentin actions on N‐methyl‐D‐aspartate receptor channels are protein kinase C‐dependent. Pain 20019385–92. [DOI] [PubMed] [Google Scholar]
- 15.Taylor C P, Gee N S, Su T Z, Kocsis J D, Welty D F, Brown J P.et al A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 199829233–249. [DOI] [PubMed] [Google Scholar]
- 16.Yellowley C E, Hancox J C, Donahue H J. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem 200286290–301. [DOI] [PubMed] [Google Scholar]
- 17.Wohlrab D, Lebek S, Kruger T, Reichel H. Influence of ion channels on the proliferation of human chondrocytes. Biorheology 20023955–61. [PubMed] [Google Scholar]
- 18.Tanaka N, Ohno S, Honda K, Tanimoto K, Doi T, Ohno‐Nakahara M.et al Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res 20058464–68. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez J C, Wilkins R J. Changes in intracellular calcium concentration in response to hypertonicity in bovine articular chondrocytes. Comp Biochem Physiol A Mol Integr Physiol 2004137173–182. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q Q, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion‐channel dependent transduction of matrix deformation signals. Exp Cell Res 2000256383–391. [DOI] [PubMed] [Google Scholar]
- 21.Azenabor A A, Yang S, Job G, Adedokun O O. Expression of iNOS gene in macrophages stimulated with 17beta‐estradiol is regulated by free intracellular Ca2+. Biochem Cell Biol 200482381–390. [DOI] [PubMed] [Google Scholar]
- 22.Mustafa S B, Olson M S. Effects of calcium channel antagonists on LPS‐induced hepatic iNOS expression. Am J Physiol 1999277351–360. [DOI] [PubMed] [Google Scholar]
- 23.Yue H, Uzui H, Shimizu H, Nakano A, Mitsuke Y, Ueda T.et al Different effects of calcium channel blockers on matrix metalloproteinase‐2 expression in cultured rat cardiac fibroblasts. J Cardiovasc Pharmacol 200444223–230. [DOI] [PubMed] [Google Scholar]
- 24.Wada Y, Kato S, Okamoto K, Izumaru S, Aoyagi S, Morimatsu M. Diltiazem, a calcium antagonist, inhibits matrix metalloproteinase‐1 (tissue collagenase) production and collagenolytic activity in human vascular smooth muscle cells. Int J Mol Med 20018561–566. [DOI] [PubMed] [Google Scholar]
- 25.Boileau C, Martel‐Pelletier J, Brunet J, Tardif G, Schrier D, Flory C.et al Oral treatment with PD‐0200347, an α2δ ligand, reduces the development of experimental osteoarthritis by inhibiting metalloproteinases and inducible nitric oxide synthase gene expression and synthesis in cartilage chondrocytes. Arthritis Rheum 200552488–500. [DOI] [PubMed] [Google Scholar]
- 26.Martel‐Pelletier J, Lajeunesse D, Pelletier J P. Etiopathogenesis of osteoarthritis. In: Koopman WJ, ed. Arthritis and allied conditions. A textbook of rheumatology. Baltimore: Lippincott, Williams & Wilkins, 20052199–2226.
- 27.Pelletier J P, Martel‐Pelletier J, Abramson S B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001441237–1247. [DOI] [PubMed] [Google Scholar]
- 28.Fanning P J, Emkey G, Smith R J, Grodzinsky A J, Szasz N, Trippel S B. Mechanical regulation of mitogen‐activated protein kinase signaling in articular cartilage. J Biol Chem 200327850940–50948. [DOI] [PubMed] [Google Scholar]
- 29.Stanton L A, Underhill T M, Beier F. MAP kinases in chondrocyte differentiation. Dev Biol 2003263165–175. [DOI] [PubMed] [Google Scholar]
- 30.Han Z, Boyle D L, Chang L, Bennett B, Karin M, Yang L.et al c‐Jun N‐terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 200110873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liacini A, Sylvester J, Li W Q, Huang W, Dehnade F, Ahmad M.et al Induction of matrix metalloproteinase‐13 gene expression by TNF‐alpha is mediated by MAP kinases, AP‐1, and NF‐kappaB transcription factors in articular chondrocytes. Exp Cell Res 2003288208–217. [DOI] [PubMed] [Google Scholar]
- 32.Yoon J B, Kim S J, Hwang S G, Chang S, Kang S S, Chun J S. Non‐steroidal anti‐inflammatory drugs inhibit nitric oxide‐induced apoptosis and dedifferentiation of articular chondrocytes independent of cyclooxygenase activity. J Biol Chem 200327815319–15325. [DOI] [PubMed] [Google Scholar]
- 33.Thornborrow E C, Patel S, Mastropietro A E, Schwartzfarb E M, Manfredi J J. A conserved intronic response element mediates direct p53‐dependent transcriptional activation of both the human and murine bax genes. Oncogene 200221990–999. [DOI] [PubMed] [Google Scholar]
- 34.Hirota Y, Tsukazaki T, Yonekura A, Miyazaki Y, Osaki M, Shindo H.et al Activation of specific MEK‐ERK cascade is necessary for TGFbeta signaling and crosstalk with PKA and PKC pathways in cultured rat articular chondrocytes. Osteoarthritis Cartilage 20008241–247. [DOI] [PubMed] [Google Scholar]
- 35.Vincenti M P, Brinckerhoff C E. Transcriptional regulation of collagenase (MMP‐1, MMP‐13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene‐specific transcription factors. Arthritis Res 20024157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes J C, Martel‐Pelletier J, Otterness I G, Lopez‐Anaya A, Mineau F, Tardif G.et al Effects of tenidap on canine experimental osteoarthritis: I. Morphologic and metalloprotease analysis. Arthritis Rheum 1995381290–1303. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier J P, Mineau F, Raynauld J P, Woessner J F, Jr, Gunja‐Smith Z, Martel‐Pelletier J. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum 199437414–423. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier J P, Lascau‐Coman V, Jovanovic D, Fernandes J C, Manning P, Currie M G.et al Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J Rheumatol 1999262002–2014. [PubMed] [Google Scholar]
- 39.Moldovan F, Pelletier J P, Hambor J, Cloutier J M, Martel‐Pelletier J. Collagenase‐3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: In vitro mimicking effect by transforming growth factor beta. Arthritis Rheum 1997401653–1661. [DOI] [PubMed] [Google Scholar]
- 40.Pelletier J P, Fernandes J C, Brunet J, Moldovan F, Schrier D, Flory C.et al In vivo selective inhibition of mitogen‐activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum 2003481582–1593. [DOI] [PubMed] [Google Scholar]
- 41.Taylor C P. Mechanisms of action of gabapentin. Rev Neurol (Paris) 199715339–45. [PubMed] [Google Scholar]
- 42.Liebmann C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal 200113777–785. [DOI] [PubMed] [Google Scholar]
- 43.Sliva D, English D, Lyons D, Lloyd F P., Jr Protein kinase C induces motility of breast cancers by upregulating secretion of urokinase‐type plasminogen activator through activation of AP‐1 and NF‐kappaB. Biochem Biophys Res Commun 2002290552–557. [DOI] [PubMed] [Google Scholar]
- 44.Benbow U, Brinckerhoff C E. The AP‐1 site and MMP gene regulation: what is all the fuss about? Matrix Biol 199715519–526. [DOI] [PubMed] [Google Scholar]
- 45.Bond M, Chase A J, Baker A H, Newby A C. Inhibition of transcription factor NF‐kappaB reduces matrix metalloproteinase‐1, ‐3 and ‐9 production by vascular smooth muscle cells. Cardiovasc Res 200150556–565. [DOI] [PubMed] [Google Scholar]
- 46.Bond M, Fabunmi R P, Baker A H, Newby A C. Synergistic upregulation of metalloproteinase‐9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF‐kappa B. FEBS Lett 199843529–34. [DOI] [PubMed] [Google Scholar]
- 47.Bond M, Baker A H, Newby A C. Nuclear factor kappaB activity is essential for matrix metalloproteinase‐1 and ‐3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun 1999264561–567. [DOI] [PubMed] [Google Scholar]
- 48.Hussain S, Assender J W, Bond M, Wong L F, Murphy D, Newby A C. Activation of protein kinase Czeta is essential for cytokine‐induced metalloproteinase‐1, ‐3, and ‐9 secretion from rabbit smooth muscle cells and inhibits proliferation. J Biol Chem 200227727345–27352. [DOI] [PubMed] [Google Scholar]
- 49.Chen C, Sytkowski A J. Erythropoietin regulation of Raf‐1 and MEK: evidence for a Ras‐independent mechanism. Blood 200410473–80. [DOI] [PubMed] [Google Scholar]
- 50.Alden K J, Garcia J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther 2001297727–735. [PubMed] [Google Scholar]
- 51.Sutton K G, Martin D J, Pinnock R D, Lee K, Scott R H. Gabapentin inhibits high‐threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol 2002135257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Field M J, Hughes J, Singh L. Further evidence for the role of the alpha(2)delta subunit of voltage dependent calcium channels in models of neuropathic pain. Br J Pharmacol 2000131282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traore K, Trush M A, George M, Jr, Spannhake E W, Anderson W, Asseffa A. Signal transduction of phorbol 12‐myristate 13‐acetate (PMA)‐induced growth inhibition of human monocytic leukemia THP‐1 cells is reactive oxygen dependent. Leuk Res 200529863–879. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Mamputu J C, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase‐2 expression induced by leptin: inhibitory effect of metformin. Diabetes 2005542227–2234. [DOI] [PubMed] [Google Scholar]
- 55.Tao Q, Spring S C, Terman B I. Comparison of the signaling mechanisms by which VEGF, H2O2, and phosphatase inhibitors activate endothelial cell ERK1/2 MAP‐kinase. Microvasc Res 20056936–44. [DOI] [PubMed] [Google Scholar]
- 56.Tiku M L, Gupta S, Deshmukh D R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic Res 199930395–405. [DOI] [PubMed] [Google Scholar]
- 57.Henrotin Y E, Bruckner P, Pujol J P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 200311747–755. [DOI] [PubMed] [Google Scholar]
- 58.Jovanovic D V, Mineau F, Notoya K, Reboul P, Martel‐Pelletier J, Pelletier J ‐ P. Nitric oxide‐induced cell death in human osteoarthritc synoviocytes is mediated by tyrosine kinase activation and hydrogen peroxide and/or superoxide formation. J Rheumatol 2002292165–2175. [PubMed] [Google Scholar]
- 59.Del Carlo M, Jr, Loeser R F. Nitric oxide‐mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum 200246394–403. [DOI] [PubMed] [Google Scholar]