The antiphospholipid syndrome (APS) is characterised by recurrent arterial or venous thromboembolism, or pregnancy loss, in association with antiphospholipid antibodies.1 The pathogenesis of the obstetric complications remains unclear—both antiphospholipid antibodies and atherosclerosis may have a role. The ankle‐brachial index (ABI) is a bedside test which reliably identifies lower extremity peripheral artery disease in asymptomatic people.2 Our hypothesis was that a diffuse vessel wall abnormality as detected by the ABI might be associated with the pregnancy morbidity in patients with APS. We studied patients with APS with previous pregnancy loss but no thrombosis and compared their ABI and vascular risk factors with those of healthy women. The study was approved by the ethics committee.
An 8 MHz Doppler probe was used to assess the ABI in 30 patients with APS who had pregnancy loss and in 30 healthy women of similar age and race. Data on cardiovascular risk factors, including hypertension, hypercholesterolaemia, diabetes, renal failure, smoking, and other variables, were collected for all subjects. Patients also had fasting blood tests for glucose, lipid profile, renal profile, and other variables. A ratio of the highest blood pressure from the posterior tibial or pedal arteries of each leg to the highest blood pressure from the brachial arteries <1.00 was considered abnormal.3 Two different observers made the measurements; one did the measurements for the APS group and the other for the control group. The observers were not blinded for the clinical data. In our hands the intraobserver variation was 0.9% and the interobserver variation was 3%, consistent with reported figures.
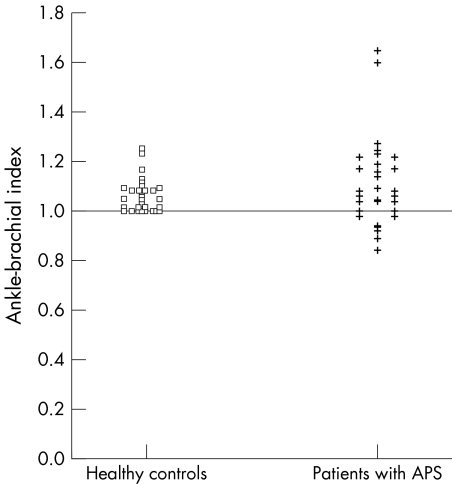
Twenty patients had primary and 10 had secondary APS. Seven (23%) patients with APS had an abnormal ABI compared with none in the control group (χ2 test, p<0.02; table 1). Four (57%) of these had primary APS and three (43%) had secondary APS. Figure 1 shows the distribution of the ABI values in patients and controls. The average age was 5.3 years higher in the APS group (Wilcoxon's test, p<0.04). The waist:hip ratio was higher in the APS group than in the control group (average values 0.82 and 0.78, respectively, Wilcoxon's test, p = 0.0073). No correlation between abnormal ABI and traditional cardiovascular risk factors or with the presence of antiphospholipid antibodies was found.
Table 1 Ankle‐brachial index results.
| APS | Controls | p Value | |
|---|---|---|---|
| (n = 30) | (n = 30) | ||
| Abnormal ABI (<1.00) | 7/30 (23) | 0/30 (0) | <0.02 |
| Primary APS | 4/7 (57) | ||
| Secondary APS | 3/7 (43) | ||
| Age (years), mean (range) | 37.5 (29–48) | 32.2 (18–49) | <0.04 |
Results are shown as No (%) unless stated otherwise.
APS, antiphospholipid syndrome; ABI, ankle‐brachial index.
Figure 1 Scattergram showing the distribution of ABI values for the cases and controls. An abnormal ABI was defined as <1.0.
Baron et al showed that 19% of patients with primary APS (mean age 40 years) and previous thrombosis had an abnormal ABI compared with 4% of healthy controls.4 Theodoridou et al found that patients with systemic lupus erythematosus (SLE) (mean age 39 years) also had a high prevalence of an abnormal ABI,5 with 37% having an ABI of <1. It is known that premature or accelerated atherosclerosis is a major cause of death in SLE.6,7
In our study the increased prevalence of an abnormal ABI in patients with APS who had pregnancy loss suggests that these patients are at increased risk of atherosclerosis and/or that a diffuse vessel wall abnormality may be contributing to the pregnancy loss. This might be in combination with other mechanisms related to antiphospholipid antibodies. Some studies have suggested that antiphospholipid antibodies may be associated with accelerated atherosclerosis in patients with APS.8,9,10 Taken together with our previous data showing that an abnormal ABI is associated with thrombosis in APS,4 we suggest that a large vessel vasculopathy may be a contributing factor to both thrombosis and pregnancy loss in APS.
References
- 1.Wilson W A, Gharavi A E, Koike T, Lockshin M D, Branch D W, Piette J C.et al International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 1999421309–1311. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Abrams J, Aurigemma G P, Bond M G, Clark L C, Criqui M H.et al Prevention conference V: beyond secondary prevention: identifying the high‐risk patient for primary prevention: non‐invasive tests of atherosclerotic burden: Writing group III. Circulation 2000101e16–e22. [DOI] [PubMed] [Google Scholar]
- 3.Sacks D, Bakal C W, Beatty P T, Becker G J, Cardella J F, Raabe R D.et al Position statement on the use of the ankle‐brachial index in the evaluation of patients with peripheral vascular disease. A consensus statement developed by the standards division of the Society of Cardiovascular and International Radiology. J Vasc Interv Radiol 200213353. [DOI] [PubMed] [Google Scholar]
- 4.Baron M A, Khamashta M A, Hughes G R V, D'Cruz D P. Prevalence of an abnormal ankle‐brachial index in patients with primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis 200564144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodoridou A, Bento L, D'Cruz D P, Khamashta M A, Hughes G R V. Prevalence and associations of an abnormal ankle‐brachial index in systemic lupus erythematosus: a pilot study. Ann Rheum Dis 2003621199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urowitz M B, Bookman A M, Koehler B E, Gordon D A, Smythe H A, Ogryzlo M A. The bimodal mortality pattern of SLE. Am J Med 197660221–225. [DOI] [PubMed] [Google Scholar]
- 7.Doherty N E, Siegel R J. Cardiovascular manifestations of systemic lupus erythematosus. Am Heart J 19851101257–1265. [DOI] [PubMed] [Google Scholar]
- 8.Vaarala O. Antiphospholipid antibodies and atherosclerosis. Lupus 19965442–447. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura E, Koike T. Accelerated atheroma and anti‐β2‐glycoprotein I antibodies. Lupus 20009210–216. [DOI] [PubMed] [Google Scholar]
- 10.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus 20009170–175. [DOI] [PubMed] [Google Scholar]