Abstract
Background
Reducing bisphosphonate dosing frequency may improve suboptimal adherence to treatment and therefore therapeutic outcomes in postmenopausal osteoporosis. Once‐monthly oral ibandronate has been developed to overcome this problem.
Objective
To confirm the 1 year results and provide more extensive safety and tolerability information for once‐monthly dosing by a 2 year analysis.
Methods
MOBILE, a randomised, phase III, non‐inferiority study, compared the efficacy and safety of once‐monthly ibandronate with daily ibandronate, which has previously been shown to reduce vertebral fracture risk in comparison with placebo.
Results
1609 postmenopausal women were randomised. Substantial increases in lumbar spine bone mineral density (BMD) were seen in all treatment arms: 5.0%, 5.3%, 5.6%, and 6.6% in the daily and once‐monthly groups (50 + 50 mg, 100 mg, and 150 mg), respectively. It was confirmed that all once‐monthly regimens were at least as effective as daily treatment, and in addition, 150 mg was found to be better (p<0.001). Substantial increases in proximal femur (total hip, femoral neck, trochanter) BMD were seen; 150 mg produced the most pronounced effect (p<0.05 versus daily treatment). Independent of the regimen, most participants (70.5–93.5%) achieved increases above baseline in lumbar spine or total hip BMD, or both. Pronounced decreases in the biochemical marker of bone resorption, sCTX, observed in all arms after 3 months, were maintained throughout. The 150 mg regimen consistently produced greater increases in BMD and sCTX suppression than the 100 mg and daily regimens. Ibandronate was well tolerated, with a similar incidence of adverse events across groups.
Conclusions
Once‐monthly oral ibandronate is at least as effective and well tolerated as daily treatment. Once‐monthly administration may be more convenient for patients and improve therapeutic adherence, thereby optimising outcomes.
Keywords: monthly, ibandronate, osteoporosis, bisphosphonate, non‐inferiority
Osteoporosis is a chronic, age related condition. Bone loss occurs without presenting symptoms and is often only diagnosed as a result of a fracture. An estimated one in three women will sustain an osteoporosis related fracture in their lifetime.1 These fractures result in increased disability and excess mortality. In 2003, the estimated total direct cost of osteoporosis related fractures in the European Union during the first year after fracture was €25 billion.2 In the USA, the annual expenditure for care of osteoporotic fractures was estimated to be $17.5 billion in 2002 (adjusted from 1995 to 2002 dollars).3
Oral bisphosphonates produce clinically significant reductions in the risk of new vertebral fractures4,5,6,7,8 and are the current mainstay of treatment for postmenopausal osteoporosis. However, long term treatment is required for optimal and sustained benefit.9,10 Long term adherence to any treatment, regardless of the disease, is known to be poor.11 Based on evidence from other chronic diseases,12 it is expected that reducing the burden of administration by further decreasing the dosing frequency of bisphosphonates may improve adherence.13 This is seen in the significant advantage provided by weekly over daily dosing.14,15,16 However, a growing body of evidence suggests that real‐world adherence to weekly oral bisphosphonates remains suboptimal. Indeed, recent studies suggest that persistence rates with weekly bisphosphonates at 1 year are less than 50%.14,15,16 Poor therapeutic adherence results in smaller decreases in bone turnover, reduced bone mineral density (BMD) gains, and a greater risk of fractures.17,18,19
Ibandronate (Boniva) is a potent, nitrogen‐containing bisphosphonate that can be administered with extended intervals between doses.20,21 Ibandronate is approved for the treatment of postmenopausal osteoporosis based on its demonstration of significant vertebral antifracture efficacy when administered orally, either daily or intermittently with extended intervals between doses of >2 months, with a safety and tolerability profile similar to placebo.4,5 A dose‐response relationship has been demonstrated with once‐monthly oral ibandronate, in a randomised, double blind, dose finding, phase I study (Monthly Oral Pilot Study (MOPS)), for systemic exposure (area under the curve and maximum concentration) and reduction of bone resorption22; confirming the feasibility of once‐monthly oral ibandronate dosing.
On the basis of these positive studies, MOBILE (Monthly Oral iBandronate In LadiEs) a randomised, phase III, non‐inferiority study was initiated. MOBILE was designed to compare the efficacy and safety of three once‐monthly oral ibandronate regimens (50 + 50 mg (given on 2 consecutive days), 100 mg, and 150 mg) with the efficacious and well tolerated daily oral ibandronate regimen (2.5 mg).4,5 The 1 year results indicated that all the once‐monthly ibandronate regimens were at least as effective as the active comparator, daily ibandronate, producing substantial increases in lumbar spine and proximal femur (total hip, femoral neck, trochanter) BMD.23 Furthermore, the once‐monthly regimens had a tolerability profile similar to the daily regimen. The 1 year analysis also suggested that the 150 mg once‐monthly regimen had a more pronounced effect for all efficacy end points than the 2.5 mg daily and 100 mg once‐monthly regimens, with similar safety and tolerability.
The purpose of this prospective, 2 year analysis was to confirm the findings of the 1 year efficacy analysis and to provide more extensive safety and tolerability information for once‐monthly ibandronate dosing.
Patients and methods
Study design
MOBILE was a 2 year, randomised, double blind, parallel group, phase III, non‐inferiority study, conducted in 65 centres in USA, Canada, Mexico, Brazil, Europe, Australia, and South Africa. The institutional review boards of the participating centres provided ethical approval of the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation of Good Clinical Practice guidelines.
Study participants and medication
All study participants were ambulatory postmenopausal women, aged 55–80 years and at least 5 years since the menopause, with osteoporosis (mean lumbar spine (L2–4) BMD T‐score between <−2.5 and >−5.0). Further details of the prespecified inclusion and exclusion criteria have previously been reported.23 All participants provided written informed consent.
At enrolment, participants were randomised into one of four oral ibandronate treatment groups, either 2.5 mg daily, 50 + 50 mg once‐monthly (single doses given on 2 consecutive days), 100 mg once‐monthly or 150 mg once‐monthly. Participants also received daily or once‐monthly oral placebo to maintain blinding, plus daily elemental calcium (500 mg) and vitamin D (400 IU) supplements. Participants were instructed to take their study medication after an overnight fast (⩾6 hours) and with 240 ml of plain water. They were then required to stay upright and fast for at least 60 minutes after dosing.
Primary efficacy end point
The primary efficacy end point was the change (%) from baseline in lumbar spine (L2–4) BMD at 1 year, as measured by dual energy x ray absorptiometry (DXA). The results for the primary efficacy end point have previously been reported.23
Secondary efficacy end points
Secondary efficacy end points included the change (%) from baseline in lumbar spine (L2–4) and proximal femur (total hip, femoral neck, trochanter) BMD at 2 years, as measured by DXA. GE Lunar (Madison, WI, USA) or Hologic (Bedford, MA, USA) instruments were used when performing DXA scans; scans were assessed by a central BMD reading centre (Synarc, Portland, USA). Responder analyses, defined as the percentage of participants maintaining or achieving increases in lumbar spine BMD, total hip BMD or lumbar spine and total hip BMD above baseline were carried out. In addition, the proportion of patients achieving defined increases in lumbar spine or total hip BMD (⩾6% and ⩾3%, respectively), previously associated with vertebral24 and non‐vertebral25 antifracture efficacy, was also prospectively evaluated.
The change (%) from baseline in serum concentrations of the biochemical marker of bone resorption C‐telopeptide of the α‐chain of type I collagen (sCTX) was also measured at 2 years. Blood samples for sCTX assessments were taken immediately before the next scheduled, once‐monthly dose, after an overnight fast (⩾6 hours), and between 8 am and 10 am. sCTX levels measured in the once‐monthly groups therefore represent the residual magnitude of suppression at the end of the dosing interval. sCTX assays (Elecsys β‐CrossLaps/serum, Elecsys 2010, Roche Diagnostic, Basel, Switzerland) were analysed at a central biomarker laboratory (Synarc, Lyon, France). The proportion of patients responding to treatment, with sCTX reductions of ⩾50% and ⩾70% from baseline, was also prospectively identified. The threshold of 50% corresponds with the least significant change (1.96√2 × coefficient of variation).26
Safety parameters
Adverse events were continuously monitored throughout the study. The relationship between treatment and intensity of all adverse events was also assessed. Clinical vertebral and non‐vertebral fractures were identified symptomatically, confirmed radiographically, and reported as adverse events. Laboratory safety variables were analysed at baseline and every 6 months thereafter. Samples were processed at a centralised safety laboratory (MDS, Les Ponts de Baillet, France).
Randomisation
Eligible patients were stratified by centre and baseline BMD status before randomisation, to ensure comparable distribution of baseline BMD across the treatment arms. Participants were randomised using adaptive minimisation via a centralised “call in” system (Interactive Voice Response System, ClinPhone Ltd, Nottingham, UK).
Statistical analysis
Analysis populations
The per‐protocol (PP) population was used as the primary analysis population for all efficacy measures as this is considered the most conservative approach to detecting actual differences among treatments when performing a non‐inferiority analysis.27 The PP population consisted of all those participants in the intention to treat (ITT) population who had no major protocol violations at baseline or during the first year of study. Major violations were categorised as a baseline lumbar spine (L2–4) BMD T‐score >−2.5; poor compliance with treatment; unconfirmed menopausal status; prohibited concomitant disease or treatment before study; lack of efficacy data or reliable BMD data. If such violations occurred during year 2 then data from individual visits were censored rather than excluding the patient completely from the analysis.
The ITT population included all participants who were randomised, received at least one dose of the study drug, and had at least one efficacy follow up data point. To confirm the robustness of the PP findings, analyses were also performed for all efficacy end points using the ITT population. The safety population included all participants who received at least one dose of the study drug, including withdrawn participants, and had at least one follow up assessment.
Analysis of primary efficacy measure at 2 years and secondary efficacy end points
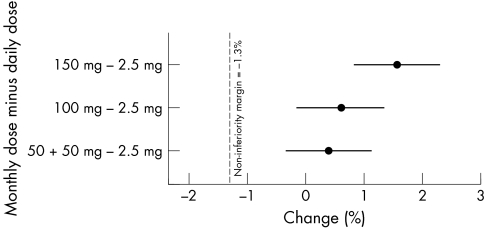
At 2 years, the change (%) from baseline in lumbar spine BMD with the once‐monthly regimens was compared with the change with the daily regimen using a non‐inferiority test. Non‐inferiority margins for the analysis of change (%) from baseline in lumbar spine BMD were based on 30% of the minimum treatment effect observed between daily oral ibandronate and placebo after 2 years in a prior clinical study (BONE).5 On this basis, non‐inferiority could be concluded if the lower boundary of the two sided 95% confidence interval (CI) for the difference in the means between the once‐monthly and daily regimens was ⩾−1.3% at 2 years.
If non‐inferiority was demonstrated for the primary efficacy measure then superiority of the once‐monthly regimens over the daily regimen was tested using analysis of variance, controlling for geographic location and baseline BMD. Changes (%) from baseline in proximal femur BMD and sCTX were summarised only, with 95% CIs for the difference in the mean BMD and median sCTX values between the once‐monthly and daily regimens calculated. The percentage of participants responding to treatment was compared using a χ2 test.
Analysis of safety variables
All adverse events reported throughout the 2 year study period were included in the safety analysis. All of the observed adverse events were evaluated by standardised tabulation of the prevalence and incidence.
Results
Patient disposition and baseline characteristics
In accordance with the original sample size calculation,23 1609 postmenopausal women were randomised into the study. Figure 1 shows the disposition of patients. A total of 1291 participants completed 2 years of treatment, with 292 (18.1%) participants withdrawing from the study. Most patients withdrew from the study during the first 3 months, regardless of treatment group.
Figure 1 Patient disposition. *50 mg on two consecutive days; †refused treatment includes “did not cooperate” and “withdrew consent”; ‡other includes “insufficient therapeutic response”, “early improvement”, “violation of selection criteria at entry”, and “other protocol violation”. AE, adverse event; trt, treatment.
The number of participants included in the PP population was 1277; the main reasons for excluding patients from the PP population, based upon 1 year data, were non‐compliance with daily or monthly schedule (∼17%), and no reliable BMD values (∼6%). Data were also censored for non‐compliance with daily study medication (∼8%) or the monthly dosing schedule during year 2 (∼7%). The number of participants included in the ITT population was 1572. Because, all patients took both daily and monthly tablets, these measures of compliance do not allow conclusions on differences in therapeutic adherence.
Baseline patient characteristics, such as age, years since menopause, history of fractures, bone turnover markers, and lumbar spine BMD, were well balanced across the treatment groups (table 1).
Table 1 Demographics (safety population).
| Demographics | Treatment group | |||
|---|---|---|---|---|
| 2.5 mg daily | 50 + 5 mg monthly | 100 mg monthly | 150 mg monthly | |
| (n = 395) | (n = 396) | (n = 396) | (n = 396) | |
| Age (years) | 65.8 | 66.0 | 66.2 | 66.2 |
| Weight (kg) | 64.1 | 64.1 | 64.0 | 63.7 |
| Height (cm) | 157.1 | 157.4 | 157.3 | 158.0 |
| Body mass index (kg/m2) | 25.9 | 25.8 | 25.9 | 25.5 |
| Time since menopause (years) | 18.3 | 18.7 | 19.1 | 18.3 |
| History of previous fractures (%)* | 48.9 | 46.3 | 45.5 | 47.0 |
| Lumbar spine (L2–4) BMD (g/cm2) | 0.755 | 0.755 | 0.756 | 0.754 |
| Lumbar spine (L2–4) BMD (T‐score) | −3.28 | −3.28 | −3.27 | −3.28 |
| sCTX (ng/ml)† | 0.49 | 0.52 | 0.51 | 0.50 |
| 25‐hydroxyvitamin D (ng/ml) | 25.7 | 24.4 | 25.1 | 24.7 |
Results are shown as the mean unless stated otherwise.
*:Percentage of patients; †median value.
Efficacy analysis
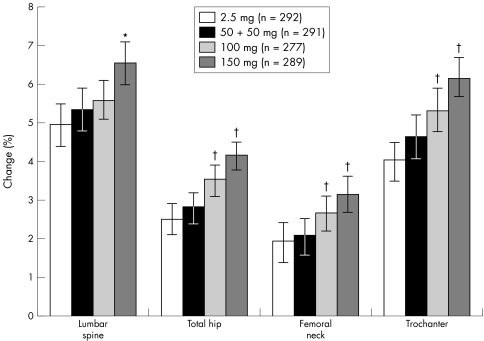
Substantial increases in lumbar spine (L2–4) BMD were seen in all treatment arms (fig 2). Compared with baseline, mean increases in lumbar spine BMD were 5.3% (95% CI 4.8 to 5.9), 5.6% (95% CI 5.1 to 6.1), and 6.6% (95% CI 6.0 to 7.1) in the 50 + 50 mg, 100 mg, and 150 mg once‐monthly groups, respectively, compared with 5.0% (95% CI 4.4 to 5.5) in the daily treatment group. All once‐monthly regimens were prospectively proved to be non‐inferior, and thus shown to be at least as effective as the daily regimen (fig 3). Furthermore, 150 mg once‐monthly was prospectively proved to be better than the daily regimen (p<0.001). A post hoc analysis showed that for the mean increase in lumbar spine (L2–4) BMD the 150 mg once‐monthly ibandronate regimen was also significantly better than the 100 mg once‐monthly treatment (p = 0.011).
Figure 2 Change (%) from baseline in lumbar spine and proximal femur BMD at 2 years (PP; mean (95% CI)). *p<0.001 versus daily treatment; †p<0.05 versus daily treatment.
Figure 3 Forest plot of the difference in the means (95% CI) of the mean change (%) from baseline in lumbar spine (L2–4) BMD between the once‐monthly and daily oral ibandronate regimens after 2 years (PP), demonstrating that all once‐monthly regimens are non‐inferior to the 2.5 mg daily dose regimen.
The ITT analysis confirmed these findings; mean increases in lumbar spine (L2–4) BMD were 5.3% (95% CI 4.7 to 5.8), 5.3% (95% CI 4.8 to 5.8), and 6.4% (95% CI 5.9 to 6.9) in the 50 + 50 mg, 100 mg, and 150 mg once‐monthly groups, respectively, compared with 4.8% (95% CI 4.3 to 5.3) in the daily treatment group. All the once‐monthly regimens were non‐inferior to the daily regimen, and the 150 mg once‐monthly regimen was superior (p<0.001). The 150 mg once‐monthly ibandronate regimen was also found, by post hoc analysis, to be statistically better than the 100 mg treatment for mean increases in lumbar spine (L2–4) BMD (p = 0.003).
Large increases in proximal femur BMD (total hip, trochanter, and femoral neck) were also seen in all treatment arms (fig 2). As at the lumbar spine, the most substantial increases in hip BMD were seen with the 150 mg regimen. From the 95% CIs for the difference between the means associated with the 100 mg and 150 mg regimens compared with the daily regimen, it was concluded that both once‐monthly regimens produced better increases in total hip, femoral neck, and trochanter BMD (p<0.05) (fig 2). Comparable findings were obtained from the ITT analysis, with substantial increases in proximal femur BMD reported for all ibandronate treatment regimens. The results of the superiority analysis were replicated with the ITT population, the one exception being the 100 mg regimen, which was no better than the daily regimen for increases in femoral neck BMD.
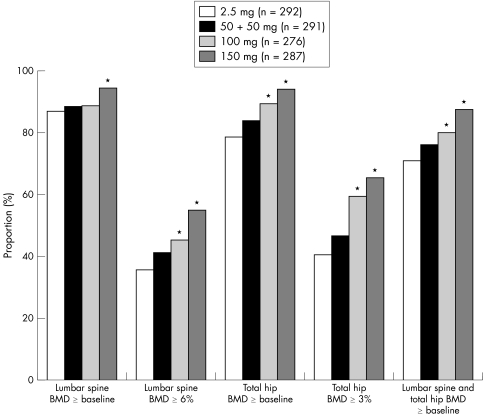
Independent of the ibandronate dosing regimen, lumbar spine BMD, total hip BMD, or both, increased above baseline in most participants (70.5–93.5%) (fig 4). In the 150 mg once‐monthly oral ibandronate group response rates at 2 years were consistently over 87% for increases in lumbar spine or total hip BMD above baseline, and were significantly greater than for the daily treatment group (p⩽0.004). A post hoc analysis found that the incidence of responders with increases above baseline in lumbar spine BMD or lumbar spine and total hip BMD in the 150 mg once‐monthly treatment group was significantly greater than in the 100 mg once‐monthly treatment group (p<0.01).
Figure 4 Participants (%) considered responders to daily or once‐monthly oral ibandronate (PP). *p<0.05 versus daily treatment.
Large proportions of patients also achieved target increases in lumbar spine (⩾6%; 35.4–54.3%) or total hip (⩾3%; 40.4–65.1%) BMD (fig 4). Overall, greater proportions of patients in the once‐monthly treatment arms achieved target increases in BMD than in the daily treatment arm, with the highest responder rates consistently seen with the 150 mg regimen (p<0.05 versus daily for all responder analyses; fig 4).
The incidence of responders to treatment was similar in the ITT analysis population; the 150 mg once‐monthly treatment group had a significantly increased percentage of patients with lumbar spine BMD, total hip BMD, or both, above baseline and with target increases (lumbar spine ⩾6%, and total hip ⩾3%) in comparison with the 2.5 mg daily treatment group (p⩽0.01).
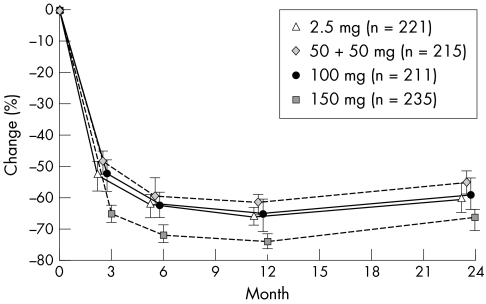
In all the treatment arms, the pronounced decreases in sCTX seen after 3 months23 were maintained throughout the 2 year study period (fig 5). Compared with baseline, median sCTX levels decreased by 56.1%, 60.5%, and 67.7% in the 50 + 50 mg, 100 mg, and 150 mg arms, respectively, compared with 61.5% in the daily group at 2 years.
Figure 5 Time course of median (95% CI) change (%) from baseline in sCTX (PP).
The percentage of patients with a ⩾50% decrease in sCTX from baseline was greater in the 150 mg once‐monthly treatment group (78.7%), than in the 50 + 50 mg (60.9%), 100 mg (63.5%), and 2.5 mg daily treatment groups (65.6%, p = 0.002). The proportion of patients with a ⩾70% decrease in sCTX from baseline followed the same pattern: 35.3%, 27.0%, 35.1%, and 48.1% for the 2.5 mg, 50 + 50 mg, 100 mg, and 150 mg treatment groups respectively. A significantly greater proportion of patients responded to the 150 mg once‐monthly regimen than to the daily regimen (p = 0.006). The ITT population analysis found comparable results for both levels of sCTX suppression.
Safety parameters
A similar proportion of patients in the once‐monthly and daily treatment arms withdrew from treatment. The numbers of withdrawals (not including deaths) due to adverse events were evenly balanced across the treatment arms: 31 (8%), 42 (11%), 36 (9%), and 39 (10%) in the 50 + 50 mg, 100 mg, 150 mg monthly, and daily treatment groups, respectively (fig 1).
The incidence of adverse events, drug related adverse events, and drug related adverse events leading to withdrawal was well balanced across the treatment arms (table 2). The most commonly reported adverse events, irrespective of relationship to treatment, were hypertension, dyspepsia and arthralgia; occurring with similar frequency across the treatment groups, and consistent with the population under study. A low rate of serious adverse events was observed. Only eight treatment related serious adverse events were reported during the 2 year study period. These were: gastric ulcer (two cases), duodenal ulcer, erosive duodenitis, gastric ulcer haemorrhage, gastritis haemorrhagic, melaena, and liver disorder. Most adverse events were considered unrelated to treatment and resulted in only a small number of patient withdrawals (table 2). In total, six deaths occurred during the study period, two during the second year, all of which were unrelated to treatment.
Table 2 Overall summary of safety (safety population).
| Treatment group | ||||
|---|---|---|---|---|
| 2.5 mg daily | 50 + 50 mg monthly | 100 mg monthly | 150 mg monthly | |
| (n = 395) | (n = 396) | (n = 396) | (n = 396) | |
| Overall | ||||
| Any adverse event | 302 (76.5) | 313 (79.0) | 318 (80.3) | 317 (80.1) |
| Any drug related adverse event: | 128 (32.4) | 119 (30.1) | 143 (36.1) | 146 (36.9) |
| Leading to withdrawal | 30 (7.6) | 20 (5.1) | 26 (6.6) | 27 (6.8) |
| Any serious adverse event | 38 (9.6) | 54 (13.6) | 55 (13.9) | 45 (11.4) |
| Any drug related serious adverse event: | 2 (0.5) | 2 (0.5) | 3 (0.8) | 1 (0.3) |
| Leading to withdrawal | 1 (0.3) | 1 (0.3) | 0 | 1 (0.3) |
| Adverse events of special interest | ||||
| Upper GI adverse events | 90 (22.8) | 79 (19.9) | 102 (25.8) | 89 (22.5) |
| Clinical osteoporotic fractures | 24 (6.1) | 29 (7.3) | 24 (6.1) | 27 (6.8) |
Results are shown as No (%).
The incidence of upper gastrointestinal (GI) adverse events was similar across the treatment arms (20–26%; table 2), and events were generally mild to moderate in severity. When only those who were taking non‐steroidal anti‐inflammatory drugs and/or aspirin at baseline (23.6–31.2%) were considered the number reporting upper GI events was slightly increased. However, the incidence of upper GI events was well balanced across the once‐monthly and daily treatment arms. In particular, there was no evidence that treatment for 2 years with once‐monthly doses of 150 mg led to an increased incidence in upper GI adverse events compared with the other once‐monthly or daily treatment groups.
The number of patients with diagnosed flu‐like illness considered by the investigator to be possibly or probably related to treatment was 1 (0.3%), 4 (1.0%), 5 (1.3%), and 13 (3.3%) for the 2.5 mg, 50 + 50 mg, 100 mg, and 150 mg groups, respectively. Such events were typically of short duration (1–4 days), mild to moderate in intensity, and associated with the first administered dose only. None of the patients experiencing flu‐like illness during year 1 had a recurrence of symptoms during year 2 of the study.
The number of clinical osteoporotic fractures was similarly low across the treatment groups (table 2). Overall, there were no clinically relevant changes in the mean haematology or clinical chemistry values during treatment in either the daily or monthly treatment groups.
Discussion
MOBILE is a randomised, double blind, comparative, non‐inferiority trial with the primary efficacy end point defined as the change (%) from baseline in lumbar spine BMD after 1 year.23 The data were analysed at 2 years to confirm the 1 year efficacy and safety findings, and to provide more extensive information about once‐monthly oral ibandronate for use in the treatment of postmenopausal osteoporosis.
The 2 year analysis categorically confirms the findings and conclusions drawn from the 1 year result.23 All the once‐monthly regimens produced substantial increases in lumbar spine BMD and were shown by a non‐inferiority test to be at least as effective as the daily oral ibandronate regimen at 1 and 2 years. As at 1 year, the increases in lumbar spine BMD observed in the 150 mg treatment arm were prospectively proved to be better than the daily regimen (p<0.001).
In addition to improvement in spinal BMD, the once‐monthly regimens produced significant increases in proximal femur (total hip, femoral neck, trochanter) BMD by the end of the 2 year study period, with the greatest gains observed with the 150 mg regimen (p<0.05). The 150 mg once‐monthly regimen consistently produced substantial improvements in proximal femur BMD over and above those seen with the daily regimen.
The increases in BMD are further supported by the marked decreases in sCTX seen in all treatment regimens. Pronounced decreases in sCTX were seen as early as 3 months23 and were maintained throughout the 2 year study period.
The prespecified responder analyses consistently demonstrated that a significantly larger proportion of postmenopausal women receiving 150 mg once‐monthly ibandronate achieve increases in lumbar spine and/or total hip BMD and decreases in sCTX of a defined magnitude after 2 years than with the daily regimen. This is particularly important considering that increases in vertebral BMD and decreases in biochemical markers of bone are both independently predictive for antifracture efficacy.24,25,28
After the second year of treatment, the safety profile of the daily and once‐monthly regimens was shown to be favourable and consistent with that reported after 1 year.23 All ibandronate regimens were generally well tolerated, with a similar incidence of adverse events and serious adverse events across the treatment groups. No upper GI safety and tolerability concerns were identified; the incidence was low and comparable across the once‐monthly and daily treatment arms, and no clinically relevant imbalances were observed even in patients receiving concomitant non‐steroidal anti‐inflammatory drugs and/or aspirin. The 2 year results furthermore confirmed a low incidence of flu‐like illness, of similar magnitude to that seen with other oral bisphosphonates,29,30 with events being of short duration and most frequently associated with the initial administration only. Overall, no relevant differences in safety and tolerability were seen between the once‐monthly and daily regimens. The findings of the overall safety and tolerability analysis are fully aligned with the favourable profile for oral ibandronate found in prior clinical studies, in which a safety profile similar to placebo was observed, independent of the dosing regimen.4
For all efficacy end points, the 150 mg once‐monthly regimen provides additional benefits in comparison with the daily and 100 mg once‐monthly regimens, without compromising safety and tolerability. A relative risk reduction in the incidence of new vertebral fractures of 62%, associated with significant increases in lumbar spine and suppression of bone turnover markers, has been demonstrated for daily oral ibandronate.4,5 The 150 mg once‐monthly regimen studied in MOBILE provided marked gains in lumbar spine BMD and decreases in sCTX over and above those demonstrated with the daily oral regimen.
A once‐monthly oral ibandronate regimen may provide further benefits through potentially greater adherence to treatment. The recently reported strong preference of patients for a once‐monthly bisphosphonate dosing regimen13 is expected to translate into improved therapeutic adherence and suggests that once‐monthly ibandronate dosing could provide additional adherence benefits over current dosing strategies, such as weekly treatment. Once‐monthly ibandronate is likely to provide an effective and patient‐friendly treatment option in postmenopausal osteoporosis.
Acknowledgements
This research was supported by F Hoffmann‐La Roche Ltd and GlaxoSmithKline. We are indebted to all those who have contributed to the conduct and analysis of the MOBILE study, including: Peter Alexandersen, Michael A Bolognese, João Brenol, Jacques P Brown, Roberto Civitelli, Edward Czerwiński, Tobie J de Villers, David V Doyle, Peter R Ebeling, Luis J Elizondo‐Alanis, Daniel Feldman, Johan Halse, David A Hanley, Gillian Hawker, Federico Hawkins, Johannes Hensen, Hans Christian Hoeck, David L Kendler, C Richard King, Howard R Knapp, Laszlo Koranyi, Norman S Koval, E Michael Lewiecki, Cesar R Libanati, Stanley Lipschitz, Roman S Lorenc, Marjorie Luckey, Liviu Macovei, Michael R McClung, James M McKenney, Jorge LA Morales‐Torres, Susan M Nattrass, Allan G Need, Ranuccio Nuti, Sebastião Radominski, Frank Raeman, Robert R Recker, John S Robinson, Sidney Rosenblatt, Adil Muhib Samara, Elliot N Schwartz, Sherwyn L Schwartz, Beata Spengler, Joan M Nolla Sole, Jacob A Stakkestad, Michael Stone, Istvan Szombati, Katalin Takacs, Daniel Uebelhart, Václav Vyskočil, and Rob Will.
Abbreviations
BMD - bone mineral density
CI - confidence interval
DXA - dual energy x ray absorptiometry
GI - gastrointestinal
ITT - intention to treat
MOBILE - Monthly Oral iBandronate In LadiEs
PP - per‐protocol
sCTX - C‐telopeptide of the α‐chain of type I collagen
Footnotes
Competing interests: The authors have received fees or reimbursement from the study sponsors, F Hoffmann‐La Roche Ltd and GlaxoSmithKline, for the following activities/items: consulting: J‐YR, SA, MG, SLS, CCh, MKD, CCo, DF, PDD, PDM; research grants: J‐YR, MG, SLS, MKD, DF, PDM; attending symposia: J‐YR, SA, JJS, MKD, CCo, DF, PDD; giving lectures: J‐YR, SA, MG, SLS, MKD, RE, CCo, DF, PDD; funds for a member of staff: JJS; organising educational programmes: DF. LR, NM, BB are employees of Hoffmann‐La Roche Ltd. PL has no competing interests.
References
- 1.Melton L J, Chrischilles E A, Cooper C, Lane A W, Riggs B L. Perspective. How many women have osteoporosis? J Bone Miner Res 199271005–1010. [DOI] [PubMed] [Google Scholar]
- 2.International Osteoporosis Foundation Osteoporosis in the European community: action plan. International Osteoporosis Foundation, 2003. Available from, http://www.osteofound.org/advocacy_policy/eu_policy_project/pdf/action_plan_03_e.pdf (accessed 12 January 2006)
- 3.Melton L J. Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 2003181139–1141. [DOI] [PubMed] [Google Scholar]
- 4.Chesnut C H, Skag A, Christiansen C, Recker R, Stakkestad J A, Hoiseth A.et al Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004191241–1249. [DOI] [PubMed] [Google Scholar]
- 5.Delmas P D, Recker R R, Chesnut C H, Skag A, Stakkestad J A, Emkey R.et al Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 200415792–798. [DOI] [PubMed] [Google Scholar]
- 6.Reginster J, Minne H W, Sorensen O H, Hooper M, Roux C, Brandi M L.et al Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 20001183–91. [DOI] [PubMed] [Google Scholar]
- 7.Black D M, Cummings S R, Karpf D B, Cauley J A, Thompson D E, Nevitt M C.et al Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 19963481535–1541. [DOI] [PubMed] [Google Scholar]
- 8.Harris S T, Watts N B, Genant H K, McKeever C D, Hangartner T, Keller M.et al Effects of risedronate treatment on vertebral and non‐vertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 19992821344–1352. [DOI] [PubMed] [Google Scholar]
- 9.Mellstrom D D, Sorensen O H, Goemaere S, Roux C, Johnson T D, Chines A A. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 200475462–468. [DOI] [PubMed] [Google Scholar]
- 10.Bone H G, Hosking D, Devogelaer J P, Tucci J R, Emkey R D, Tonino R P.et al Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 20043501189–1199. [DOI] [PubMed] [Google Scholar]
- 11.Haynes R B, McDonald H, Garg A X, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2002(2)CD000011. [DOI] [PubMed]
- 12.Claxton A J, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001231296–1310. [DOI] [PubMed] [Google Scholar]
- 13.Åkesson K, Beusterien K, Hebborn A, Kline Leidy N, Simon J. Patient preference for once‐monthly over once‐weekly bisphosphonate treatment [abstract]. Osteoporos Int 200516(suppl 3)S83 [Google Scholar]
- 14.Cramer J A, Amonkar M M, Hebborn A, Suppapanya N. Does dosing regimen impact persistence with bisphosphonate therapy among postmenopausal osteoporotic women? [abstract]. J Bone Miner Res 200419(suppl 1)S448 [Google Scholar]
- 15.Ettinger M P, Gallagher R, Amonkar M, Smith J C, MacCosbe P E. Medication persistence is improved with less frequent dosing of bisphosphonates, but remains inadequate [abstract]. Arthritis Rheum 200450(suppl)S513 [Google Scholar]
- 16.Bartl R, Goette S, Hadji P, Hammerschmidt T. Persistence and compliance with daily‐ and weekly‐administered bisphosphonates in German women with osteoporosis [abstract]. Osteoporos Int 200516(suppl 3)S45 [Google Scholar]
- 17.Caro J J, Ishak K J, Huybrechts K F, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 2004151003–1008. [DOI] [PubMed] [Google Scholar]
- 18.Eastell R, Garnero P, Vrijens L, van de Langerijt L, Pols H A P, Ringe J D.et al Influence of patient compliance with risedronate therapy on bone turnover marker and bone mineral density response: the Impact Study [abstract]. Calcif Tissue Int 200372P297 [Google Scholar]
- 19.Sebaldt R J, Shane L G, Pham B Z, Cook R, Thabane L, Petrie A.et al Longer‐term effectiveness outcomes of non‐compliance and non‐persistence with daily‐regimen bisphosphonate therapy in patients with osteoporosis treated in tertiary specialist care [abstract]. Osteoporos Int 200415(suppl 1)S107 [Google Scholar]
- 20.Mühlbauer R C, Bauss F, Schenk R, Janner M, Bosies E, Strein K.et al BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res 199161003–1011. [DOI] [PubMed] [Google Scholar]
- 21.Bauss F, Russell G G. Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int 200415423–433. [DOI] [PubMed] [Google Scholar]
- 22.Reginster J Y, Wilson K M, Dumont E, Bonvoisin B, Barrett J. Monthly oral ibandronate is well tolerated and efficacious in postmenopausal women: results from the monthly oral pilot study. J Clin Endocrinol Metab 2005905018–5024. [DOI] [PubMed] [Google Scholar]
- 23.Miller P D, McClung M R, Macovei L, Stakkestad J A, Luckey M, Bonvoisin B.et al Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1‐year results from the MOBILE Study. J Bone Miner Res 2005201315–1322. [DOI] [PubMed] [Google Scholar]
- 24.Wasnich R D, Miller P D. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 200085231–236. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg M C, Greenspan S, Wasnich R D, Miller P, Thompson D E, Ross P D. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 2002871586–1592. [DOI] [PubMed] [Google Scholar]
- 26.Garnero P, Mulleman D, Munoz F, Sornay‐Rendu E, Delmas P D. Long‐term variability of markers of bone turnover in postmenopausal women and implications for their clinical use. The OFELY study. J Bone Miner Res 2003181789–1794. [DOI] [PubMed] [Google Scholar]
- 27.ICH ICH harmonized tripartite guideline. Statistical principles for clinical trials. Statist Med 1999181905–1942. [PubMed] [Google Scholar]
- 28.Delmas P D, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti‐resorptive therapy. Bone 200434599–604. [DOI] [PubMed] [Google Scholar]
- 29.Website Alendronate prescribing information. http://www.fosamax.com (accessed 12 January 2006)
- 30.Wensite Risedronate prescribing information. http://www.actonel.com (accessed 12 January 2006)