Abstract
A recently released immunoassay detecting total serum hepatitis C virus (HCV) core antigen was used to prospectively monitor virological responses to antiviral treatment in patients with chronic HCV infection. Sustained responders cleared core protein from serum within the first month of therapy and maintained stably negative values for the entire duration of follow-up after treatment discontinuation. However, patients who relapsed or failed to respond showed transient negative values and could not be accurately discriminated either because of the intrinsic lower sensitivity of the core-antigen assay than those of molecular assays or because of differentially regulated secretion of immunoreactive core protein from infected hepatocytes.
Hepatitis C virus (HCV) infection frequently evolves to chronic liver disease which, in a proportion of cases, may progress to cirrhosis and hepatocellular carcinoma (1). Spontaneous resolution of persistent infection is anecdotical (3), and the current standard of care involves a long-term combination treatment with various formulations of alpha interferon and ribavirin (4). Therapeutic responses to antiviral drugs are currently monitored by qualitative or quantitative HCV RNA determination and—in most studies—early decline or disappearance of HCV RNA was found to be predictive of sustained virological response (9, 10). Recently, Fried et al. (6) conferred a negative predictive value on a less than 2 log10 reduction of HCV RNA after 12 weeks of treatment with pegylated alpha interferon and ribavirin. However, molecular assays for HCV RNA monitoring are not always available in clinical settings and require expert handling as well as dedicated personnel and laboratory space to avoid environmental contamination. A standardized enzyme immunoassay for quantitative detection of circulating HCV core protein, originally developed for blood bank screening purposes, has been recently released. The assay is highly specific, even though it is less sensitive than molecular assays (2, 4). The finding of a strong correlation with HCV RNA levels suggests that serum core antigen may represent an excellent surrogate marker of HCV replication (2). Unfortunately, only limited information is currently available on HCV core-antigen application to antiviral treatment monitoring of patients with chronic hepatitis C. Here we prospectively examined HCV core antigenemia compared to qualitative and quantitative HCV RNA testing in selected, well-characterized patients with chronic HCV infection treated with standard or pegylated alpha interferon and ribavirin.
Seven patients (three males and four females, median age, 56 years; range, 39 to 64 years) who were part of a large, independent Italian multicenter trial comparing standard and 12-kDa-pegylated alpha 2b interferon (Schering-Plough Co., Kenilworth, N.J.) in combination with ribavirin (unpublished data) were considered for this analysis. All were infected with genotype 1b (INNO-LiPA, HCV II; Bayer Corporation, Tarrytown, N.Y.), as this was a requirement for enrollment, and had chronic hepatitis of varying grading and staging scores at liver biopsy (6a). According to accepted criteria to evaluate therapeutic responses in this setting (5, 6), there were two sustained responders, three relapsers, and two nonresponders. Circulating HCV core antigen was detected by a commercially available enzyme immunoassay (Ortho trak-C assay; Ortho Clinical Diagnostics, Raritan, N.J.). The assay was carried out exactly as indicated by the manufacturer. The lower detection limit was 1.5 pg/ml. The upper detection limit was 100 pg/ml. Sera showing values exceeding this limit were diluted to obtain values comprised by the curve, and the data were extrapolated. Samples were tested in duplicate, although the excellent reproducibility led the manufacturer to recommend single determinations. In addition, the current version of the test is equally efficient at detecting core antigen derived from different genotypes (2). HCV RNA levels were determined by a quantitative signal amplification technique (Versant HCV RNA 3.0 bDNA, detection limit 615 IU/ml; Bayer Corporation) and were determined qualitatively by nested reverse transcription-PCR as described previously (7). The sensitivity of our in-house nested PCR assay was 365 IU/ml, according to a European Quality Control Panel, a detection threshold which was lower than that of the commercial quantitative assay. Patients were monitored during treatment for 1 year and for 1 subsequent year after treatment discontinuation for sustained responders or until biochemical and virological relapse occurred. Primary virological nonresponders stopped treatment at 6 months, as planned, and were monitored for 7 months thereafter.
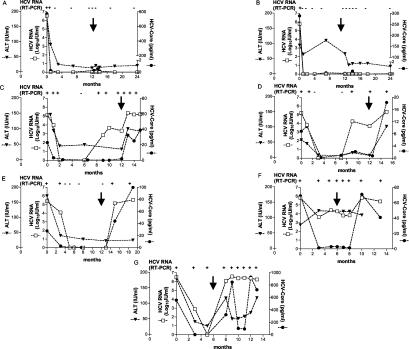
Serum HCV core protein levels were variably elevated at baseline, ranging from 1.6 to 775.1 pg/ml regardless of the final therapeutic outcome, and generally paralleled those of HCV RNA. There was no correlation between baseline HCV RNA or core protein levels and response to therapy. In sustained responder patients, serum HCV RNA and core-antigen concentrations rapidly declined, reaching stably negative values within 1 month from initiation of treatment (Fig. 1A and B). However, one patient (A) was still HCV RNA positive by nested PCR at the same time that HCV core and quantitative HCV RNA values were already below cutoff levels. Relapser patients were representative of three typical clinical situations. While biochemical response was observed in all patients with complete normalization of alanine aminotransferase (ALT), one remained HCV RNA positive, with the exception of a single time point, until treatment discontinuation (Fig. 1C), whereas one further patient became HCV RNA negative by PCR 3 months after starting therapy to become positive again at month 9 on treatment (Fig. 1D). The last relapser patient was found to be negative at month 5 on therapy and remained so until 1 month after treatment discontinuation (Fig. 1E). Unfortunately, core antigen levels could not accurately discriminate these patients from those who developed a sustained virological response since, with the exception of a single time point, they always decreased to below the detection limit 3 months after starting therapy. Primary nonresponders never completely normalized ALT values. One patient with the lowest baseline viremia of the lot maintained fluctuating core-antigen levels just above the detection limit, subsequently reaching levels higher than baseline after stopping therapy in close correlation with HCV RNA values (Fig. 1F). The other nonresponder showed very high core-antigen levels, which paralleled those of HCV RNA after an initial transient decline while on therapy (Fig. 1G).
FIG. 1.
Kinetics of serum HCV RNA (IU/ml), core antigen (pg/ml), and ALT (IU/ml) in patients treated with alpha interferon and ribavirin. Patients A and B were sustained responders as they normalized ALT and cleared HCV RNA during treatment and remained in remission for 1 year after therapy discontinuation. Patients C through E were defined as relapsers since they normalized ALT and transiently cleared HCV RNA while on treatment but returned to pretreatment features after stopping antivirals. Patients F and G were defined as nonresponders since they never normalized ALT and never cleared HCV RNA during treatment by qualitative PCR. Qualitative PCR data (+ or −) are indicated at the top of each panel. Patients B, C, D, and G received standard alpha 2b interferon, whereas patients A, E, and F received 12-kDa-pegylated alpha 2b interferon. Arrows indicate treatment discontinuation.
To our knowledge, this is the first study in which patients with chronic HCV infection treated with antiviral drugs were prospectively monitored by HCV core antigen testing. Collectively, these findings confirm that HCV core antigen is a reliable surrogate marker of HCV replication only for HCV RNA titers above approximately 10,000 IU/ml, determined by a signal amplification technique, as shown in several previous studies (2, 4, 8)—although we did occasionally, but not reproducibly, detect core-antigen levels above cutoff in some samples for HCV RNA values around 5,000 IU/ml. It is important to emphasize that the HCV core antigen-to-RNA ratio may differ among patients, suggesting variations in the amount of core protein per RNA molecule in different sera, in agreement with the presence of excess core protein not associated with viral genomes and vice versa (2). Indeed, it is also likely that secretion of the HCV core protein from infected hepatocytes is tightly regulated, which would explain on the one hand its delayed appearance in serum despite high HCV RNA levels (Fig. 1C, D, and F) and on the other hand its occasional wide fluctuations in the face of relatively stable RNA titers (Fig. 1F and G). Long-term follow-up of our patients with chronic HCV infection with different treatment outcomes suggests that the intrinsic lower sensitivity of the core antigen assay than those of molecular assays restricts its use as an HCV RNA surrogate test only in specific clinical settings. To this end, it is noteworthy that early loss of core antigen, as seen in patients A and B, may be indicative of subsequent sustained response; however, the simple loss of detectable core antigen in the peripheral blood is by no means suggestive of sustained response to antiviral therapy as shown in both relapser and nonresponder patients. Further studies on viral kinetics in larger cohorts are mandatory to confirm that early and sustained core-antigen clearance may be predictive of successful therapy outcome.
Acknowledgments
We thank Ortho Clinical Diagnostics, Italy, for providing us with the HCV core antigen kit, and we thank Lara Firmo for editorial assistance.
This work was supported by Ministero dell'Università e della Ricerca Scientifica (COFIN-MIUR prot. MM06261448_003) and Ministero della Salute (numbers 015RFM/00/01, 08920401, and103).
REFERENCES
- 1.Alberti, A., L. Chemello, and L. Benvegnù. 1999. Natural history of hepatitis C. J. Hepatol. 31(Suppl. 1):17-24. [DOI] [PubMed] [Google Scholar]
- 2.Bouvier-Alias, M., K. Patel, H. Dahari, S. Beaucourt, P. Larderie, L. Blatt, C. Hezode, G. Picchio, D. Dhumeaux, A. U. Neumann, J. G. McHutchison, and J.-M. Pawlotsky. 2002. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36:211-218. [DOI] [PubMed] [Google Scholar]
- 3.Cividini, A., C. Rebucci, E. Silini, and M. U. Mondelli. 2001. Is the natural history of hepatitis C virus carriers with normal aminotransferase really benign? Gastroenterology 121:1526-1527. [DOI] [PubMed] [Google Scholar]
- 4.Cividini, A., A. Cerino, A. Muzzi, M. Furione, C. Rebucci, L. Segagli, M. Gatti, V. Barnaba, and M. U. Mondelli. 2003. Kinetics and significance of serum hepatitis C virus core antigen in patients with acute hepatitis C. J. Clin. Microbiol. 41:2144-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bisceglie, A. M., and J. H. Hoofnagle. 2002. Optimal therapy of hepatitis C. Hepatology 36:S121-S127. [DOI] [PubMed] [Google Scholar]
- 6.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Gonçales, D. Häussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxì, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 6a.Knodell, R. G., K. G. Ishak, W. C. Black, T. S. Chen, R. Craig, N. Kaplowitz, T. W. Kiernan, and J. Wollman. 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431-435. [DOI] [PubMed] [Google Scholar]
- 7.Silini, E. M., F. Bono, A. Cerino, V. Piazza, E. Solcia, and M. U. Mondelli. 1993. Virological features of HCV infection in hemodialysis patients. J. Clin. Microbiol. 31:2913-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka, E., C. Ohue, K. Aoyagi, K. Yamaguchi, S. Yagi, K. Kiyosawa, and H. J. Alter. 2000. Evaluation of a new enzyme immunoassay for hepatitis C virus (HCV) core antigen with clinical sensitivity approximating that of genomic amplification of HCV RNA. Hepatology 32:388-393. [DOI] [PubMed] [Google Scholar]
- 9.Tong, M. J., L. M. Blatt, J. G. McHutchison, R. L. Co, and A. Conrad. 1997. Prediction of response during interferon α 2b therapy in chronic hepatitis C patients using viral and biochemical characteristics: a comparison. Hepatology 26:1640-1645. [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem, S., J. H. Lee, A. Franke, B. Rüster, O. Prümmer, and G. Herrmann. 1998. Quantification of the initial decline of serum hepatitis C virus RNA and response to interferon alfa. Hepatology 27:1149-1156. [DOI] [PubMed] [Google Scholar]