Abstract
Objective
To determine clinical and radiographic determinants of hyperkyphosis in patients with ankylosing spondylitis.
Methods
Spinal hyperkyphosis was assessed by occiput to wall distance (OWD) in 135 patients participating in the OASIS cohort and defined as OWD >0. Disease activity was assessed by the Bath ankylosing spondylitis disease activity index (BASDAI). Wedging of the vertebrae was calculated as the Ha/Hp ratio. Structural damage of the spine was assessed by the modified Stoke ankylosing spondylitis spine score (mSASSS). Hip involvement was assessed by the Bath ankylosing spondylitis radiology index (BASRI) and defined as a score >2. Data were analysed by multiple regression analysis on van der Waerden‐normal OWD values, with mean Ha/Hp ratio, mSASSS, hip involvement, and BASDAI as explanatory variables, and age, sex, and disease duration after diagnosis as covariates.
Results
61 patients (45.2%) had an OWD >0 cm. Of these, 81% were male, v 57% in the group with normal OWD (p<0.001). Forty two patients had wedged thoracic vertebrae, and 27 of these (44%) had an increased OWD, compared with 15 of 74 with a normal OWD (20%) (p = 0.005). OWD was correlated with mean wedging of the thoracic spine (r = −0.45, p = 0.01), mSASSS (r = 0.56, p = 0.01), and hip involvement (r = 0.2, p = 0.05). Multivariate analysis showed that mSASSS (standardised β (stβ) = 0.52; p<0.001), wedging of the thoracic spine (stβ = −0.28; p = 0.01), and BASDAI (stβ = 0.15; p = 0.05) were independent determinants of OWD.
Conclusions
Radiological damage of the cervical and lumbar spine, thoracic wedging, and disease activity are determinants of hyperkyphosis in patients with ankylosing spondylitis. These findings could be important in determining treatment goals in this disease.
Keywords: ankylosing spondylitis, occiput to wall distance, osteoporosis, radiological damage
Hyperkyphosis of the upper part of the spine is a common clinical problem among patients with ankylosing spondylitis.1,2,3 In our prevalence cohort of patients with ankylosing spondylitis (Outcome in Ankylosing Spondylitis International Study (OASIS) cohort) with a mean disease duration of 9.4 years, 49% of the patients have some degree of hyperkyphosis, if it is expressed as an occiput to wall distance (OWD) of more than 0 cm.4 The prominent position of the head and neck may give rise to functional and psychological impairment in these patients.5 They may be unable to see straight ahead and may have difficulties in activities of daily living; furthermore, severe hyperkyphosis may result in compression of the abdominal viscera.6
The degree of hyperkyphosis in patients with ankylosing spondylitis is related to radiological damage.7 In general, hyperkyphosis is also associated with vertebral osteoporosis, and it is increasingly recognised that osteoporosis is a problem in patients with ankylosing spondylitis.8,9,10,11,12 Vertebral deformities are regarded as one of the classical hallmarks of vertebral osteoporosis. In a population based study, the relative risk of vertebral morphometric deformities in patients with ankylosing spondylitis was 7.6 compared with the control population.13 Other investigators found a prevalence of vertebral deformities in 10–17% of the patients with ankylosing spondylitis seen in the clinic.14,15,16,17 In a pilot study we have shown that thoracic but not lumbar vertebral deformities are related to an increase of OWD in patients with ankylosing spondylitis.17 However, it is not known how all potential contributory factors (such as disease activity, structural damage visible on radiographs, hip involvement, and vertebral wedging) interrelate with respect to explaining increased OWD in patients with ankylosing spondylitis. We therefore investigated the independent contribution of various factors that may explain hyperkyphosis in a cross sectional study of patients with ankylosing spondylitis.
Methods
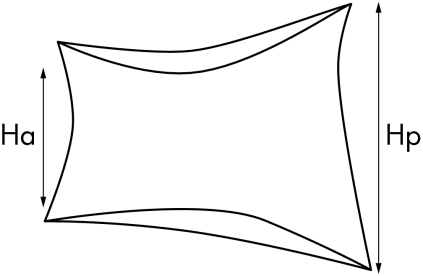
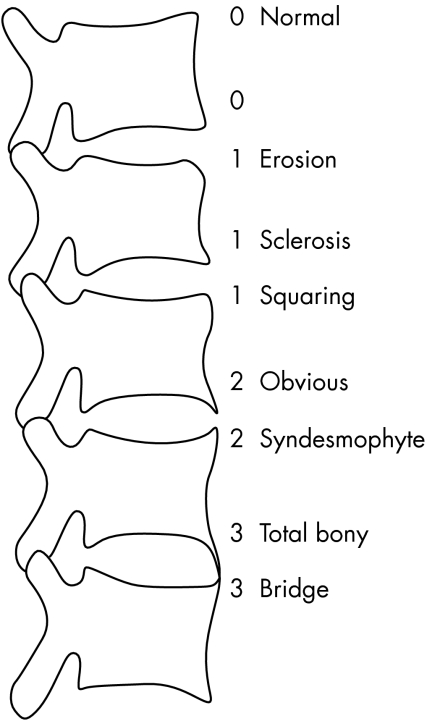
We included 139 patients of the OASIS cohort—an international longitudinal, observational study on outcome in ankylosing spondylitis, with a male to female ratio of 2:1, a mean disease duration of 9.4 years (defined as years since diagnosis), and a mean duration of complaints of 17.9 years. Consecutive patients in four secondary and tertiary referral centres, fulfilling the modified New York criteria for ankylosing spondylitis, were included.18 Patients were followed according to a fixed protocol.4 Data from the four year assessment were used in the present analysis, and include the Bath ankylosing spondylitis disease activity index (BASDAI),19 the Bath ankylosing spondylitis functional index (BASFI),20 lateral radiographs of the cervical, thoracic, and lumbar spine, and radiographs of the pelvis. To assess structural damage, the modified Stoke ankylosing spondylitis spine score (mSASSS) was used.21 This method scores the anterior site of the lumbar spine (lower border T12 to upper border S1) and cervical spine (lower border C2 to upper border T1) on a lateral view. The anterior corners of each vertebra are examined and scored 1 for an erosion, sclerosis, or squaring, 2 for a syndesmophyte, and 3 for total bony bridging, giving a maximum possible score of 72. The mSASSS was applied by one observer (AW) (fig 1). In a previous experiment we determined that intraobserver (intraclass correlation coefficient (ICC) = 0.98) and interobserver (ICC = 0.99) reliability on mSASSS status scores of this observer were excellent.22 Radiographic hip involvement determined according to the Bath ankylosing spondylitis radiology index (BASRI)‐hip23 was as follows: 0, no involvement; 1, (possible) focal joint space narrowing; 2, definite narrowing leaving a circumferential joint space >2 mm; 3, narrowing with circumferential joint space ⩽2 mm or bone on bone apposition of <1 cm; 4, bone deformity or bone on bone apposition ⩾1 cm. Grades 1 and 2 increase by one grade if two of the following bony changes were present: erosions, osteophytes, protrusion. Scores are applied to both hips. For the purpose of this article, hip involvement was defined as a BASRI‐hip >2. Anterior (Ha) and posterior (Hp) height of the vertebrae was measured on lateral radiographs of the thoracic (T4–12) and lumbar spine (L1–5) in millimetres by one observer (DV) (fig 2) Imaging of the first three thoracic vertebrae is mostly inadequate so we omitted them in the analyses. Wedging of a vertebra was calculated as the Ha/Hp ratio. This was defined as mild if the ratio was >0.75 but ⩽0.80, moderate if the ratio was >0.60 but ⩽0.75, and severe if the ratio was ⩽0.60.24 We calculated the mean wedge (mean Ha/Hp ratio) of all thoracic and lumbar vertebrae per patient, as well as for the lumbar and thoracic spine separately.
Figure 1 Assessment of modified Stoke ankylosing spondylitis spine score (mSASSS).
Figure 2 Measurement of vertebral heights.
To assess interobserver reliability of measuring anterior and posterior height two readers measured 70 vertebrae in 10 patients with ankylosing spondylitis. These vertebrae were chosen by an independent observer who tried to include the entire spectrum of deformities. The smallest detectable difference, calculated according to the limits of agreement method by Bland and Altman, was 0.14 for the Ha/Hp ratio. The ICC for mean vertebral wedging was 0.93 (95% confidence interval (CI), 0.85 to 0.96) for absolute heights and 0.84 (0.74 to 0.96) for Ha/Hp ratios.
To quantify hyperkyphosis the distance between occiput and wall (OWD) was assessed with the patient standing with the heels and back against the wall, with hips and knees as straight as possible. The chin was held at the usual carrying level and the patient exerted maximum effort to touch the head against the wall. The distance between the wall and the occiput was measured in centimetres to one decimal place. The better of two tries was recorded.25,26 Patients were grouped as having a normal OWD ( = 0 cm) or an increased OWD (>0 cm).
Statistical analysis
We used χ2 tests to test differences in proportions. Spearman's correlation coefficients were calculated to investigate univariate associations between OWD and mean wedging, mSASSS, and BASDAI. Linear regression analysis was undertaken to investigate the independent contribution of BASDAI, mean wedging, hip involvement, and mSASSS to explain variation in the dependent variable OWD. Covariates in the analysis were age, disease duration, and sex. Variables that were not normally distributed were first normalised by the Van der Waerden technique.
Results
Of the 139 patients, four could not be included because of incomplete data. Table 1 presents some baseline characteristics of the patients. Of the 135 patients with complete data, 50 of the 90 male patients (56%) and 11 of the 45 female patients (24%) had an OWD >0 cm (p = 0.001). Compared with patients with a normal OWD, patients with an increased OWD were older, had a longer mean disease duration, and were more often of male sex. Disease activity (BASDAI) was similar in the two groups with a higher level of physical limitation (BASFI) and radiographic damage of the spine (mSASSS). In our cohort, 12 patients (9%) had severe radiological hip involvement; eight of these had an OWD of >0 cm.
Table 1 Clinical and radiological data on all patients in relation to normal and increased occiput to wall distance.
| Variable | OWD = 0 | OWD >0 | p Value* |
|---|---|---|---|
| (n = 74) | (n = 61) | ||
| Age (y) | 45.7 (10.5) | 53.5 (11.9) | <0.001 |
| Mean disease duration | 13.3 (9.7) | 16.5 (9.6) | 0.06 |
| after diagnosis (y) | |||
| Sex (% male) | 57% | 81% | <0.001 |
| BASDAI | 3.3 (2.3) | 3.6 (2.4) | 0.28 |
| BASFI | 2.8 (2.3) | 4.8 (2.5) | <0.001 |
| MSASSS | 8.0 (10.9) | 28.5 (22.0) | <0.001 |
| Wedge thoracic spine | 0.94 (0.04) | 0.90 (0.06) | <0.001 |
| Wedge lumbar spine | 1.03 (0.05) | 1.05 (0.06) | 0.013 |
| At least one wedged vertebra (n) | 15/74 | 27/61 | 0.005 |
| Severe hip involvement (n) | 4/70 | 8/53 | 0.12 |
Values are mean (SD) unless stated otherwise.
*For the difference between patients with OWD = 0 v >0.
BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; mSASSS, modified Stoke ankylosing spondylitis spine score; OWD, occiput to wall distance; y, years.
Overall, 42 patients had 89 vertebral wedgings, defined as a Ha/Hp ratio ⩽0.80. When comparing fracture rates in patients with normal OWD (n = 74) and increased OWD (n = 61), 15 patients in the normal OWD group (20%) had vertebral fractures compared with 27 (44%) with OWD >0 cm. Of these 89 fractures, 59 (66%) were mild, 29 (33%) moderate, and one (1%) severe. All fractures were found in the thoracic spine except for four (three moderate, one mild) in the first lumbar vertebra and one mild fracture in the fourth lumbar vertebra (table 2). When comparing the number of fractures with OWD we found significant correlations, both when analysing all fractures and when analysing fracture groups (no, 1, or >1 fracture and yes/no fractures) versus OWD (p = 0.002, p = 0.01, p = 0.003, respectively).
Table 2 Distribution of the number of vertebral fractures and their severity rating.
| Vertebra | No of | Percentage of | Mild* | Moderate† | Severe‡ |
|---|---|---|---|---|---|
| fractures | total fractures | fracture | fracture | fracture | |
| T4 | 4 | 3 | 4 | ||
| T5 | 3 | 2 | 2 | 1 | |
| T6 | 13 | 10 | 6 | 7 | |
| T7 | 15 | 11 | 8 | 7 | |
| T8 | 14 | 10 | 6 | 8 | |
| T9 | 7 | 5 | 5 | 2 | |
| T10 | 9 | 7 | 8 | 1 | |
| T11 | 12 | 9 | 9 | 3 | |
| T12 | 7 | 5 | 7 | ||
| L1 | 4 | 3 | 1 | 3 | |
| L2 | |||||
| L3 | |||||
| L4 | 1 | 1 | 1 | ||
| L5 |
*Ha/Hp ratio >0.75 and ⩽0.80.
†Ha/Hp ratio >0.60 and ⩽0.75.
‡Ha/ Hp ratio ⩽0.60.
Spearman's correlation coefficients were calculated to investigate univariate associations between OWD and mean wedging in the thoracic and lumbar spine, mSASSS, hip involvement, and BASDAI. OWD showed significant correlations with all factors except mean lumbar wedging and BASDAI (table 3). To explore the independent contribution of different variables in explaining the variation in OWD (as a continuous measure), linear regression analysis was undertaken. Because age, sex, and disease duration may be associated with OWD spuriously, these variables were included as covariates. Table 4 shows the main results of this analysis. Variation in OWD was primarily explained by mSASSS. There was, however, an independent contribution of both mean thoracic wedging and cross sectional assessed BASDAI. In this analysis severe hip involvement did not independently contribute to explaining variation in OWD, although a positive trend could be recognised.
Table 3 Univariate correlation coefficients between occiput to wall distance, wedging, disease activity, and radiological hip involvement.
| mSASSS | Mean wedging | Mean wedging | BASDAI | Hip | |
|---|---|---|---|---|---|
| thoracic spine | lumbar spine | score >2 | |||
| OWD | 0.56** | −0.45** | 0.16 | 0.10 | 0.20* |
| MSASSS | 1 | −0.32** | 0.05 | 0.05 | 0.16 |
| Mean wedging of thoracic spine | 1 | −0.10 | 0.10 | −0.02 | |
| Mean wedging of lumbar spine | 1 | −0.001 | 0.10 | ||
| BASDAI | 1 | −0.03 |
**p = 0.01; *p = 0.05.
BASDAI, Bath ankylosing spondylitis disease activity index; mSASSS, modified Stoke ankylosing spondylitis spine score; OWD, occiput to wall distance.
Table 4 Multivariate analysis of variables determining occiput to wall distance in patients with ankylosing spondylitis.
| Variable | Standardised β | p Value |
|---|---|---|
| MSASSS | 0.47 | 0.01 |
| Mean wedging | 0.28 | 0.01 |
| of thoracic spine | ||
| BASDAI | 0.15 | 0.05 |
| Hip involvement | 0.12 | 0.08 |
Adjusted for age, sex, and disease duration after diagnosis.
Model R2 = 0.42.
BASDAI, Bath ankylosing spondylitis disease activity index; mSASSS, modified Stoke ankylosing spondylitis spine score.
OWD was merely associated with wedging of the thoracic part of the vertebral column. However, the mSASSS does not take the thoracic spine into account, only the cervical and lumbar spine. We analysed the contribution of the mSASSS of the cervical spine and the lumbar spine separately in explaining OWD by regression models, including the same variables as in the main model. The contribution of both site specific mSASSSs in explaining variation in OWD was approximately similar (stβ = 0.62 for the cervical mSASSS and 0.57 for the lumbar mSASSS).This is not unexpected as the cervical and lumbar mSASSS values were highly correlated (r = 0.64).
Discussion
In the entire OASIS cohort, spinal hyperkyphosis (OWD >0) occurs in half the patients.4 In our study we found a clear correlation between radiological damage assessed on the anterior site of the cervical and lumbar spine (mSASSS) and hyperkyphosis. Furthermore, mean thoracic wedging was significantly and independently contributory to explaining OWD, while lumbar wedging deformities did not contribute to OWD. Wedging was assessed in the thoracic spine as well as in the other parts, but mSASSS does not score thoracic spine. The reason is that radiographic changes in this particular area of the spine are difficult to score and reproducibility is not good. Braun et al published data on magnetic resonance imaging changes in the thoracic spine and they found that the thoracic spine was prominently involved in comparison with the cervical and lumbar spine.27 Missing the thoracic spine scores could have been crucial if we had not found a correlation between mSASSS and OWD, as in such a scenario preferential thoracic spine damage could still be an explanation. However, we found a correlation between radiological changes and OWD, even without including thoracic spine data, and we believe that if we had included the thoracic spine, these data would only have strengthened our findings.
In a multivariate analysis, in addition to mSASSS and mean thoracic wedging, disease activity (BASDAI) independently, although marginally, contributed to spinal hyperkyphosis. Considering the hip involvement in ankylosing spondylitis as a possible factor leading to limitation of movement, one can assume the influence of fixed flexion of the hips on the OWD. Therefore, we investigated the correlation between hip involvement and hyperkyphosis. We analysed this by comparing OWD in patients with severe hip involvement (BASRI >2) versus no or mild hip involvement (BASRI ⩽2). This analysis showed a trend, but did not reach statistical significance in both the univariate and multivariate analyses, possibly due to the low prevalence of hip involvement. It should be stressed that the variables that we have investigated here only in part explain hyperkyphosis. Other potentially contributory factors include inflammation of ligaments, muscles and entheses. And in a previous article we described the role of disc deformities in hyperkyphosis.17 In this study we did not analyse these soft tissues, as we focused on vertebral wedging and radiographic damage.
A shortcoming of our study is the cross sectional design. It would be preferable to have a prospective follow up of patients with ankylosing spondylitis in an early phase of their disease and to observe the development of the spine deformity. On the other hand, others could not find a relation between radiological damage and disease duration, age at diagnosis, or acute phase response in such prospective studies.7 In previous analyses Boonen et al28 concluded that there was no change in self reported health status over a period of four years. In a long term follow up study of the OASIS cohort, in which patients were followed every two months during the first two years and every year thereafter, mean BASDAI did not change (stβ = 0.007 (95% CI, –0.013 to 0.027)), which is why we believe a cross sectional approach is justified.
Possible confounders of the relation between wedging and OWD are age and sex, as OWD may increase with age, and as ankylosing spondylitis is a predominantly male disease with a more serious course in male than in female patients.1 However, we did not find a relation between age, sex, and OWD, either univariate or multivariate.
In ankylosing spondylitis the burden of illness is a result of longstanding inflammation and its consequences. Further, cumulative inflammation results in radiological damage reflected in mSASSS scores. In our study we found an independent significant relation between these factors and hyperkyphosis.
In analogy with periarticular bone loss in rheumatoid arthritis, we hypothesise that vertebral wedging is the result of periarticular bone loss in the spine.29 Inflammatory processes are found to be associated with increased bone loss and bone turnover.9,30 This results in reduced bone mineral density.
Osteoporosis is shown to be associated with inflammatory rheumatic diseases, such as rheumatoid arthritis31,32 and ankylosing spondylitis.11 Vertebral deformities are considered to be the main feature of osteoporosis. Our study confirmed an independent significant relation between wedging of (thoracic) vertebrae and hyperkyphosis.
Conclusions
We found three independent significant contributory factors to hyperkyphosis: structural damage of the spine, wedging of thoracic vertebrae, and cross sectional disease activity. In addition we found a significantly higher BASFI in patients with an OWD of >0 cm. Therefore, in order to prevent or limit functional decline, future studies on new treatments in ankylosing spondylitis should include prevention, if possible, of the development of structural damage as well as prevention of the development of vertebral osteoporosis and its effect on hyperkyphosis.
Acknowledgments
We thank T Schoonbrood MD for her assistance in scoring the vertebral radiographs.
Abbreviations
BASDAI - Bath ankylosing spondylitis disease activity index
BASFI - Bath ankylosing spondylitis functional index
BASRI - Bath ankylosing spondylitis radiology index
mSASSS - modified Stoke ankylosing spondylitis spine score
Ha - anterior height of vertebra
Hp - posterior height of vertebra
ICC - intraclass correlation coefficient
OASIS - Outcome in Ankylosing Spondylitis International Study
OWD - occiput to wall distance
References
- 1.Carette S, Graham D, Little H, Rubenstein J, Rosen P. The natural disease course of ankylosing spondylitis. Arthritis Rheum 198326186–190. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson M, Bywaters E G L. Clinical features and course of ankylosing spondylitis. Ann Rheum Dis 195817209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer D G, Park W M, Dick H M, Papazoglou S N, Buchanan W W. Radiological manifestations in 200 patients with ankylosing spondylitis: correlation with clinical features and HLA‐B27. J Rheumatol 19796305–315. [PubMed] [Google Scholar]
- 4.Spoorenberg A, van der Heijde D, de Klerk E, Dougados M, deVlam K, Mielants H.et al A comparative study of the usefulness of the Bath Ankylosing Spondylitis Functional Index and the Dougados Functional Index in the assessment of ankylosing spondylitis. J Rheumatol 199926961–965. [PubMed] [Google Scholar]
- 5.McMaster M J. Osteotomy of the cervical spine in ankylosing spondylitis. J Bone Joint Surg Br 199779197–203. [DOI] [PubMed] [Google Scholar]
- 6.Kim K T, Suk K S, Cho Y M, Hong G P, Park B J. Clinical outcome results of pedicle subtraction osteotomy in ankylosing spondylitis with kyphotic deformity. Spine 200227612–618. [DOI] [PubMed] [Google Scholar]
- 7.Averns H L, Oxtoby J, Taylor H G, Jones P W, Dziedzic K, Dawes P T. Radiological outcome in ankylosing spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J Rheumatol 199635373–376. [DOI] [PubMed] [Google Scholar]
- 8.Bronson W D, Walker S E, Hillman L S, Keisler D, Hoyt T, Allen S H. Bone mineral density and biochemical markers of bone metabolism in ankylosing spondylitis. J Rheumatol 199825929–935. [PubMed] [Google Scholar]
- 9.Lee Y S, Schlotzhauer T, Ott S M, van Vollenhoven R F, Hunter J, Marcus R.et al Skeletal status of men with early and late ankylosing spondylitis. Am J Med 1997103233–241. [DOI] [PubMed] [Google Scholar]
- 10.Devogelaer J P, Maldague B, Malghem J, Nagant de Deuxchaisnes C. Appendicular and vertebral bone mass in ankylosing spondylitis. A comparison of plain radiographs with single‐ and dual‐photon absorptiometry and with quantitative computed tomography. Arthritis Rheum 1992351062–1067. [DOI] [PubMed] [Google Scholar]
- 11.Will R, Palmer R, Bhalla A K, Ring E F J, Calin A. Osteoporosis in early ankylosing spondylitis: a primary pathologic event? Lancet. 1989 Dec 23/30 21483–1485. [DOI] [PubMed] [Google Scholar]
- 12.Maghraoui A, Borderie D, Cherruau B, Eduoard R, Dougados M, Roux C. Osteoporosis, body composition and bone turnover in Ankylosing Spondylitis. J Rheumatol 1999262205–2209. [PubMed] [Google Scholar]
- 13.Cooper C, Carbone L, Michet C J, Atkinson E J, O'Fallon W M, Melton L J. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol 1994211877–1882. [PubMed] [Google Scholar]
- 14.Ralston S H, Urquhart G D K, Brzeski M, Sturrock R D. Prevalence of vertebral compression fractures due to osteoporosis in ankylosing spondylitis. BMJ 1990300563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly S, Doyle D V, Denton A, Rolfe I, McCloskey E V, Spector T D. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann Rheum Dis 199453117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra D, Elvins D M, Speden D J, Collins A J. The prevalence of vertebral fractures in mild ankylosing spondylitis and their relationship to bone mineral density. Rheumatology 20003985–89. [DOI] [PubMed] [Google Scholar]
- 17.Geusens P, Vosse D, van der Heijde D M, Vanhoof J, van Tubergen A, Raus J.et al High prevalence of thoracic vertebral deformities and discal wedging in ankylosing spondylitis patients with hyperkyphosis. J Rheumatol 2001281856–1861. [PubMed] [Google Scholar]
- 18.van der Linden S M, Valkenburg H A, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 198427361–368. [DOI] [PubMed] [Google Scholar]
- 19.Calin A, Nakache J P, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath ankylosing spondylitis disease activity index) an appropriate instrument? J Rheumatol 199438878–882. [DOI] [PubMed] [Google Scholar]
- 20.Calin A, Garrett S, Whitelock H, Kennnedy G, O'Hea J, Mallorie P.et al A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath ankylosing spondylitis functional index. J Rheumatol 1994212281–2285. [PubMed] [Google Scholar]
- 21.Creemers M C W, Franssen M J A M, van't Hof M A, Gribnau F W J, van de Putte L B A, van Riel P L C M. Assessment of outcome in ankylosing spondylitis: an extended radiological scoring system. Ann Rheum Dis 200564127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanders A J B, Landewé R B M, Spoorenberg A, Dougados M, van der Linden S, Mielants H.et al What is the most appropriate radiological scoring method for ankylosing spondylitis? A comparison of the available methods based on the OMERACT filter. Arthritis Rheum 2004502622–2632. [DOI] [PubMed] [Google Scholar]
- 23.MacKay K, Mack C, Brophy S, Calin A. The Bath ankylosing spondylitis radiology index (BASRI). A new, validated approach to disease assessment. Arthritis Rheum 1998412263–2270. [DOI] [PubMed] [Google Scholar]
- 24.Genant H K, Wu C Y, Kuijk van C, Nevitt M C. Vertebral fracture assessment using a semi quantitative technique. J Bone Miner Res 199381137–1148. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N.Musculoskeletal clinical metrology. Dordrecht: Kluwer Academic publishers, 1993
- 26.Heuft‐Dorenbosch L, Vosse D, Landewe R, Spoorenberg A, Dougados M, Mielants H.et al Measurement of spinal mobility in ankylosing spondylitis: comparison of occiput‐to‐wall and tragus‐to‐wall distance. J Rheumatol 2004311779–1784. [PubMed] [Google Scholar]
- 27.Braun J, Baraliakos X, Golder W, Hermann K G, Listing J, Brandt J.et al Analysing chronic spinal changes in ankylosing spondylitis: a systematic comparison of conventional x rays with magnetic resonance imaging using established and new scoring systems. Ann Rheum Dis 2004631046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonen A, van der Heijde D, Landewé R, Mielants H, Dougados M, van der Linden S. Health status in patients with ankylosing spondylitis shows no relevant change over a period of 4 years. Clin Exp Rheumatol. 2004;22: 682 P4, 12
- 29.Haugeberg G, Strand A, Kvien T K, Kirwan J R. Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis. Results from a randomized placebo‐controlled trial. Arch Intern Med 20051651293–1297. [DOI] [PubMed] [Google Scholar]
- 30.Toussirot E, Ricard‐Blum S, Dumoulin G, Cedoz J P, Wendling D. Relationship between urinary pyridinium cross‐links, disease activity and disease subsets of ankylosing spondylitis. Rheumatology 19993821–37. [DOI] [PubMed] [Google Scholar]
- 31.Deodhar A A, Woolf A D. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol 199635309–322. [DOI] [PubMed] [Google Scholar]
- 32.Laan R F J M, Buijs W C A M, Verbeek A L, Draad M P, Corstens F H, van de Putte L B.et al Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis 19935221–26. [DOI] [PMC free article] [PubMed] [Google Scholar]