Abstract
Objective
To investigate the relation between temporal artery biopsy (TAB) length and diagnostic sensitivity for giant cell arteritis.
Methods
Histological TAB reports generated from four hospital pathology departments were reviewed for demographics, histological findings, and formalin fixed TAB lengths. A biopsy was considered positive for giant cell arteritis if there was a mononuclear cell infiltrate predominating at the media–intima junction or in the media.
Results
Among 1821 TAB reports reviewed, 287 (15.8%) were excluded because of missing data, sampling errors, or age <50 years. Mean TAB length of the 1520 datasets finally analysed (67.2% women; mean (SD) age, 73.1 (10.0) years) was 1.33 (0.73) cm. Histological evidence of giant cell arteritis was found in 223 specimens (14.7%), among which 164 (73.5%) contained giant cells. Statistical analyses, including piecewise logistic regression, identified 0.5 cm as the TAB length change point for diagnostic sensitivity. Compared with TAB length of <0.5 cm, the respective odds ratios for positive TAB without and with multinucleated giant cells in samples ⩾0.5 cm long were 5.7 (95% confidence interval, 1.4 to 23.6) and 4.0 (0.97 to 16.5).
Conclusions
A fixed TAB length of at least 0.5 cm could be sufficient to make a histological diagnosis of giant cell arteritis.
Keywords: giant cell arteritis, temporal artery biopsy, sensitivity
The diagnosis of giant cell arteritis is most solidly established by the demonstration of granulomatous inflammation in a temporal artery biopsy (TAB). For between 7% and 44% of cases of giant cell arteritis, however, TAB examination is unremarkable1 and it is unclear to what extent these non‐informative specimens reflect false negative histological findings. One of several factors that has been advanced to explain false negative TAB results is that, because of the “skipping” nature of the inflammatory involvement in giant cell arteritis,2,3 histological changes may be missed in a TAB taken in an arteritis‐free segment.
The resultant widely accepted dogma is that a long TAB segment should be excised to maximise the chance of visualising disease components. Although it is most commonly recommended that samples of 2–3 cm4 or 3–5 cm5 length be taken, the TAB length yielding optimal diagnostic sensitivity remains unknown. We therefore undertook this study in an attempt to determine the relation between TAB length and diagnostic sensitivity for giant cell arteritis.
Methods
We reviewed the written histological TAB reports from four pathology departments in three university hospitals (centres 1–3), each having internal medicine, rheumatology, and ophthalmology units, and one public ophthalmology hospital (centre 4). These departments customarily measured the lengths of formalin fixed TAB samples on macroscopic examination and recorded them in their reports. Using those departments' computerised databases, we retrieved all consecutive TAB reports that had been generated during well defined periods. Selected time periods covered either the entire interval from computerisation of histological reports until study onset or were chosen so as to obtain approximately 300 to 400 reports per centre. All four departments routinely applied a similar protocol for microscopic examination by dividing the artery, when sufficiently long, into several segments, paraffin embedding, and transverse cutting of 3–5 µm thick sections stained with haematoxylin and eosin and sometimes saffron; longitudinal sections of the artery were not examined in any of the centres.
The selected TAB reports were printed out and two of us (AM and MS) analysed them together. The abstracted information included sex, patient's age at biopsy, TAB length, unilateral or bilateral TAB, and histological findings. Histological findings were recorded with respect to the presence of inflammatory cell infiltrate, giant cells, intimal thickening, and fragmentation of the internal elastic lamina. Details on the precise number of sections and levels examined were not mentioned in most reports. For patients who underwent bilateral TAB, the combined length of the two specimens was recorded. After all data had been entered, we searched for those patients who had undergone sequential TAB within a 30 day period. Assuming that those sequential TABs had been taken within the same diagnostic episode, their individual sample lengths were also added and histological findings were pooled with those of the first TAB. TAB were considered to be giant cell arteritis positive when a mononuclear cell infiltrate predominating at the media–intima junction or in the media had been seen; the presence of multinucleated giant cells was not mandatory.4 Because of their uncertain pathological significance, samples showing mild lymphocyte infiltration in the adventitial or periadventitial tissue exclusively were not classified as giant cell arteritis.3,6
Statistics
Quantitative variables were compared using Student's t test. Logistic regression analysis was used to model the relation between TAB length and diagnostic yield. To identify a potential change point in the risk of obtaining a positive TAB, we used a piecewise linear approach7 adapted to the context of logistic regression. Accordingly, we ran a backward stepwise logistic regression of a model including the variable “TAB length” and five transformed covariates for possible change points (0.5, 1.0, … , 2.5 cm). A potential change point was considered when the best fitted model used the untransformed and one transformed covariate. All confidence intervals (CI) were calculated at the 95% level.
Results
We reviewed a total of 1821 histological TAB reports from the centres 1–4 (453, 475, 592, and 287, respectively), covering sampling periods of 8 to 13 years. Among these, 287 (15.8%) were excluded from this analysis because the TAB length was missing (n = 200), the birth date unrecorded (n = 18), sampling errors were present (n = 23), or age was less than 50 years at biopsy (n = 80). Among the remaining 1534 samples (obtained from 1501 patients), we identified 13 individuals who had two (n = 12) or three TABs (n = 1) taken within 30 days. The principal demographic data and histological findings of the 1520 TAB datasets ultimately retained are given in table 1. Histological findings consistent with giant cell arteritis were recorded in 223 TABs (14.7%; table 1); all but three of these also had at least one of the following features: multinucleated giant cells; intimal thickening; fragmentation of the internal elastic lamina. Samples containing multinucleated giant cells always also had an inflammatory infiltrate consistent with giant cell arteritis.
Table 1 Demographic and histological findings for 1520 TAB data sets gathered from 4 pathology departments.
| Variable | All 4 centres | Centre 1 | Centre 2 | Centre 3 | Centre 4 |
|---|---|---|---|---|---|
| TAB datasets analysed (n) | 1520 | 405 | 394 | 459 | 262 |
| Period of TAB sampling | 1991 to 2004 | 1996 to 2003 | 1991 to 2003 | 1997 to 2004 | 1997 to 2004 |
| Demographics | |||||
| Age at biopsy (y) (mean (SD)) | 73.1 (10.0) | 74.1 (9.8) | 73.5 (9.7) | 73.0 (10.6) | 71.4 (9.8) |
| Female (%) | 67.2 | 71.6 | 71.1 | 67.8 | 53.1 |
| Bilateral TAB | |||||
| Simultaneously (n) | 57 | 12 | 10 | 3 | 32 |
| Within 30 days (n) | 13 | 1 | 7 | 5 | 0 |
| TAB data | |||||
| Length (cm) (mean (SD)) | 1.33 (0.72) | 1.52 (0.80) | 1.43 (0.71) | 1.28 (0.88) | 1.13 (0.67) |
| Positive TAB (%) | 14.7 | 12.4 | 16.0 | 13.1 | 18.7 |
| Mononuclear cell infiltrate (%) | 100 | 100 | 100 | 100 | 100 |
| Fragmentation of the IEL (%) | 95.0 | 78.7 | 100 | 100 | 100 |
| Intimal thickening (%) | 94.4 | 80.9 | 95.1 | 100 | 100 |
| Multinucleated giant cells (%) | 73.5 | 66.7 | 82.5 | 83.3 | 57.1 |
IEL, internal elastic lamina; TAB, temporal artery biopsy; y, years.
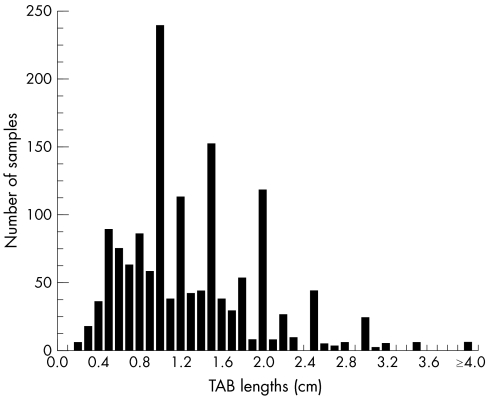
The distribution of the TAB lengths is shown in fig 1 and demonstrates a tendency of measures to concentrate around key values. The mean (SD) TAB length was 1.33 (0.72) cm (median 1.20 cm; range 0.1 to 6.5). Among the 70 bilateral TABs taken either simultaneously or sequentially within 30 days, the mean combined length of both segments was 2.69 (1.14) cm (median 2.50; range 0.6 to 6.5). Mean TAB lengths of samples positive and negative for giant cell arteritis did not differ significantly for the entire dataset (1.34 (0.64) v 1.33 (0.73) cm; p = 0.72) or among the 70 bilateral TABs (2.61 (0.79) v 2.71 (1.20) cm; p = 0.79). Table 2 lists the positive biopsy rates and odds ratios of detecting giant cell arteritis for six classes of TAB lengths stratified by 0.5 cm increments. Piecewise regression analysis retained the level of 0.5 cm as the change point for diagnostic yield (p = 0.03), with additional statistical analyses confirming this value as the most pertinent cut off. Compared with TAB <0.5 cm, the odds ratio for obtaining a positive TAB for longer samples was 5.7 (95% CI, 1.4 to 23.6) (p = 0.016). Similar results were obtained for the odds ratio of detecting positive TABs with giant cells (table 2).
Figure 1 Distribution of the temporal artery biopsy (TAB) lengths, based on 1520 datasets.
Table 2 Odds ratios of obtaining a positive temporal artery biopsy (TAB) as a function of the sample length (derived from 1520 TAB datasets).
| TAB length | Diagnostic sensitivity | |||
|---|---|---|---|---|
| All giant cell arteritis | Giant cell arteritis with giant cells | |||
| n/total n (%) | OR (95% CI) | n/total n (%) | OR (95% CI) | |
| Class | ||||
| <0.5 cm* | 2/66 (3.0%) | 1 | 2/66 (3.0%) | 1 |
| 0.5–0.9 cm | 56/381 (14.7%) | 5.5 (1.3 to 23.2) | 38/381 (10.0%) | 3.5 (0.8 to 15.1) |
| 1.0–1.4 cm | 79/486 (16.3%) | 6.2 (1.5 to 25.9) | 61/486 (12.6%) | 4.6 (1.1 to 19.2) |
| 1.5–1.9 cm | 45/290 (15.5%) | 5.9 (1.4 to 24.9) | 33/290 (11.4%) | 4.1 (0.96 to 17.6) |
| 2.0–2.4 cm | 23/172 (13.4%) | 4.9 (1.1 to 21.6) | 19/172 (11.0%) | 4.0 (0.9 to 17.6) |
| ⩾2.5 cm | 18/125 (14.4%) | 5.4 (1.2 to 24.0) | 11/125 (8.8%) | 3.1 (0.7 to 14.4) |
| Change point | ||||
| <0.5 cm* | 2/66 (3.0%) | 1 | 2/66 (3.0%) | 1 |
| ⩾0.5 cm | 220/1454 (15.1%) | 5.7 (1.4 to 23.6%)† | 162/1454 (11.1%) | 4.0 (0.97 to 16.5*)‡ |
*Reference value.
†p = 0.016; ‡p = 0.055.
CI, confidence interval; OR, odds ratio.
Discussion
This review of 1520 TAB datasets did not show a clear cut linear relation between increasing TAB length and giant cell arteritis positivity. Our search for an optimal cut off suggested that the best diagnostic sensitivity might be offered for TAB of at least 0.5 cm long. These results were further supported by our subanalysis of the accuracy to detect full blown giant cell arteritis based on the presence of multinucleated giant cells.
The average fixed TAB length of 1.33 cm observed here might appear disappointing but is in close accord with reported mean or median TAB lengths of 1.0 to 1.5 cm6,8,9,10 and highlights that, in practice, harvesting of long arterial segments is not readily achieved. Our finding that 15% of biopsied patients had giant cell arteritis also fits with published figures of 11–20%.6,8,10,11,12 Higher rates of 33% positive TAB have been reported,13 with these differences either pointing toward a higher quality in performing and analysing TAB, more restrictive indications for ordering them, or the use of broader histological definitions for the diagnosis of giant cell arteritis.
Although surprising, our finding that TAB length is potentially less important than has been thought is supported by previous observations. Indeed, the existence of skip lesions in giant cell arteritis has not been confirmed by all histological studies.14 According to investigators who did find them, it would appear that skipped areas were around 1 mm long2 and, consequently, it could be expected that even short TAB might be sufficient to contain histological signs of arteritis. Furthermore, although conflicting data have been published,9,11 the authors of several studies found that the mean lengths of positive and negative TAB did not differ,6,8,12,13 as confirmed herein. This lack of difference seems to suggest that short sample lengths might not overtly explain negative TAB findings. Notably, our results are also in close agreement with the claim that giant cell arteritis can be detected in samples as short as 0.4 cm.8
Our study has potential drawbacks. Because we were blinded to clinical data and as TAB can sometimes be diagnostic for other vasculitides,4 we cannot certify that all biopsies had been carried out for suspected giant cell arteritis. To overcome this limitation, we discarded a priori TAB reports done in patients less than 50 years old, and the age and sex distribution of the patients ultimately retained resembled that of the population in which giant cell arteritis usually occurs.5 We were also unable to verify the extent to which histological examination was equally thorough, regardless of sample size. Most importantly, it cannot be excluded that our results might have suffered from an indication bias, with longer arterial segments having been removed in cases where the index of suspicion of giant cell arteritis was lower. Nonetheless, we think it unlikely that a surgeon's decision concerning the length of artery to be biopsied could have been systematically influenced by prebiopsy or intraoperative diagnostic probability.
To summarise, this study challenges the idea that the possibility of a histological diagnosis of giant cell arteritis increases steadily with TAB length and might indicate that, beyond 0.5 cm, fixed sample length does not substantially affect diagnostic accuracy. Our results should be interpreted within the confines of the histological definition applied herein and the TAB length range investigated, and taking into consideration the formalin fixation induced shortening of the biopsy specimen length by around 8%.15 In the light of the assertion that a long TAB is particularly critical in the setting of low diagnostic suspicion,5 further research is warranted to determine whether our findings are applicable to all patterns of presentation of giant cell arteritis.
Acknowledgements
Supported by grants from the French Vasculitis Study Group and by the GIS‐Institut des maladies rares.
Abbreviations
TAB - temporal artery biopsy
References
- 1.Lee A G, Brazis P W. Temporal arteritis: a clinical approach. J Am Geriatr Soc 1999471364–1370. [DOI] [PubMed] [Google Scholar]
- 2.Albert D M, Ruchman M C, Keltner J L. Skip areas in temporal arteritis. Arch Ophthalmol 1976942072–2077. [DOI] [PubMed] [Google Scholar]
- 3.Klein R G, Campbell R J, Hunder G G, Carney J A. Skip lesions in temporal arteritis. Mayo Clin Proc 197651504–510. [PubMed] [Google Scholar]
- 4.Weyand C M, Goronzy J J. Giant‐cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003139505–515. [DOI] [PubMed] [Google Scholar]
- 5.Salvarani C, Cantini F, Boiardi L, Hunder G G. Polymyalgia rheumatica and giant‐cell arteritis. N Engl J Med 2002347261–271. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarty A, Franks A J. Temporal artery biopsy: is there any value in examining biopsies at multiple levels? J Clin Pathol 200053131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T. BMDP program for piecewise linear regression. Comput Methods Programs Biomed 19862353–55. [DOI] [PubMed] [Google Scholar]
- 8.Chambers W A, Bernardino V B. Specimen length in temporal artery biopsies. J Clin Neuroophthalmol 19888121–125. [Google Scholar]
- 9.Sudlow C. Diagnosing and managing polymyalgia rheumatica and temporal arteritis. Sensitivity of temporal artery biopsy varies with biopsy length and sectioning strategy. BMJ 1997315549. [PMC free article] [PubMed] [Google Scholar]
- 10.McDonnell P J, Moore G W, Miller N R, Hutchins G M, Green W R. Temporal arteritis. A clinicopathologic study. Ophthalmology 198693518–530. [DOI] [PubMed] [Google Scholar]
- 11.Kent R B, Thomas L. Temporal artery biopsy. Am Surg 19905616–21. [PubMed] [Google Scholar]
- 12.Albertini J G, Ramsey M L, Marks V J. Temporal artery biopsy in a dermatologic surgery practice. Dermatol Surg 199925501–508. [DOI] [PubMed] [Google Scholar]
- 13.Achkar A A, Lie J T, Hunder G G, O'Fallon W M, Gabriel S E. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med 1994120987–992. [DOI] [PubMed] [Google Scholar]
- 14.Cohen D N, Smith T R. Skip areas in temporal arteritis: myth versus fact. Trans Am Acad Ophthalmol Otolaryngol 197478772–783. [Google Scholar]
- 15.Danesh‐Meyer H V, Savino P J, Bilyk J R, Eagle R C, Sergott R C. Shrinkage: fact or fiction? Arch Ophthalmol 20011191217. [PubMed] [Google Scholar]