Abstract
Objectives
To investigate the anti‐inflammatory effects of the active leflunomide metabolite A771726 (Lef‐M) in combination with methotrexate (MTX) on synovial macrophages (SM) from rheumatoid arthritis (RA) patients co‐cultured with an activated T cell line (Jurkat cell line).
Methods
Pro‐inflammatory cytokines (TNFα, IL1β, IL6), adhesion molecule ICAM‐1, cyclooxygenase isoenzymes (COX1, COX2), and the nuclear factor κB (NF‐κB) complex were analysed on SM co‐cultured with a T cell line, as intracellular protein expression by immunocytochemistry (ICC) and western blot analysis, as extracellular protein expression by ELISA assay, and as mRNA expression by reverse transcriptase‐multiplex PCR (RT‐MPCR) after treatment with Lef‐M (1, 10, 30 μmol/l) alone or in combination with MTX (50 ng/ml).
Results
The most significant intracellular decrease in cytokines was observed by ICC in SM treated with the combination of Lef‐M (1, 10, 30 μmol/l) and MTX (50 ng/ml) versus untreated SM (TNFα 29%, 37%, 49%, IL1β 56%, 43%, 50%, and IL6 59%, 62%, 71%, respectively). Furthermore, a significant decrease was confirmed concerning cytokine levels evaluated by ELISA in the medium of SM treated with the combination Lef‐M+MTX (TNFα 40%, 41%, 44%; IL1β 10%, 20%, 60%; IL6 37%, 41%, 49%, respectively). Western blot and RT‐PCR analysis confirmed these results. Concordant decreased expression was observed for ICAM‐1, COX1, COX2, and the NF‐κB complex after Lef‐M+MTX treatment.
Conclusions
The combination of MTX and Lef‐M shows additive inhibitory effects on the production of inflammatory mediators from SM co‐cultured with a T cell line. These observations might support the positive results obtained in RA clinical studies by combination therapy.
Keywords: leflunomide, macrophages, methotrexate, rheumatoid arthritis, TNFα
Migration of activated lymphocytes and monocytes into the synovial tissue in rheumatoid arthritis (RA) is the first stage in synovial inflammation which is then followed by subsequent degradation of the joints.1,2 These lymphocytes and monocytic cells require an increase in de novo synthesis of pyrimidines in order to progress from G1 to the S phase of the cell cycle.3 Leflunomide (Lef) (N‐(4‐trifluoro‐methylphenyl)‐5‐methylisoxazole‐4‐carboxamide), mainly through its active metabolite A771726 (Lef‐M), at low therapeutic doses reversibly inhibits the enzyme dihydro‐orotate dehydrogenase (DHODH), the rate limiting step in de novo synthesis of pyrimidines in different cell lines.4
However, recent studies suggest that the observed anti‐inflammatory effects exerted by Lef‐M are strongly related to its ability to inhibit osteoclastogenesis as well as metalloproteinase and inflammatory cytokine production by cultured RA synovial cells and to inhibit activation following cell‐cell contact between T lymphocytes and monocytes.5,6,7
In addition, further studies indicate that Lef‐M seems to interfere with nuclear factor κB (NF‐κB) complex activation and to down regulate the glycosylation of adhesion molecules such as ICAM‐1.8,9,10
Recently, Lef‐M was found to influence the trans‐endothelial migration of peripheral blood mononuclear cells (PBMC) and to decrease cell adhesion molecule activity, such as monocytic CD44 expression and PBMC‐hyaluronan binding by inhibiting DHODH in treated RA patients.11 All these effects might act together to block cell activation and cell traffic into inflamed RA synovial tissue.
However, given the high failure rate of RA monotherapy and the multifactorial nature of the pathogenesis of RA, combinations of different therapeutic agents are increasingly being administered to inhibit the complex processes of the disease. In particular, Lef has been shown to be useful in combination with methotrexate (MTX) in RA patient management.12,13,14
MTX is the most common disease modifying antirheumatic drug used in RA therapy and acts by inhibiting dihydrofolate reductase and hence decreasing the supply of reduced folates for purine.15 A number of anti‐inflammatory effects exerted by MTX seem to be related to the induction of extracellular adenosine increase and its interaction with specific cell surface receptors with subsequent inhibition of IL8 production by PBMC, IL6 secretion by human monocytes, leukotriene B4 synthesis in neutrophils, and decreased synovial collagenase gene expression.16 In addition, MTX seems to exert anti‐inflammatory and antiproliferative effects particularly on activated monocytes.17,18
Recently, RA patients treated with a combination of MTX and Lef exhibited significant suppression of several major chemokines including monocyte derived chemokine (MCP‐1) and macrophage derived chemokine (MDC‐1).19 Positive correlations among reductions in plasma chemokines and clinical outcome measures were also found.19
Therefore, we decided to investigate the effects of Lef‐M and its combination with MTX in a co‐culture of an activated T cell line and RA synovial macrophages (SM). The study focused on mRNA expression and detection of intra‐ and extracellular protein for different mediators of the inflammatory reaction, such as cytokines (TNFα, IL1β, IL6), adhesion molecule ICAM‐1, cyclooxygenase isoenzymes (COX1, COX2), and the NF‐κB pathway, as a complex of transcriptional molecules modulating cellular responses in activated cells.20
Methods
Co‐culture of the T cell line and SM
Synovial macrophages were obtained from five RA patients who fulfilled the American College of Rheumatology criteria for adult RA.21 The ethical committee approved the study and informed consent was obtained from patients.
Mean±standard deviation (SD) disease duration was 4±2 years and disease activity as assessed by the DAS28 score was 4.1. No RA patients had been treated with oral or intra‐articular corticosteroids during the 3 months preceding therapeutic arthroscopic synoviectomy.
After synoviectomy, the synovial RA tissue samples were gently cut into small pieces (2–5 mm), washed in Dulbecco's phosphate buffered saline (DPBS; Sigma‐Aldrich, Sigma Chemical Division, Milan, Italy) and incubated in collagenase (0.75 mg/ml; type IV from Clostridium histolyticum; Sigma‐Aldrich) for 1 h at 37°C. The digest was passed through a wire 150 mesh to separate the synovial cells from the debris tissue. The cells were then washed three times with DPBS, resuspended in RPMI‐1640 medium (Sigma‐Aldrich) supplemented with 10% fetal bovine serum (containing <0.5 EU/ml endotoxin), 2 mmol/l l‐glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin (Sigma‐Aldrich). The synovial cells were seeded into multiwell flat bottom plates (5×105 cells/well) and cultured in 5% CO2 air humidified atmosphere at 37°C. Viability of the cells (95–97%) was tested by trypan blue exclusion. After 1 h, non‐adherent cells were washed out, while adherent cells (SM) were co‐cultured for 24 h in a trans‐well system (Corning‐Costar, Acton, MA, USA) together with a T cell line (Jurkat) previously activated with concanavalin‐A (1 μg/ml) for 20 h, in the absence of as well as in the presence of different concentrations (1, 10, 30 μmol/l) of A771726 (Aventis Pharma Deutschland, Frankfurt am Main, Germany). Co‐cultures (T cell line+macrophages) were also treated with the three different concentrations of Lef‐M (1, 10, 30 μmol/l) in combination with MTX (50 ng/ml).
The utilised concentrations of Lef‐M were close to the serum levels obtained with therapeutic doses in RA patients.10 The MTX dose (50 ng/ml) has already been determined as the concentration achievable in the serum of RA patients treated with low dose MTX.17,18 However, the capacity of cultured cells to take up MTX might be underestimated when studies are performed in medium with high concentration of folic acid.
At the end of the co‐culture incubation period, the Jurkat T cell line was removed with several washes in PBS and SM were harvested, washed in PBS, and used for the different test assays. Experiments were performed in triplicate: each experiment, performed with SM obtained from an individual patient, was replicated three times.
Immunocytochemistry assay
Macrophages were harvested mechanically and then incubated on glass slides for 45 min at 4°C. The cellular spots were then fixed in acetone for 30 s, air dried, and stored at −20°C until use. After rehydration in PBS to prevent non‐specific antibody binding, the spots were incubated with rabbit normal serum for 20 min. The spots were then incubated with anti TNFα (lot no. C161), IL1β (lot no. J100), IL6 (lot no. G170), and ICAM‐1 (lot no. C221) antibodies diluted 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 45 min.
Linked antibodies were detected by biotinylated universal (pan‐specific) antibody and subsequently horseradish peroxidase streptavidin (Vector Laboratories, Burlingame, CA, USA). Each step was followed by two washes in PBS. The staining reaction was developed by the diaminobenzidine system (Ylem, Rome, Italy). Finally, slides were counterstained with haematoxylin, fixed with ethanol, and cover slipped with Eukitt. Controls were treated identically, however the primary antibodies were omitted.
Image analysis
Image analysis was performed with the Leica Q‐Win image analysis system (Leica, Cambridge, UK). About 100 cells were analysed for each sample, and pixels/μm2 (positive area) were quantified by the Leica Q‐Win software. The single cells were randomly selected by the operators using the cursor and then automatically measured as positive area.
Immune enzymatic assay
After 24 h of treatment, the culture medium was harvested and stored at −20°C until analysis. The enzyme immunoassay for quantitative determination of TNFα, IL1β, and IL6 was carried out with a microplate kit system (Diaclone, Besançon, France) (CVintra: 2.15; CVextra: 3.85). The results were obtained with a multiwell plate automatic processor (Thecno Genetics, Milan, Italy).
Western blot analysis
After the different treatments, macrophage pellets were lysed in a buffer containing 20 mmol/l Tris‐HCl pH 8, 150 mmol/l NaCl, 1 mmol/l phenylmethylsulphonylfluoride, 5 mg/ml aprotinin, and 0.5% Nonidet P‐40 (Promega, Milan, Italy) for 1 h at 4°C. The lysates were then centrifuged for 10 min at 13 000 rpm. Protein samples (20 μg) were diluted with sample buffer and separated by 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. The proteins were transferred to a Hibond‐C nitrocellulose membrane (Amersham Pharmacia Biotech Europe, Freiburg, Germany). After transfer, the membrane was blocked for 1 h at room temperature in PBS containing 5% non‐fat powdered milk.
For immunoblot analysis, the membrane was incubated with anti TNFα, IL1β, and IL6 polyclonal antibodies diluted 1:500 (Santa Cruz Biotechnology) overnight at 4°C, washed in 0.05% PBS/Tween 20 pH 7.4 and incubated for 1 h at room temperature with secondary horseradish peroxidase labelled goat antibody (Santa Cruz Biotechnology). After three further washes with PBS/Tween, bound secondary antibody was detected by emitting chemiluminescent reaction (Amersham Pharmacia Biotech Europe).
mRNA analysis
After treatment at the different culture conditions, aliquots were harvested from the samples, washed in DPBS twice, and treated with Trizol mRNA lysis buffer for total RNA extraction. All samples were analysed by reverse transcriptase‐multiplex PCR (RT‐MPCR; MBL, Maxim Biotech, San Francisco, CA) for temporal and spatial distribution of mRNA expression of different cytokines (TNFα, IL1β, IL6, IL8, GM‐CSF), COX1, COX2, NF‐κB sub unity p50/p52, and NF‐κB inhibitors. This method is an accurate and valid system for detecting multiple gene expression by amplifying all the genes under the same conditions. The PCR primers designed by Maxim Biotech have similar Tm and no obvious 3′ end overlap to enhance multiple amplification. The 680 bp (TNFα), 555 bp (IL1β), 424 bp (GM‐CSF), 360 bp (IL6), 300 bp (IL8), 161 bp (TGFβ), 278 bp (COX2), 218 bp (COX1), 183 bp (NF‐κB inhibitors), and 143 bp (NF‐κB p50/p52) PCR products can be generated from human RNA or the positive control, which is included in the kits.
Statistical analysis
The results were analysed by the ANOVA non‐parametric test (Bonferroni test) and represent mean values±SD of five different experiments. Each experiment, performed with SM obtained from an individual patient, was repeated three times (in triplicate).
Results
Effects of Lef‐M alone and with MTX
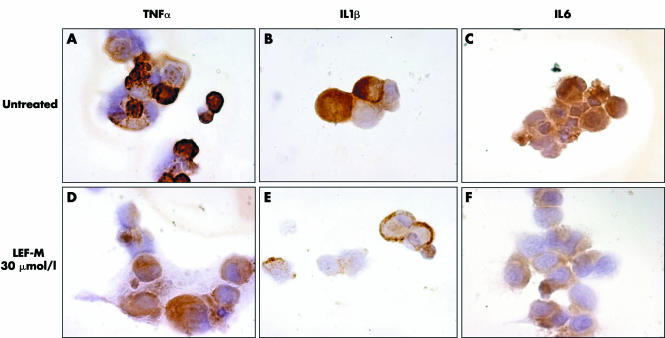
Synovial macrophages co‐cultured with an activated T cell line showed a significant reduction in cytokine content, evaluated as intracytoplasmatic protein expression by immunocytochemistry (ICC) following treatment with Lef‐M at different concentrations (1, 10, 30 μmol/l) (fig 1).
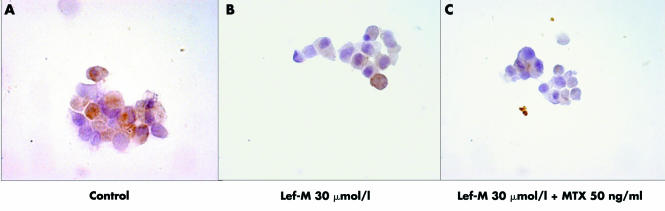
Figure 1 Intracytoplasmatic detection of TNFα, IL1, and IL6 by ICC assay in co‐cultured synovial macrophages (SM) not treated (A, B, and C, respectively) and treated with Lef‐M (1, 10, 30 μmol/l) (D, E, and F, respectively). (magnification 100×)
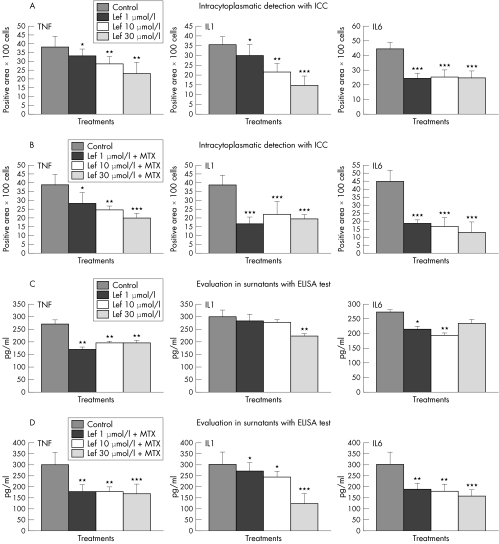
The observed changes included a dose dependent decrease in TNFα of 13% (p<0.05), and 24% and 40% (p<0.01) and in IL1β of 16% (p<0.05), 39% (p<0.01), and 58% (p<0.001) following treatment with 1, 10, and 30 μmol/l Lef‐M, respectively. For IL6 we observed a non‐dose dependent decrease of 46%, 43%, and 44% (all p<0.001) when compared with controls (untreated co‐culture) (fig 2A).
Figure 2 (A) Evaluation of TNFα, IL1β, and IL6 intracytoplasmatic expression in co‐cultured SM treated with 1, 10, and 30 μmol/l Lef‐M and untreated SM (control) by ICC and image analysis. Results are expressed as means±SD of different synovial macrophage (SM) populations obtained from five different RA patients. Each experiment, using cells obtained from an individual patient, was performed three times. *p<0.05 v control; **p<0.01 v control; ***p<0.001v control. (B) Evaluation of TNFα, IL1β, and IL6 intracytoplasmatic expression in co‐cultured SM treated with Lef‐M 1, 10, and 30 μmol/l in combination with MTX (50 ng/ml) and untreated SM (control) by ICC and image analysis. Results are expressed as means±SD of different SM populations obtained from five patients. Each experiment, using cells obtained from an individual patient, was performed three times. *p<0.05 v control; **p<0.01 v control; ***p<0.001 v control. (C) Evaluation of TNFα, IL1β, and IL6 levels in surnatants of co‐cultured SM treated with Lef‐M 1, 10, and 30 μmol/l and untreated SM (control) by ELISA assay. *p<0.05 v control; **p<0.01 v control. (D) Evaluation of TNFα, IL1β, and IL6 levels in surnatants of co‐cultured SM treated with Lef‐M 1, 10, and 30 μmol/l in combination with MTX (50 ng/ml) and untreated SM (control) by ELISA assay. *p<0.05 v control; **p<0.01 v control; ***p<0.001 v control.
The combined treatment with Lef‐M and MTX induced a further reduction in cytokine intracytoplasmatic expression at the level of macrophages (TNFα 29% (p<0.05), 37% (p<0.01), 49% (p<0.001); IL1β 56%, 43%, 50% (all p<0.001); IL6 59%, 62%, 71% (all p<0.001)). In particular, a dose dependent decrease in TNFα expression was observed, whereas the decrease in IL1β and IL6 was not found to be dose dependent (fig 2B).
A decrease in cytokine concentration was confirmed in the media of the co‐culture after treatment with Lef‐M (different concentrations) when compared with untreated cells (TNFα 37% (p<0.01), and 28% and 27% (p<0.05); IL1β 6% and 8% (NS), 26% (p<0.05); IL6 21% (p<0.05), 29% (p<0.01), 14% (NS)). The reduction in IL1β levels was dose dependent, in particular in relation to the 30 μmol/l doses, while the decrease in TNFα and IL6 was quite similar for all Lef‐M concentrations (fig 2C). Interestingly, treatment of the co‐culture with Lef‐M in combination with a unidose of MTX induced a further significant cytokine reduction, particularly for TNFα (40% and 41% (p<0.01), 44% (p<0.001)), IL1β (10% and 20% (p<0.05), 60% (p<0.001)), and IL6 (37% and 41% (p<0.01), 49% (p<0.001)) (fig 2D).
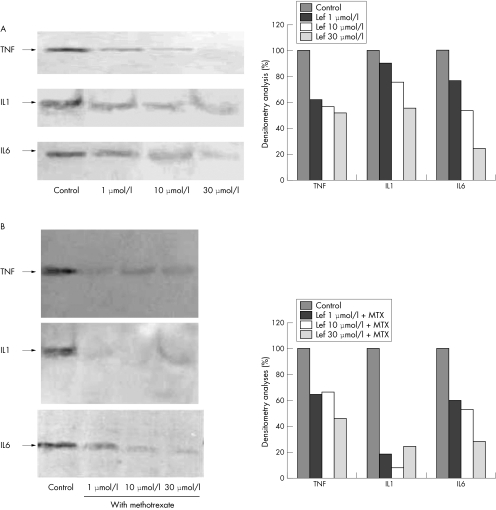
The data acquired with western blot analysis for both Lef‐M alone and Lef‐M in combination with MTX were obtained with densitometry analyses and confirmed a down regulation of cytokine intracytoplasmatic expression versus untreated cells; a greater decrease in signal was evident in the cells treated with Lef‐M+MTX. The data were expressed as area and normalised versus control (fig 3A,B).
Figure 3 (A) Western blot analysis of TNFα, IL1β, and IL6 protein expression in co‐cultured SM untreated (control) and treated with Lef‐M 1, 10, and 30 μmol/l. Right: densitometry analyses. (B) Western blot analysis of TNFα, IL1β, and IL6 protein expression in co‐cultured SM untreated (control) and treated with Lef‐M (1, 10, and 30 μmol/l) in combination with MTX (50 ng/ml). Right: densitometry analyses.
Comparative analysis of data obtained from ICC, western blot, and ELISA assay confirms a reduction in cytokine production in the co‐cultured SM treated with Lef‐M in combination with MTX.
Effects of Lef‐M alone and with MTX on cytokine mRNA expression
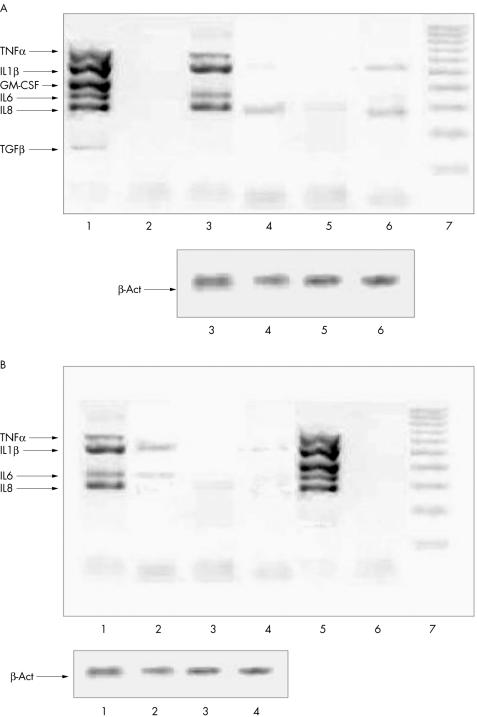
The effects of Lef‐M, alone or in combination with MTX, on mRNA expression in co‐cultured SM were assessed by multiplex RT‐PCR analysis for expression of different genes. A decrease in the mRNA for all cytokines when compared with the untreated co‐cultured SM was detected. mRNA expression decreased in a non‐dose dependent manner (fig 4A). Interestingly, the combination of Lef‐M and MTX induced a further evident and additive down regulation of mRNA expression for all tested cytokines (fig 4B).
Figure 4 (A) Expression of cytokine mRNAs evaluated by multiplex RT‐PCR. Lane 1, positive control; lane 2, negative control; lane 3, untreated co‐cultured SM; lane 4, co‐cultured SM treated with Lef‐M (1 μmol/l); lane 5, with Lef‐M (10 μmol/l); lane 6, with Lef‐M (30 μmol/l); lane 7, markers of molecular weight. Bottom: expression of the housekeeping gene β‐actin. (B) Expression of cytokine mRNAs evaluated by multiplex RT‐PCR. Lane 1, untreated co‐cultured SM; lane 2, co‐cultured SM treated with Lef‐M (1 μmol/l) in combination with MTX (50 ng/ml); lane 3, with Lef‐M (10 μmol/l) and MTX (50 ng/ml); lane 4, with Lef‐M (30 μmol/l) and MTX (50 ng/ml); lane 5, positive control; lane 6, negative control; lane 7, markers of molecular weight. Bottom: expression of the housekeeping gene β‐actin.
Effects of Lef‐M alone and with MTX on ICAM‐1 protein expression
ICC analysis showed that treatment with Lef‐M induced decreased expression of the ICAM‐1 protein at all tested concentrations when compared with untreated controls. Treatment with Lef‐M (different doses) in combination with MTX (unidose) confirmed a more evident additive decrease in ICAM‐1 expression (fig 5A–C). These data were confirmed by image analysis (data not shown).
Figure 5 Intracytoplasmatic detection of ICAM‐1 evaluated by ICC assay. (A) ICAM‐1 detection in untreated co‐cultured SM. (B) ICAM‐1 detection in co‐cultured SM treated with Lef‐M (30 μmol/l) alone. (C) ICAM‐1 detection in co‐cultured SM treated with Lef‐M (30 μmol/l) in combination with MTX (50 ng/ml). (magnification 50×)
Effects of Lef‐M alone and with MTX on COX1, COX2, and NF‐κB complex mRNA expression
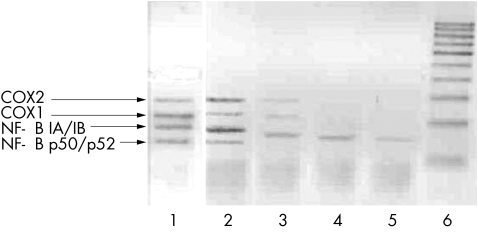
In the present study, analysis with RT‐MPCR for expression of different genes showed an evident dose dependent decrease in COX mRNA expression in co‐cultured SM after treatment with Lef‐M (fig 6).
Figure 6 Expression of COX1 and COX2, NF‐κB inhibitors, and NF‐κB complex mRNAs evaluated by multiplex RT‐PCR. Lane 1, positive control; lane 2, untreated co‐cultured SM (control); lane 3, co‐cultured SM treated with Lef‐M (1 μmol/l); lane 4, with Lef‐M (10 μmol/l); lane 5, with Lef‐M (30 μmol/l); lane 6, markers of molecular weight.
In addition, mRNA expression for the NF‐κB subunit p50/p52 disappeared after 24 h in treated co‐cultured SM at all Lef‐M concentrations and mRNA expression of NF‐κB inhibitors decreased in a dose dependent manner (fig 6).
Interestingly, in the combination treatment with Lef‐M and MTX, a total loss of mRNA signal was observed for all gene expression analysed here (data not shown).
Discussion
The present study shows that the pro‐inflammatory activity of cultured RA SM is enhanced in the presence of an activated T cell line and that the addition of the active metabolite of Lef (A771726) exerts a significant anti‐inflammatory effect by decreasing the macrophage production of pro‐inflammatory cytokines (TNFα, IL1β, IL6), adhesion molecule ICAM‐1, COXs, and NF‐κB expression, as a complex of transcriptional molecules modulating cellular responses in activated cells.
The treatment of these co‐cultures with the combination of Lef‐M and MTX induced a further significant decrease in the inflammatory mediators mentioned above, supporting a possible additive action of MTX, as already postulated a few years ago.22 The effects of the combination Lef‐M+MTX versus the presence of Lef‐M alone was found to be particularly impressive and significant as regards the production of the pro‐inflammatory cytokines (TNFα, IL1β, and IL6) by the activated RA macrophages.
Since the same dose of MTX (50 ng/ml) was used in all combination experiments, the observed inhibitory influences reported here were mainly driven by Lef‐M, as its dose related effects (1, 10, 30 μmol/l) were still evident after MTX addition. Furthermore, in our previous studies, no significant anti‐inflammatory effects of MTX (50 ng/ml) alone were found on cultured RA macrophages when compared to activated monocytic cells, suggesting a less potent action of MTX on differentiated cells (that is, macrophages).17,18
The results of this study strongly support a recent clinical investigation showing that RA patients treated with a combination of MTX and Lef exhibited significant suppression of several major chemokines including MCP‐1 and MDC‐1.19 These RA patients had received MTX 15 mg/week for not less than 3 months before entry into the study. A loading dose of 100 mg/day of Lef was then given for 3 days, followed by 10 mg/day for the remainder of the study period. Moreover, positive correlations among reductions in plasma chemokines and clinical outcome measures were also found.19 The study suggests that combination therapy with Lef and MTX exhibits anti‐inflammatory activity in the suppression of chemokine expression and subsequent recruitment of inflammatory cells into the inflammatory sites in RA.19 In further studies it would be of interest to use an experimental model involving RA SM in co‐cultures with peripheral blood lymphocytes of the same RA patients in order to investigate the effects of Lef‐M and MTX combination therapy.
Several clinical studies have recently confirmed that combination therapy with Lef and MTX improves clinical responses in active RA patients and that it is safe.13,14,23
Therefore, the results of the present in vitro study seem to confirm the recent observation of a significantly reduced number of macrophages, together with decreased expression of TNFα, IL1β, and ICAM‐1, in synovial tissue samples obtained from patients with RA after 4 months of treatment with Lef or MTX.24
Concerning ICAM‐1 expression, Lef‐M by depleting the pyrimidine pool seems to down regulate the glycosylation of adhesion molecules, further reducing cell‐cell contact activation and homing of inflammatory cells during the inflammatory reaction.10,25 Lef‐M was found to influence the trans‐endothelial migration of PBMC by inhibiting DHODH in treated RA patients (mainly monocytes).11 Fluorocytometric analysis of PBMC subsets within the migrated population showed a decrease in monocytes, but not in B or T cells, after Lef‐M treatment. Furthermore, incubation with Lef‐M of the PBMC from RA patients also decreased other cell adhesion molecule activity, such as monocytic CD44 expression and PBMC‐hyaluronan binding.11
The present study shows that the combination of Lef‐M and MTX induces an additive decreased expression of ICAM‐1 on macrophages co‐cultured with an activated T cell line. Therefore, the further anti‐inflammatory mechanism exerted by both drugs in RA seems to be related to the decrease in possible cell‐cell contact activation.26
Recently, dynamic gadolinium enhanced magnetic resonance imaging (DRMRI) analysis of synovial tissue in patients with RA treated with Lef as well as MTX showed a significant reduction in the inflammatory reaction for both drugs.27
Because the DRMRI result correlates with the fact that leucocytes infiltrate RA synovial tissue, it was confirmed that Lef as well as MTX exert anti‐inflammatory activity mainly on monocytes/macrophages. Actually, very similar effects on basic mechanisms of inflammation have been observed for both Lef‐M and MTX in combination or as a single treatment.10,15,17,18,28
The results of the present study support an additive effect of MTX when combined with Lef‐M concerning anti‐inflammatory activity with effects on macrophages co‐cultured with a T cell line. However, the additive or synergistic effect of the combination should be confirmed in a study comparing each drug alone at different doses as well as when combined.
The significant decrease in inducible COX2 and in COX1 found for the first time in the present study on mRNA expression, confirms earlier observations that suggest a wide anti‐inflammatory effect both for Lef‐M, as shown here, and for MTX (data not shown).10,29,30
Since Lef‐M has also been shown to suppress the activation of NF‐κB, a potent mediator of inflammation when stimulated by inflammatory agents, the present study suggests that the inhibition is realised at the level of mRNA expression, supporting another important target of the Lef mechanisms of action.31,32,33
The more detailed effects of Lef‐M and MTX (alone or in combination) on NF‐κB complex activation, IκB‐α phosphorylation (ser 32), and NF‐κB DNA binding should be investigated further.
In conclusion, the combination of MTX and Lef‐M shows additive inhibitory effects on the production of inflammatory mediators by SM co‐cultured with a T cell line. These observations possibly support the positive results obtained in RA combination clinical studies and might allow a reduced daily dosage of both drugs when combined.34,35
Supplementary Material
Abbreviations
DHODH - dihydro‐orotate dehydrogenase
DPBS - Dulbecco's phosphate buffered saline
DRMRI - dynamic gadolinium enhanced magnetic resonance imaging
ICC - immunocytochemistry
Lef - leflunomide
Lef‐M - leflunomide metabolite
MCP‐1 - monocyte derived chemokine
MDC‐1 - macrophage derived chemokine
MTX - methotrexate
NF‐κB - nuclear factor κB
PBMC - peripheral blood mononuclear cells
RA - rheumatoid arthritis
RT‐MPCR - reverse transcriptase‐multiplex PCR
SD - standard deviation
SM - synovial macrophages
Footnotes
This study was supported by a bursary for a young scientist from Aventis
Competing interests: none declared
References
- 1.Hitchon C A, el‐Gabalawy H S. The histopathology of early synovitis. Clin Exp Rheumatol 200321S28–S36. [PubMed] [Google Scholar]
- 2.Ospelt C, Neidhart M, Gay R E, Gay S. Synovial activation in rheumatoid arthritis. Front Biosci 200492323–2334. [DOI] [PubMed] [Google Scholar]
- 3.Fairbanks L D, Bofil M, Ruckerman K, Simmonds H A. Importance of ribonucleotide availability to proliferating T‐lymphocytes from healthy humans. J Biol Chem 199549253–279. [PubMed] [Google Scholar]
- 4.Davis J P, Cain G A, Pitts W J, Magolda R L, Copeland R A. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry 1996351270–1273. [DOI] [PubMed] [Google Scholar]
- 5.Urushibara M, Takayanagi H, Koga T, Kim S, Isobe M, Morishita Y.et al The antirheumatic drug leflunomide inhibits osteoclastogenesis by interfering with receptor activator of NF‐kappa B ligand‐stimulated induction of nuclear factor of activated T cells c1. Arthritis Rheum 200450(3)794–804. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam O, Yaron I, Shirazi I, Judovitch R, Caspi D, Yaron M. Active leflunomide metabolite inhibits interleukin 1beta, tumour necrosis factor alpha, nitric oxide, and metalloproteinase‐3 production in activated human synovial tissue cultures. Ann Rheum Dis 200362(5)440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger D, Begue‐Pastor N, Benavent S, Gruaz L, Kaufmann M T, Chicheportiche R.et al The active metabolite of leflunomide, LEF‐M, inhibits the production of prostaglandin E(2), matrix metalloproteinase 1 and interleukin 6 in human fibroblast‐like synoviocytes. Rheumatology (Oxford) 200342(1)89–96. [DOI] [PubMed] [Google Scholar]
- 8.Manna S K, Mukhopadhyay A, Aggarwal B B. Leflunomide suppresses TNF‐induced cellular responses: effects on NF‐kappa B, activator protein‐1, c‐Jun N‐terminal protein kinase, and apoptosis. J Immunol 2000165(10)5962–5969. [DOI] [PubMed] [Google Scholar]
- 9.Breedveld F C, Dayer J M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis 200059(11)841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutolo M, Sulli A, Ghiorzo P, Pizzorni C, Craviotto C, Villaggio B. Anti‐inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann Rheum Dis 200362(4)297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grisar J, Aringer M, Koller M D, Stummvoll G H, Eselbock D, Zwolfer B.et al Leflunomide inhibits transendothelial migration of peripheral blood mononuclear cells. Ann Rheum Dis 200463(12)1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer J M, Genovese M C, Cannon G W, Caldwell J R, Cush J J, Furst D E.et al Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 2002137(9)726–733. [DOI] [PubMed] [Google Scholar]
- 13.Kremer J, Genovese M, Cannon G W, Caldwell J, Cush J, Furst D E.et al Combination leflunomide and methotrexate (MTX) therapy for patients with active rheumatoid arthritis failing MTX monotherapy: open‐label extension of a randomized, double‐blind, placebo controlled trial. J Rheumatol 200431(8)1521–1531. [PubMed] [Google Scholar]
- 14.Van Riel P. Leflunomide improves the clinical response in patients with active rheumatoid arthritis treated with methotrexate. Clin Exp Rheumatol 200321(6)695–696. [PubMed] [Google Scholar]
- 15.Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub R H. Anti‐inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 200160(8)729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronstein B N. The mechanism of action of methotrexate. Rheum Dis Clin North Am 199723(4)739–745. [DOI] [PubMed] [Google Scholar]
- 17.Cutolo M, Sulli A, Craviotto C, Felli L, Pizzorni C, Seriolo B.et al Antiproliferative‐antiinflammatory effects of methotrexate and sex hormones on cultured differentiating myeloid monocytic cells (THP‐1). Ann N Y Acad Sci 2002966232–237. [DOI] [PubMed] [Google Scholar]
- 18.Cutolo M, Bisso A, Sulli A, Felli L, Briata M, Pizzorni C.et al Antiproliferative and antiinflammatory effects of methotrexate on cultured differentiating myeloid monocytic cells (THP‐1) but not on synovial macrophages from patients with rheumatoid arthritis. J Rheumatol 200027(11)2551–2557. [PubMed] [Google Scholar]
- 19.Ho C Y, Wong C K, Li E K, Tam L S, Lam C W. Suppressive effect of combination treatment of leflunomide and methotrexate on chemokine expression in patients with rheumatoid arthritis. Clin Exp Immunol 2003133(1)132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacher S, Schmitz M L. The NF‐kappaB pathway as a potential target for autoimmune disease therapy. Curr Pharm Des 200410(23)2827–2837. [DOI] [PubMed] [Google Scholar]
- 21.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831(3)315–322. [DOI] [PubMed] [Google Scholar]
- 22.Kremer J M. Methotrexate and leflunomide: biochemical basis for combination therapy in the treatment of rheumatoid arthritis. Semin Arthritis Rheum 199929(1)14–26. [DOI] [PubMed] [Google Scholar]
- 23.Weinblatt M E, Kremer J M, Coblyn J S, Maier A L, Helfgott S M, Morrell M.et al Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum 199942(7)1322–1328. [DOI] [PubMed] [Google Scholar]
- 24.Kraan M C, Reece R J, Barg E C, Smeets T J M, Farnell J, Rosenburg R.et al Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Arthritis Rheum 200041820–1830. [DOI] [PubMed] [Google Scholar]
- 25.Elder R T, Xu X, Gong H, Finnegan A, Chong A S F. The immunosuppressive metabolite of leflunomide, A771726, affects murine T cells through two biochemical mechanisms. J Immunol 199715922–27. [PubMed] [Google Scholar]
- 26.Dimitrijevic M, Bartlett R R. Leflunomide, a novel immunomodulating drug, inhibits homotypic adhesion of mononuclear cells in rheumatoid arthritis. Transplant Proc 1996283086. [PubMed] [Google Scholar]
- 27.Recee R J, Kraan M C, Radjenovic A, Veale D J, O'Connor P J, Ridgway J P.et al Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum 200246366–372. [DOI] [PubMed] [Google Scholar]
- 28.Smith M D, Kraan M C, Slavotinek J, Au V, Weedon H, Parker A.et al Treatment‐induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatology (Oxford) 200140367–374. [DOI] [PubMed] [Google Scholar]
- 29.Halminton L C, Vojnovic I, Warner T D. A771726, the active metabolite of leflunomide, directly inhibits activity of cyclo‐oxygenase‐2 in vitro and in vivo in a substrate‐sensitive manner. Br J Pharmacol 19991271589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weithmann K U, Jeske S, Schlotte V. Effects of leflunomide on constitutive and inducible pathways of cellular eicosanoid generation. Agents Actions 199441(3–4)164–170. [DOI] [PubMed] [Google Scholar]
- 31.Manna S K, Aggarwal B B. Immunosuppressive leflunomide metabolite (A771726) blocks TNF‐dependent nuclear factor‐kB activation and gene expression. J Immunol 19991622095. [PubMed] [Google Scholar]
- 32.Manna S K, Mukhopadhyay A, Aggarwal B B. Leflunomide suppresses TNF‐induced cellular response: effects on NF‐kB, activator protein‐1, c‐Jun, N‐terminal protein kinase and apoptosis. J Immunol 20001655962–5969. [DOI] [PubMed] [Google Scholar]
- 33.Fairbanks L D, Bofill M, Ruckemann K, Simmonds H A. Importance of ribonucleotide availability to proliferating T‐lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibition. J Biol Chem 199527029682–29689. [PubMed] [Google Scholar]
- 34.Kalden J R, Smolen J S, Emery P, van Riel P L, Dougados M, Strand C V.et al Lefunomide in combination therapy. J Rheumatol Suppl 20047125–30. [PubMed] [Google Scholar]
- 35.Dayer J M, Cutolo M. Is there a rationale to using leflunomide in early rheumatoid arthritis? Clin Exp Rheumatol 200523404–412. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.